Florence Urio*1,2, Matilda Mkombachepa1,3, Gration Rwegasira1, Twilumba Makene4, Billy Ngasala4, Teddy Mselle2, Julie Makani1,5 and Lucio Luzzatto5.
1 Muhimbili Sickle Cell Programme, Muhimbili University of Health and Allied Sciences, Tanzania.
2 Department of Biochemistry, Muhimbili University of Health and Allied Sciences, Tanzania.
3 Department of Parasitology, Muhimbili National Hospital.
4 Department of Parasitology and Medical Entomology, Muhimbili University of Health and Allied Sciences, Tanzania.
5 Department of Hematology and Blood Transfusion, Muhimbili University of Health and Allied Sciences, Tanzania.
Correspondence to: Florence Urio. Department of Biochemistry and
Muhimbili Sickle Cell Programme, Muhimbili University of Health and
Allied Sciences, P. O. Box 65001, Dar-es-Salaam, Tanzania. Tel:
+255716894860. E-mail:
flosu28@gmail.com
Published: May 1, 2021
Received: February 11, 2021
Accepted: April 16, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021036 DOI
10.4084/MJHID.2021.036
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Malaria morbidity and mortality, almost entirely from Plasmodium
falciparum, are still rampant in Africa: therefore, it is important to
study the biology of the parasite and the parasite-host cell
interactions. In vitro cultivation of Plasmodium falciparum is most
useful for this purpose, as well as for investigating drug resistance
and possible new therapies. Here we report that the Trager & Jensen
continuous culture of P. falciparum can be established in a laboratory
in Tanzania with minimal facilities and with modest expenditure.
Methodology:
This was an in-vitro set up of continuous culture of Plasmodium
falciparum study, carried out in 2016-2020 at Muhimbili university of
health and allied sciences, Dar-es salaam. Parasite samples were
obtained from patients with acute malaria, frozen parasites, and live
cultures. Data was collected and analyzed using GraphPad Prism version
8.
Results: We have
successfully achieved exponential growth of existing strains that are
used worldwide, as well as of parasites in clinical samples from
patients with acute malaria. In the aim to optimize growth we have
compared human serum and bovine serum albumin as components of the
culture media. Additionally, culture synchronization has been achieved
using sorbitol.
Conclusion:
This experimental system is now available to our institution and to
researchers aiming at investigating drug sensitivity and mechanisms of
protection against Plasmodium falciparum that accrue from various genes
expressed in red cells.
|
Introduction
All
countries in tropical Africa are severely affected by malaria, where
Plasmodium falciparum accounts for most of malaria morbidity and
mortality,[1] with an estimated 400,000 deaths per year.[2]
In Tanzania, we are far from elimination of malaria: this is one good
reason why we need to understand better the biology of the parasite and
of parasite-host cell interactions.
The introduction of continuous culture of P. falciparum
by William Trager over 40 years ago has been of tremendous value
in malaria research since viable parasites can be studied at each stage
of the intra-erythrocytic cycle.[2] Compared to long-established laboratory strains, clinical parasite isolates tend to have low rates of multiplication in vitro, at least initially. The precise mechanisms underlying adaptation to in vitro
culture are still incompletely known but adaptation may be associated
with loss or gain of large chromosomal regions, as well as with
specific mutations,[3,4,5] meaning that established strains are almost certainly genetically different from fresh isolates.
RPMI 1640 has been the reference medium for P falciparum cultures, and it has been used to investigate parasite behaviour, drug action, and potential targets for future therapies.[6,7]
Human serum is known to enhance parasite growth; however, it may
contain inhibitory antibodies especially if obtained from donors in
malaria-endemic countries:[8] in view of this, various alternatives have been tested.[9,10,11,12,13,14,15] Among these, the most popular is commercially available lipid-enriched bovine serum albumin, Albumax I.[2,16,17,18]
The main aim of our study was to define what are the minimum facilities required to obtain continuous culture of P falciparum
in a laboratory in a low resource setting. We report here that both
established strains and parasites from patients reporting to hospital
with acute malaria can be grown successfully.
Methods
Study area.
This work has been carried out in the Molecular Biology Research
Laboratory in the MPL Building at Muhimbili University of Health and
Allied Sciences (MUHAS) in Dar-es-Salaam, Tanzania
Study design. In-vitro set up of continuous culture of Plasmodium falciparum in 2016-2020.
Source of Samples. (a) Samples from consenting patients with acute malaria (P. falciparum) were obtained from Emergency Department at Muhimbili National Hospital (MNH).
