Rola Husni7, Remie Chrabieh7, Rita Wilson Dib2, Jose Vazquez2, Thais Guimaraes3, Ana Fernández4, Rita Khoury7, Lina Asmar5, Georges Khazen7, Nadia Samaha6, Issam Raad1 and Ray Hachem1.
1 Department
of Infectious Diseases, Infection Control, and Employee Health, The
University of Texas M.D. Anderson Cancer Center, Houston, Texas.
2 Department of Internal Medicine, Medical College of Georgia, Augusta University, GA, USA.
3 Department of Infectious Diseases Hospital do Servidor Publico estadul de Sao Paulo, Sao Paulo, Brazil.
4 Department of Infectious Diseases, Hospital Universitario Puerta de Hierro Segovia de Arana Majadahonda (Madrid).
5 Lianasmar Consulting.
6 Georgetown University, Washington, DC, USA.
7 Gilbert & Rose-Marie Chagoury School of Medicine, Lebanese American University, Beirut, Lebanon..
Correspondence to: Ray Hachem, MD. Department of Infectious
Diseases, Infection Control and Employee Health, Unit 402, The
University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd.,
Houston, TX 77030. E-mail:
rhachem@mdanderson.org
Published: May 1, 2021
Received: February 3, 2020
Accepted: April 9, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021031 DOI
10.4084/MJHID.2021.031
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Candida bloodstream infection (BSI) remains one of the leading causes
of BSI in critically ill and immunosuppressed cancer patients. In light
of the changing epidemiology and rising resistant species, duration of
treatment and appropriate timing of stepdown therapy from intravenous
(IV) to oral antifungal agents are crucial for utmost disease control
and overall survival.
Method:
We performed a multicenter retrospective study, with 119
non-neutropenic patients enrolled from four different medical
institutions in Brazil, Lebanon, Spain and the United States, to assess
the duration of IV therapy and appropriate time to step-down to oral
therapy in adult patients, 14 years of age and older, with documented
candidemia. The analysis was done using the statistical program R and
SAS v9.4. Descriptive statistics are presented as frequencies and
tables and the Fisher exact test was used to test the association
between the categorical variables: organism, cancer, country,
antifungal drug and duration of therapy, and time of step-down.
Results: Candida albicans
contributed to 45% of bloodstream infection versus 55% of infection
caused by Candida non-albicans. The three most common Candida
non-albicans are: Candida glabrata 24%, Candida parapsilosis 13% and Candida tropicalis
8%. Most (57%) of the patients were admitted to ICU, whereas 52% had
underlying malignancy. Multivariate analysis showed that a stay at ICU
or an underlying cancer requiring chemotherapy were independently
associated with failure and death (p <0.001). The average total
duration of therapy was 14 days in all patients and 16 days in those
who responded and survived. Forty-five patients were stepped down to
either fluconazole and/or voriconazole in association with clinical and
microbiologic resolution of the candidemia. The average (and median)
day of step-down was 5 days. Patients who had a stepdown had more
favorable outcomes (78% survival) as compared to those with no stepdown
(56% survival) (P = 0.022). However, the 20 patients who received 1-4
days of first IV treatment before a stepdown to oral azoles had a
comparable outcome (20% mortality) to the 25 patients who received
>5 days of treatment (24% mortality - p = 0.75).
Conclusion:
Our data support the IDSA guidelines in that the total duration of
treatment for candidemia should be at least 14 days after a negative
blood culture. However, in non-neutropenic cancer patients with
candidemia, a step-down to oral azole therapy can safely take place
early (within 4 days of initiating IV therapy) as long as the patient
had clinical and microbiologic resolution of the bloodstream infections.
|
Introduction
Candida
bloodstream infections have become one of the leading causes of
bloodstream infections (BSI) in critically ill and immunosuppressed
patients.[1,2] They are also associated with high morbidity and mortality rates, which range from 10 to 47%.[3,4]
The main objective of this multicenter study was to examine the
real-world use of antifungal agents in the treatment of candidemia and
assess the appropriate duration of treatment as well as the outcome on
patients who were step-down to oral therapy in non-neutropenic patients
with BSI caused by candida
Given the seriousness of the infection,
determining the appropriate type of therapy with step-down, and
ensuring timely treatment is both essential and crucial.
The
most recent guidelines published in 2016 by the Infectious Disease
Society of America (IDSA) for the management of candidemia recommend a
minimum of 14 days of antifungal therapy after a negative blood culture
in clinically stable patients.[5] Furthermore, the
IDSA guidelines suggest a step-down strategy from intravenous (IV)
antifungals to oral therapy within five to seven days as long as the
signs and symptoms associated with the candidemia resolve with negative
blood cultures.[5] However, these recommendations have
become a routine practice over the last few decades, even though there
have not been prospective randomized studies that evaluated and
determined the appropriate total duration of therapy and time to
step-down to oral therapy.[6-9]
The early initiation of antifungal agents has been associated with favorable survival outcomes,[10]
but the question remains when it should be stopped. To the best of our
knowledge, there are no randomized studies comparing the different
durations of treatment, and a limited number of studies have examined
the appropriate timing to step-down from IV to oral antifungal therapy.
Therefore, the objective of this multicenter international study
was to describe the epidemiology of candidemia cases over the past five
years in four centers located in four different countries and
continents (Brazil, Lebanon, Spain, and USA) in order to evaluate the
duration of IV therapy and determine the appropriate time to step-down
to oral therapy in non-neutropenic adult patients with documented
candidemia.
Methodology
A
multicenter, retrospective study was conducted with 119 patients
enrolled with approximately 20-30 patients from four different
international institutions, including Lebanon, Brazil, Spain, and USA.
Patients 14 years and older with documented candidemia and received at
least one dose of antifungal therapy were included. Patients with
neutropenia at the onset of infection diagnosis or with documented
candida endocarditis, osteomyelitis, meningitis, or disseminated
candidiasis diagnosed within 72 hours from the first positive blood
culture for candida were excluded from the study. Data on demographic
measures, in addition to the occurrence of cancer, other underlying
diseases, and different treatment types, duration and outcomes, were
collected. Step-down therapy refers to switching from IV to oral
antifungal therapy. Response to therapy was defined by clinical
improvement and microbiological eradication of patients with candidemia
treated with appropriate antifungal therapy.
Cox regression
analysis was used to identify the independent predictors of the
response to antifungal therapy (success vs. failure). In both
multivariate analyses, factors with a p-value <0.2 in univariate
analysis were included in each initial multivariate model, and then the
full model was reduced to the final model by a backward variable
elimination procedure. A p-value of less than .05 was considered
statistically significant.
The study was approved by the IRB at
the different sites. The analysis was performed using the statistical
program R, and SAS v 9.4 (SAS Institute Inc., Cary, NC, USA),
descriptive statistical software. The Fisher exact test was used to
test the association between the categorical variables organism,
cancer, country, antifungal drug, and duration of therapy and the
results are presented in tables as frequencies.
Results
In
this retrospective study, a total of 119 patients were enrolled from 4
different countries: Lebanon (34 patients), Brazil (33), Spain (32),
and the USA (20). Table 1 outlines
the demographics of study patients from the four reported country