(b)
Frozen parasites: These were obtained from (Kenya Medical Research
Institute-KEMRI), Kilifi, Kenya; (Ifakara Health Institute-IHI)
Bagamoyo Tanzania and (National Institute for Medical Research-NIMR)
Korogwe, Tanzania.
(c) Live cultures: these were obtained from
(University of Witwatersrand) Johannesburg, South Africa; (University
of Ghana) Accra, Ghana; (University of Milan) Milano, Italy; (National
Institute of Health) Rome, Italy and (NIMR) Korogwe, Tanga
Continuous Culture of Plasmodium falciparum. For (a) and (c) we have followed the original Trager & Jensen methodology;[2] for (b) we have used in addition the thawing techniques detailed in Protocols.[19]
Clinical isolates.
Fresh clinical isolates were obtained from patients with acute malaria
residing in Dar es salaam before initiation of anti-malarial drugs. For
our attempts to establish continuous cultures from such isolates we
selected patients who had at least 30000 parasites/microlitre (Figure 1).
This was estimated in the Parasitology diagnostic laboratory from the
white blood cell count of each patient, and it was then confirmed in
the malaria culture lab before starting cultures.
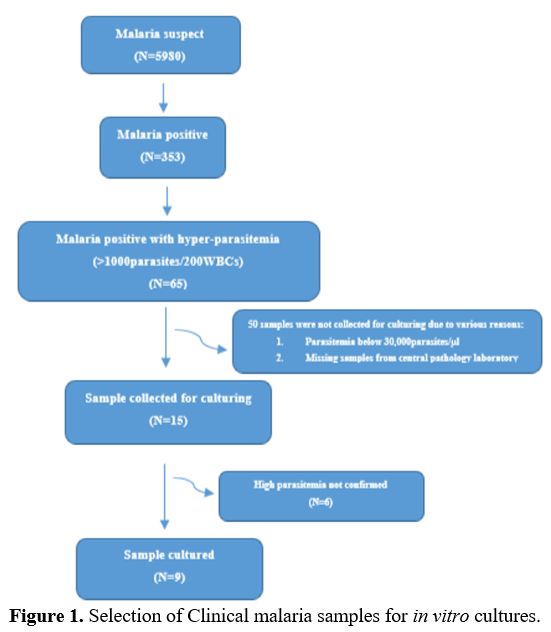 |
Figure 1. Selection of Clinical malaria samples for in vitro cultures.
|
Culture technique.
All Venous blood samples (from malaria patients or from normal donors)
were collected in EDTA tubes. After initial centrifugation the buffy
coat was removed, and the red cells were washed three times in RPMI
1640. To prepare a 25% hematocrit of uninfected red cell suspension,
1-2mls of RPMI 1640 was added to the red cell suspension. The
hematocrit was then confirmed by using an automated hematology analyzer
(Sysmex XT 2000i Kobe, Japan). The prepared red cell suspension was
used for up to 8 days. The culture medium contained NaHCO3 (25 mmol/liter)
and was supplemented with HEPES (25mmol/liter), Gentamicin (80 mg/2ml)
and L-glutamine (200mM). Infected red cells were diluted with medium
and fresh non-infected red cells to a hematocrit of 2 to 4% (0.08 to
0.16 ml of packed red cells were added to 4ml of ‘complete malaria
culture media’ cMCM) and to an initial parasitemia of 0.1% to 1%.
Cultures were grown in 25 cm2 flasks,
or in small petri dishes, or in 6 well microtiter plates. The cMCM
included, in addition to the above, either 10% (vol/vol) group A human
serum, or 10% Albumax II solution, or a combination of both in equal
parts. Human serum was obtained from donors who had not had malaria for
at least the past one year. Flask screw caps were loosened before
transfer to the candle jar. The cMCM was replaced on an alternate day
and if the culture had parasitemia of 3% and above, group O+ve red
cells were added to lower the parasitemia.
The development and
growth of parasites was assessed using the light microscope. Percentage
parasite count was calculated by counting 300-1000 red cells.
Ethics approval and consent to participate.
The study was granted ethical approval by Muhimbili University of
Health and Allied Science (MUHAS) Institutional Review Board (Reference
number: 2016-7-21/AEC/Vol.x/04).
Results
Laboratory set-up.
For petri dishes or flasks containing red cells in a nutrient medium a
major threat is contamination by bacteria (despite gentamycin in the
medium) or by fungi: therefore, a Biosafety cabinet (Class II) is the
main piece of equipment required (Figure 2A, B).