centers. The median age for 119 patients was 68 years (range, 19 - 91),
and the median duration for their total treatment was 14 days (range,
1- 36). Candida albicans was prominent among 45% of the patients, and 55% were infected with Candida non-albicans. The three most common Candida non-albicans reported were Candida glabrata (24%), Candida parapsilosis (13%), and Candida tropicalis (8%) (Figure 1). The two most common identified sources of infection were the central line (50%) and abdomen (18%). Candida albicans
was notably the most commonly isolated species (spp), causing
candidemia in the centers located in Brazil (67%) and in Spain (53%),
whereas Candida non-albicans was predominant in the USA (85%) and Lebanon (68%). There was a significant association between the Candida organism type and geographical area (Fisher exact test p-value=<0.001).
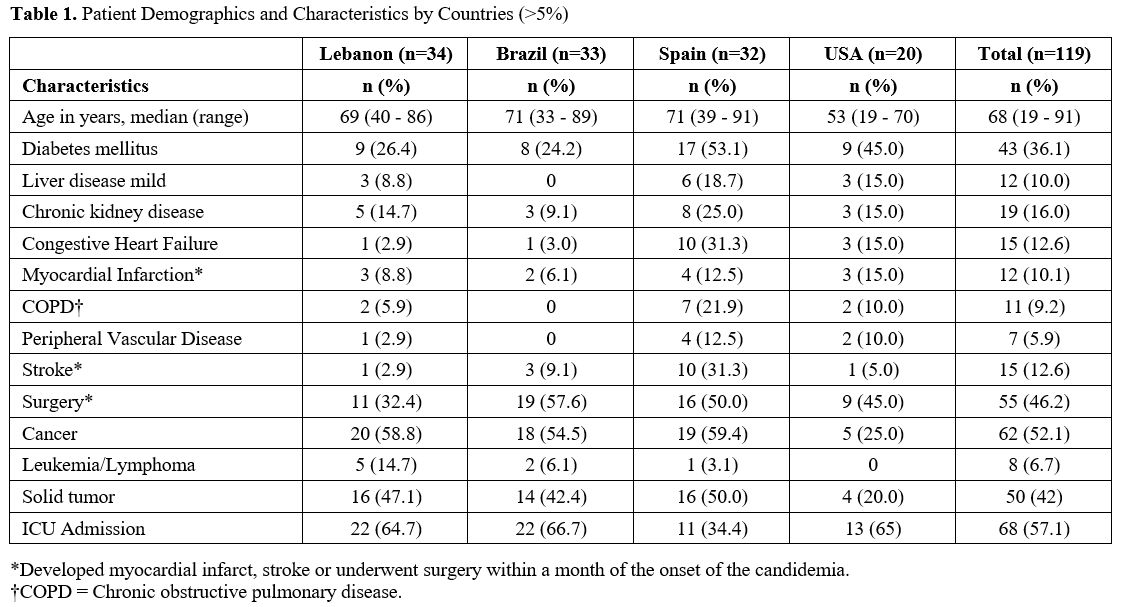 |
Table 1. Patient Demographics and Characteristics by Countries (>5%). |
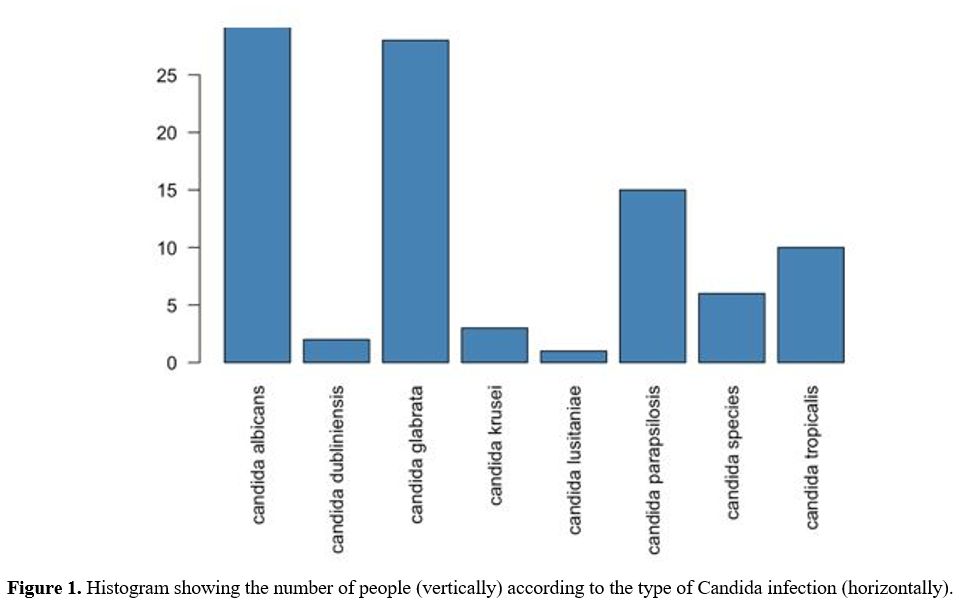 |
Figure 1. Histogram showing the number of people (vertically) according to the type of Candida infection (horizontally). |
Candida glabrata was more commonly found in the Lebanese and American samples compared with samples obtained from patients in Brazil and Spain. Candida albicans had a much higher frequency in Brazil (67%) compared with the USA (15%) and Lebanon (32%). Figure 1 gives the distribution of organisms by country.
Echinocandins
(48%) were the most commonly used antifungal medications, followed by
azoles (39%) and amphotericin B (6%). Most patients (97%) received
their first antifungal through the IV route, while 3% were treated with
oral antifungals. In hospitals in USA and Lebanon, echinocandins were
mainly used as the first antifungal drug, whereas azoles were
predominantly used in Brazil and Spain. No combination therapy was used
in this study.
The mortality rate was 38% but ranged from a high of 64% in Brazil to a low of 16% in Spain (Table 2).
Of the patients who died, 29% were definitely attributable to the
candidemia, 35% of the cases were caused by the infection and 36% of
cases were not attributed to the Candida infection. The highest
mortality rate was observed in Brazil (64%) (Table 2) despite the fact that most (73%) of the candidemia cases in Brazil originated from a removable catheter-related source (Table 3).
In contrast, the USA's mortality rate was more than three-fold lower
than Brazil's (20%) despite the fact that only 10% of the candidemia in
the US was line related (Table 3).
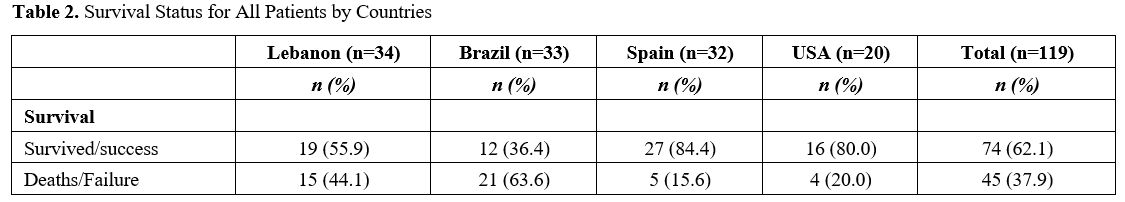 |
Table 2. Survival Status for All Patients by Countries |
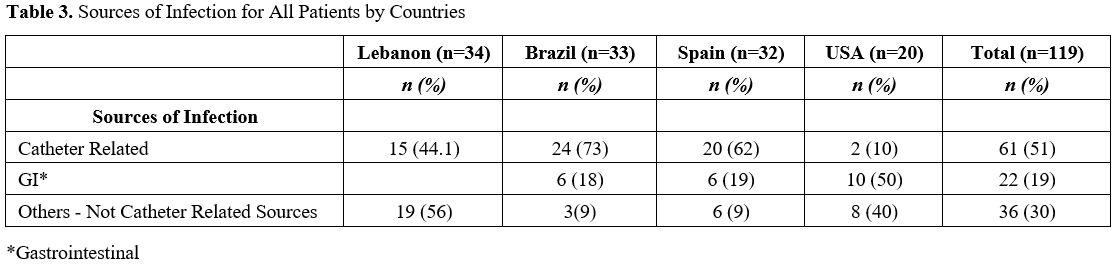 |
Table
3. Sources of Infection for All Patients by Countries. |
Of the 119 patients with candidemia, 57% were admitted to the ICU and 52% had underlying cancer during their hospitalization (Table 1).
The median duration of stay in ICU was 15 days (range, 1 – 34). Of the
107 patients treated for at least 24 hours, 70 (65%) patients responded
to treatment and survived. Of those responders, 32 (46%) were admitted
to ICU (p <0.001). However, 36/37 (97%) of the patients who were
treated and died were admitted to ICU. There was a significant
association between overall mortality and ICU stay (p-value < 0.01).
Of the 45 patients who died (including the 8 not treated), 80% were
admitted to the ICU for an average of 15 days. Multivariate analysis
showed that a stay at ICU or an underlying cancer requiring
chemotherapy were independently associated with failure and death
outcome (HR (95% CI), 5.90 (2.22, 15.71) for ICU, and 6.63 (2.43,
18.11) for chemotherapy P <0.001).
Among treated patients,
the overall average duration of therapy was 14 days and 16 days in
those who had a response to therapy. The median time on treatment for
patients who died was 13 days (range, 1 – 24). The mean total duration
of ICU hospital stay of the patients who did not receive stepdown
therapy was 12 days, with a standard deviation of 8.76. Of the 107
treated patients whose antifungal was changed due to clinical response,
45 (42%) were stepped down to oral therapy. Those 45 patients, who were
stepped down to oral antifungal drugs, received either fluconazole or
voriconazole, while 13% and 3% of patients were stepped up to
echinocandins, and amphotericin B, respectively. The median and mean
duration for the step-down from IV to oral antifungal therapy was 5
days of IV treatment.
Looking at the total population, patients
who were treated and had a stepdown medication had more favorable
outcomes (78% survival) as compared to those with no stepdown (56%
survival) (P = 0.022). However, the 20 patients who received 1-4 days
of first IV treatment before a stepdown to oral azoles had an outcome
(20% mortality) which is comparable to the 25 patients who received
>5 days of treatment (24% mortality - p = 0.75).
Discussion
Current
practice in the treatment of candidemia, when it comes to therapy
duration and the time to step-down from IV antifungal therapy to oral
drugs, has been based on inference rather than evidence. For example,
in a milestone study by Rex et al. published in 1994 in the New England
Journal of Medicine whereby fluconazole and amphotericin B were shown
to be equally effective in the treatment of candidemia, the duration of
therapy in both arms was mandated to be 2 weeks after the last negative
blood culture.[7] This treatment duration (2 weeks)
has become a routine practice for years to come in the absence of hard
data that would highlight the appropriate total duration of therapy and
time to step-down to oral therapy.
Hence, the recent IDSA
guidelines recommended a total duration of treatment for candidemia of
at least 14 days after a negative blood culture. The guidelines also
recommend a step-down to oral azole therapy within 5-7 days as long as
the patient has achieved clinical stability characterized by resolution
of signs and symptoms associated with the infection and clearance of
the candida (which should be susceptible to the azole to be used
orally) from the bloodstream.[5] In 2012 the European
Society for Clinical Microbiology and Infectious Diseases (ESCMID)
suggested stepping down to oral after 10 days of IV therapy if the
patient was stable clinically and isolated candida demonstrated
susceptibility to the oral antifungal drug.[11]
Patients in the present study were treated according to the IDSA guidelines[5]
because the overall average duration of treatment for candidemia in
this current trial was 14 days, and it was 16 days in those patients
who responded to therapy and survived. Furthermore our data do support
step-down to oral azoles in patients with clinical and microbiologic
resolution since patients, who had a step-down, presented a favorable
outcome, even with a more favorable outcome and better survival
compared to those patients who did not undergo any stepdown [p =
0.022]. This improved outcome associated with stepdown to oral therapy
could be related to the fact that a stepdown is dependent on clinical
improvement and microbiologic eradication of the candida from the
bloodstream, and supports de-escalation in selected patients with good
prognostic factors such as the ones described.