The cabinet is equipped with a HEPA (High Efficiency Particulate Air)
filter, capable of retaining 0.3-micron particles with 99.99%
efficiency. We made sure that the airflow met specifications and that
the cabinet was regularly serviced. We installed a UV lamp which was
turned on at least 30 minutes before use. Then, with the UV lamp turned
off, we exposed open blood-agar and nutrient agar microbiology plates
for 12 hours and confirmed there was no bacterial growth. The cabinet
was always kept free of any unnecessary items. All manipulations
involving cultures or reagents needed for cultures were carried out in
this cabinet with sterile precautions. We always wear gloves and
sleeveless gowns on sleeveless arms. Reduced oxygen is known to be
essential for optimal growth of P falciparum.[2] Rather than continuous flow of a gas mixture from an ad hoc
cylinder, we chose the so-called ‘candle jar’ approach for several
reasons. (i) It is free of charge. (ii) Supply of the appropriate gas
mixture cylinders may be erratic. (iii) In a sealed candle jar, if it
is sterile to begin with, the cultures are completely protected from
contamination (the same is not necessarily true in CO2 incubators with continuous gas flow). By the candle jar method O2 is 17% and CO2 is 3%.[20] The jar we used was a vacuum desiccator made of heavy glass (Figure 2C)
with a 2 cm ground glass edge, and the lid has a similar edge (we found
vacuum desiccators made of plastic not equally reliable). In order to
obtain a perfect seal, we apply a thin but generous layer of high
vacuum grease (Dow Corning Corporation, USA) to both edges and to the
ground glass device incorporating the tap. The jar, when open, is
handled only under the biosafety cabinet. We lay the flasks or dishes
inside the jar, light a white candle, and put in place the lid with the
tap open; when the flame goes out, we immediately close the tap.
The
sealed jar is then carefully transferred to the incubator, that must
have a good temperature control, and must be checked to be never
outside the range of 36.8-37.1°C (Figure 2D).
 |
Figure 2. Laboratory set-up for Plasmodium falciparum culture. A: Telstar Biosafety Cabinet Class II A with UV light on when not in use. B: Same cabinet when in use. C: Close-up of candle jar with lighted candle. D: Candle jar (flame off) in 37 °C incubator.
|
Culture of established P falciparum strains. Thanks to the courtesy of many colleagues (Table 1) we have obtained several culture samples, some live and some frozen. The data in Table 1
indicate that, despite our precautions, infection was a significant
problem especially at the beginning. In some cases, cultures may have
failed because frozen parasites were no longer viable as a result of
prolonged storage or problems associated with transportation.
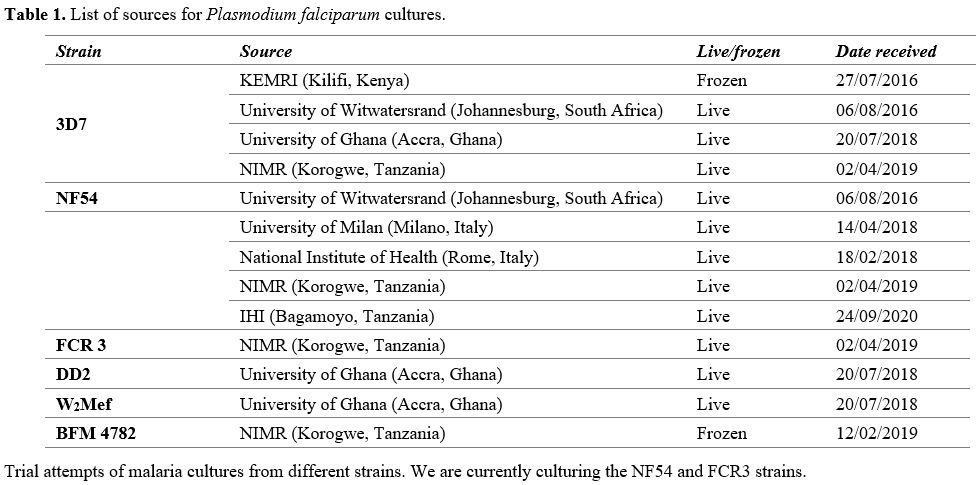 |
Table
1. List of sources for Plasmodium falciparum cultures.
|
Cultures of P falciparum from clinical isolates. In our attempts to culture parasites from patients we have selected, for obvious reasons, those who had high parasitemia (Table 2).
In 9 attempts (leaving aside one in which the culture suffered early
bacterial contamination), we initially observed gametocytes in all the
cultured clinical isolates for up to 30 days. We also saw the
production of new rings (Figure 3A)
in 8 cases: however, in 4 of these parasite growths stopped after one
to five cycles. In the remaining 4 cases we obtained continuous
cultures, but two of these were later lost (again because of bacterial
contamination). With PAT-2 and PAT-6 we were able to document
protracted exponential growth (Figure 4) with high parasite counts (supplementary table 1).