However, although
our data show that the mean and median duration for stepdown to oral
antifungal therapy was 5 days (which is in line with the IDSA
guidelines of 5-7 days), there was no indication that a particular
minimal time period of IV therapy is necessary before the stepdown
should occur. When we compared the 20 patients in our study who
received 1–4 days of IV therapy before stepping down to oral azoles
with the 25 patients who received 5 days or more of IV therapy before
the stepdown, there was no difference in outcome and survival. Hence,
stepdown could occur early and at any point in time as long as the
patient demonstrates clinical and microbiologic resolution of the
bloodstream infection.
In an open label non-comparative trial that
evaluated the response to intravenous anidulafungin followed by
stepdown to oral fluconazole, the step-down criteria consisted of 24
hours without fever associated with hemodynamic stability and
documentation of negative blood cultures as well as resolution of the
neutropenia. The time range for the stepdown was 1–6 days in that
study.[12] Another multicenter prospective randomized
trial compared voriconazole IV to amphotericin B and allowed a stepdown
from IV voriconazole to oral voriconazole as well as a stepdown from IV
amphotericin to oral fluconazole. Based on the data, a stepdown on day
3 was proposed if the patient was clinically stable with negative blood
cultures.[13] Hence it is clear from our study and
the literature that the documented clinical and microbiologic
resolution of the candidemia is what determines appropriate timing for
the stepdown to oral antifungal therapy.
Traditionally, Candida albicans has been the most common Candida infection worldwide, followed by candida non-albicans species in both pediatric and adult patients.[14,15] However, over the past 10 years, there has been a change in the epidemiology of candidemia. Specifically, a decrease in the candida albicans infection rate and an increase in the Candida non-albicans infection rate has been reported, particularly for C. parapsilosis, C. glabrata, C. tropicalis, and C. krusei.[15,16] In this study, we found that Candida albicans infections are still the most common Candida species, with 45% of patients being infected with Candida albicans and 55% with Candida non-albicans. The three most common Candida non-albicans were Candida glabrata (24%), Candida parapsilosis (13%), and Candida tropicalis (8%), which are consistent with the literature (Figure 1).[14-17]
Our data showed that 57% of our patients were admitted to the ICU and 52% had an underlying cancer (Table 1).
Hence, the main micro-epidemiological changes in the intensive care and
oncology units (to which most of our patients belonged) has been the
change in the main type of candida species from C. albicans to C. non-albicans species, such as C. glabrata, C. tropicalis, C. krusei, and C. parapsilosis.[18-21]
In the past, fluconazole, have shown efficacy in preventing invasive
candida infections in cancer patients, however, their use has been
associated with the emergence of resistant candida species,
particularly candida non-albicans, such as Candida glabrata and Candida krusei.[19,20] Sun et al. revealed a global decline in Candida albicans infection rates with an associated rise in Candida parapsilosis infections among adult patients with malignancy.[22]
In our current study, Lebanon and the USA had higher cases of
non-albicans candida spp. compared to Brazil and Spain, where albicans
spp. remained the most common, which is also consistent with the
literature.[23-26]
The two most common sources
of candidemia in our study were the central line (possible skin origin)
(50%) and gastrointestinal (18%). Since 57% of our patients were
admitted to the ICU where central venous catheters are commonly used,
it is not surprising that the vascular catheter was implicated as a
source for the candidemia in more than half of our patients (Tables 1 and 2).
Candida species also belong to the normal gastrointestinal flora, and
several risk factors lead to overgrowth of candida and their consequent
spread into the bloodstream. Patients with underlying malignancies are
at an increased risk of invasive candida infections because
chemotherapeutic agents disrupt the gastrointestinal normal flora. More
than half of our patients had underlying malignancy and hence it is not
unusual to have 18% of our patient having a gastrointestinal source for
the candidemia.
As shown in the present study, the mortality
rate associated with candidemia was around 38% which is similar to the
published literature in high-risk patients. By multivariate analysis we
have demonstrated that patients with an ICU admission or with
underlying cancer requiring chemotherapy were significantly associated
with the highest mortality. Hence, the high mortality rate in this
study is likely related to the fact that the majority of the population
analyzed were very sick (57% admitted to the ICU), and have multiple
comorbidities (52% with underlying cancer, 46% with prior surgery and
36% with underlying diabetes mellitus).
However, similar to the
epidemiological distribution of invasive candida infections, mortality
and cure rates may also vary among populations and geographical areas.[30] For instance, the rate of death increased from 29% in the USA to 60% in South Africa and 54-72% in Brazil.[30]
Similar findings were observed in our study, in which Lebanon and
Brazil had higher rates of death compared to the USA and Spain. In some
ways, these results are not surprising because of the numerous factors
that would likely affect the nature, effectiveness, and accuracy of
care given in developing countries.[31,32]
Andes
et al. conducted an individual patient-level review to assess the
clinical outcome, mortality, and factors associated with the success of
treatment.[33] Many factors were identified to affect
the success of treatment and survival of patients, such as early
removal of the central venous catheter and early use of antifungals,
specifically echinocandin.[31,33-35]
In contrast, other studies suggested that advanced age, the presence of
comorbidities, and delaying treatment until positive blood cultures,
are factors that could increase mortality.[32-34,36]
Conclusion
In
summary, our study showed that the mortality associated with candidemia
may be high especially among high-risk patients who are critically ill
or cancer patients receiving chemotherapy. Our data support the IDSA
guidelines and previous reviews[37] in that the total
duration of treatment for candidemia should be at least 14 days after a
negative blood culture. However, our data showed that in
non-neutropenic cancer patients with candidemia, a step-down to oral
azole therapy can safely take place early as long as the patient had
clinical and microbiologic resolution of the bloodstream infection.
References
- Vincent, J.L., J. Rello, J. Marshall, E. Silva, et
al., International study of the prevalence and outcomes of infection in
intensive care units. JAMA, 2009. 302(21): 2323-9. https://doi.org/10.1001/jama.2009.1754 PMid:19952319
- Martin,
G.S., D.M. Mannino, S. Eaton, and M. Moss, The epidemiology of sepsis
in the United States from 1979 through 2000. N Engl J Med, 2003.
348(16): 1546-54. https://doi.org/10.1056/NEJMoa022139 PMid:12700374
- Gudlaugsson,
O., S. Gillespie, K. Lee, J. Vande Berg, et al., Attributable mortality
of nosocomial candidemia, revisited. Clin Infect Dis, 2003. 37(9):
1172-7. https://doi.org/10.1086/378745 PMid:14557960
- Pappas,
P.G., J.H. Rex, J. Lee, R.J. Hamill, et al., A prospective
observational study of candidemia: epidemiology, therapy, and
influences on mortality in hospitalized adult and pediatric patients.
Clin Infect Dis, 2003. 37(5): 634-43. https://doi.org/10.1086/376906 PMid:12942393
- Pappas,
P.G., C.A. Kauffman, D.R. Andes, C.J. Clancy, et al., Clinical Practice
Guideline for the Management of Candidiasis: 2016 Update by the
Infectious Diseases Society of America. Clin Infect Dis, 2016. 62(4):
e1-50. https://doi.org/10.1093/cid/civ933 PMid:26679628 PMCid:PMC4725385
- Kuse,
E.R., P. Chetchotisakd, C.A. da Cunha, M. Ruhnke, et al., Micafungin
versus liposomal amphotericin B for candidaemia and invasive
candidosis: a phase III randomised double-blind trial. Lancet, 2007.
369(9572): 1519-1527. https://doi.org/10.1016/S0140-6736(07)60605-9
- Rex,
J.H., J.E. Bennett, A.M. Sugar, P.G. Pappas, et al., A randomized trial
comparing fluconazole with amphotericin B for the treatment of
candidemia in patients without neutropenia. Candidemia Study Group and
the National Institute. N Engl J Med, 1994. 331(20): 1325-30. https://doi.org/10.1056/NEJM199411173312001 PMid:7935701
- Pappas,
P.G., C.M. Rotstein, R.F. Betts, M. Nucci, et al., Micafungin versus
caspofungin for treatment of candidemia and other forms of invasive
candidiasis. Clin Infect Dis, 2007. 45(7): 883-93. https://doi.org/10.1086/520980 PMid:17806055
- Betts,
R.F., M. Nucci, D. Talwar, M. Gareca, et al., A Multicenter,
double-blind trial of a high-dose caspofungin treatment regimen versus
a standard caspofungin treatment regimen for adult patients with
invasive candidiasis. Clin Infect Dis, 2009. 48(12): 1676-84. https://doi.org/10.1086/598933 PMid:19419331
- Morrell,
M., V.J. Fraser, and M.H. Kollef, Delaying the empiric treatment of
candida bloodstream infection until positive blood culture results are
obtained: a potential risk factor for hospital mortality. Antimicrob
Agents Chemother, 2005. 49(9): 3640-5. https://doi.org/10.1128/AAC.49.9.3640-3645.2005 PMid:16127033 PMCid:PMC1195428
- Cornely,
O.A., M. Bassetti, T. Calandra, J. Garbino, et al., ESCMID* guideline
for the diagnosis and management of candida diseases 2012:
non-neutropenic adult patients. Clin Microbiol Infect, 2012. 18 Suppl
7: 19-37. https://doi.org/10.1111/1469-0691.12039 PMid:23137135
- Vazquez,
J., A.C. Reboli, P.G. Pappas, T.F. Patterson, et al., Evaluation of an
early step-down strategy from intravenous anidulafungin to oral azole
therapy for the treatment of candidemia and other forms of invasive
candidiasis: results from an open-label trial. BMC Infect Dis, 2014.
14: 97. https://doi.org/10.1186/1471-2334-14-97 PMid:24559321 PMCid:PMC3944438
- Kullberg,
B.J., J.D. Sobel, M. Ruhnke, P.G. Pappas, et al., voriconazole versus a
regimen of amphotericin B followed by fluconazole for candidaemia in
non-neutropenic patients: a randomised non-inferiority trial. Lancet,
2005. 366(9495): 1435-42. https://doi.org/10.1016/S0140-6736(05)67490-9
- Wisplinghoff,
H., T. Bischoff, S.M. Tallent, H. Seifert, et al., Nosocomial
bloodstream infections in US hospitals: analysis of 24,179 cases from a
prospective nationwide surveillance study. Clin Infect Dis, 2004.
39(3): 309-17. https://doi.org/10.1086/421946 PMid:15306996
- Marchetti,
O., J. Bille, U. Fluckiger, P. Eggimann, et al., Epidemiology of
candidemia in Swiss tertiary care hospitals: secular trends, 1991-2000.
Clin Infect Dis, 2004. 38(3): 311-20. https://doi.org/10.1086/380637 PMid:14727199
- Pfaller,
M.A. and D.J. Diekema, Epidemiology of invasive candidiasis: a
persistent public health problem. Clin Microbiol Rev, 2007. 20(1):
133-63. https://doi.org/10.1128/CMR.00029-06 PMid:17223626 PMCid:PMC1797637
- Horn,
D.L., D. Neofytos, E.J. Anaissie, J.A. Fishman, et al., Epidemiology
and outcomes of candidemia in 2019 patients: data from the prospective
antifungal therapy alliance registry. Clin Infect Dis, 2009. 48(12):
1695-703. https://doi.org/10.1086/599039 PMid:19441981
- Sipsas,
N.V. and D.P. Kontoyiannis, Invasive fungal infections in patients with
cancer in the Intensive Care Unit. Int J Antimicrob Agents, 2012.
39(6): 464-71. https://doi.org/10.1016/j.ijantimicag.2011.11.017 PMid:22337064 PMCid:PMC3855365
- Slavin,
M.A., T.C. Sorrell, D. Marriott, K.A. Thursky, et al., Candidaemia in
adult cancer patients: risks for fluconazole-resistant isolates and
death. J Antimicrob Chemother, 2010. 65(5): 1042-51. https://doi.org/10.1093/jac/dkq053 PMid:20202987
- Sipsas,
N.V., R.E. Lewis, J. Tarrand, R. Hachem, et al., candidemia in patients
with hematologic malignancies in the era of new antifungal agents
(2001-2007): stable incidence but changing epidemiology of a still
frequently lethal infection. Cancer, 2009. 115(20): 4745-52. https://doi.org/10.1002/cncr.24507 PMid:19634156
- Leroy,
O., J.P. Mira, P. Montravers, J.P. Gangneux, et al., Comparison of
albicans vs. non-albicans candidemia in French intensive care units.
Crit Care, 2010. 14(3): R98. https://doi.org/10.1186/cc9033 PMid:20507569 PMCid:PMC2911735
- Sun,
M., C. Chen, W. Xiao, Y. Chang, et al., Increase in Candida
Parapsilosis Candidemia in Cancer Patients. Mediterr J Hematol Infect
Dis, 2019. 11(1): e2019012. https://doi.org/10.4084/mjhid.2019.012 PMid:30671218 PMCid:PMC6328045
- Guinea,
J., Global trends in the distribution of Candida species causing
candidemia. Clin Microbiol Infect, 2014. 20 Suppl 6: 5-10. https://doi.org/10.1111/1469-0691.12539
- Richardson,
M. and C. Lass-Florl, Changing epidemiology of systemic fungal
infections. Clin Microbiol Infect, 2008. 14 Suppl 4: 5-24. https://doi.org/10.1111/j.1469-0691.2008.01978.x PMid:18430126
- Cuenca-Estrella,
M., D. Rodriguez, B. Almirante, J. Morgan, et al., In vitro
susceptibilities of bloodstream isolates of Candida species to six
antifungal agents: results from a population-based active surveillance
programme, Barcelona, Spain, 2002-2003. J Antimicrob Chemother, 2005.
55(2): 194-9. https://doi.org/10.1093/jac/dkh548 PMid:15618284
- Pfaller,
M.A., S.A. Messer, G.J. Moet, R.N. Jones, et al., Candida bloodstream
infections: comparison of species distribution and resistance to
echinocandin and azole antifungal agents in Intensive Care Unit (ICU)
and non-ICU settings in the SENTRY Antimicrobial Surveillance Program
(2008-2009). Int J Antimicrob Agents, 2011. 38(1): 65-9. https://doi.org/10.1016/j.ijantimicag.2011.02.016 PMid:21514797
- Bassetti,
M., M. Merelli, E. Righi, A. Diaz-Martin, et al., Epidemiology, species
distribution, antifungal susceptibility, and outcome of candidemia
across five sites in Italy and Spain. J Clin Microbiol, 2013. 51(12):
4167-72. https://doi.org/10.1128/JCM.01998-13 PMid:24108614 PMCid:PMC3838046
- Cisterna,
R., G. Ezpeleta, O. Telleria, and G. Spanish Candidemia Surveillance,
Nationwide sentinel surveillance of bloodstream Candida infections in
40 tertiary care hospitals in Spain. J Clin Microbiol, 2010. 48(11):
4200-6. https://doi.org/10.1128/JCM.00920-10 PMid:20826636 PMCid:PMC3020865
- Nucci,
M., F. Queiroz-Telles, A.M. Tobon, A. Restrepo, et al., Epidemiology of
opportunistic fungal infections in Latin America. Clin Infect Dis,
2010. 51(5): 561-70. https://doi.org/10.1086/655683 PMid:20658942
- Lamoth,
F., S.R. Lockhart, E.L. Berkow, and T. Calandra, Changes in the
epidemiological landscape of invasive candidiasis. J Antimicrob
Chemother, 2018. 73(suppl_1): i4-i13. https://doi.org/10.1093/jac/dkx444 PMid:29304207
- Garey,
K.W., M. Rege, M.P. Pai, D.E. Mingo, et al., time to initiation of
fluconazole therapy impacts mortality in patients with candidemia: a
multi-institutional study. Clin Infect Dis, 2006. 43(1): 25-31. https://doi.org/10.1086/504810 PMid:16758414
- Kaur,
H. and A. Chakrabarti, Strategies to Reduce Mortality in Adult and
Neonatal Candidemia in Developing Countries. J Fungi (Basel), 2017.
3(3). https://doi.org/10.3390/jof3030041 PMid:29371558 PMCid:PMC5715942
- Andes,
D.R., N. Safdar, J.W. Baddley, G. Playford, et al., Impact of treatment
strategy on outcomes in patients with candidemia and other forms of
invasive candidiasis: a patient-level quantitative review of randomized
trials. Clin Infect Dis, 2012. 54(8): 1110-22. https://doi.org/10.1093/cid/cis021 PMid:22412055
- Hirano,
R., Y. Sakamoto, K. Kudo, and M. Ohnishi, Retrospective analysis of
mortality and Candida isolates of 75 patients with candidemia: a single
hospital experience. Infect Drug Resist, 2015. 8: 199-205. https://doi.org/10.2147/IDR.S80677 PMid:26185460 PMCid:PMC4501221
- Labelle,
A.J., S.T. Micek, N. Roubinian, and M.H. Kollef, Treatment-related risk
factors for hospital mortality in Candida bloodstream infections. Crit
Care Med, 2008. 36(11): 2967-72. https://doi.org/10.1097/CCM.0b013e31818b3477 PMid:18824910
- Kollef,
M., S. Micek, N. Hampton, J.A. Doherty, et al., Septic shock attributed
to Candida infection: importance of empiric therapy and source control.
Clin Infect Dis, 2012. 54(12): 1739-46. https://doi.org/10.1093/cid/cis305 PMid:22423135
- Dib,
R.W., R. Hachem, A.M. Chaftari, and I. Raad, Appropriate Duration of
Intravenous Treatment of Candidemia and Timing of Step-down to Oral
Therapy in Non-neutropenic Patients. Mediterr J Hematol Infect Dis,
2018. 10(1): e2018028. https://doi.org/10.4084/mjhid.2018.028 PMid:29755705 PMCid:PMC5937951
[TOP]