The multiplication factor per cycle (48hrs) of clinical isolates ranged
from 1.6 to 5.5, whereas it was 8.0-11.1 for the NF54 strain.
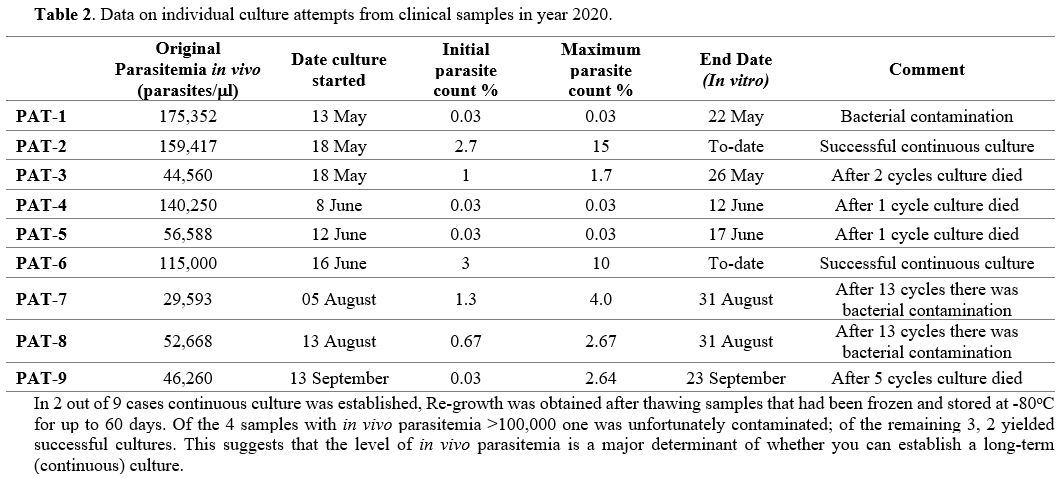 |
Table 2. Data on individual culture attempts from clinical samples in year 2020. |
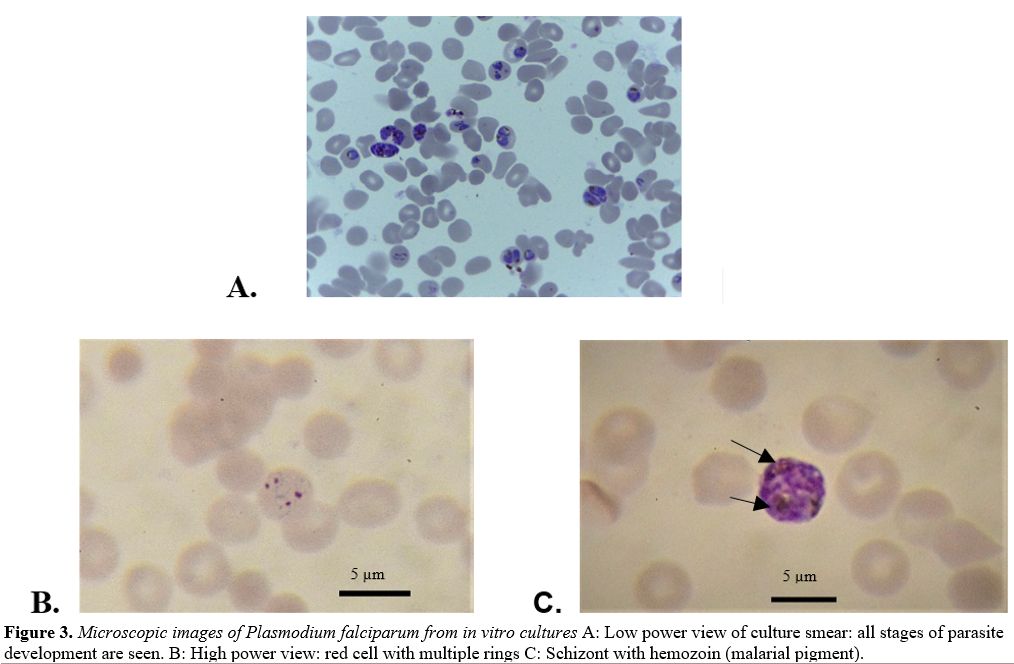 |
Figure 3. Microscopic images of Plasmodium falciparum from in vitro cultures A: Low power view of culture smear: all stages of parasite development are seen. B: High power view: red cell with multiple rings C: Schizont with hemozoin (malarial pigment). |
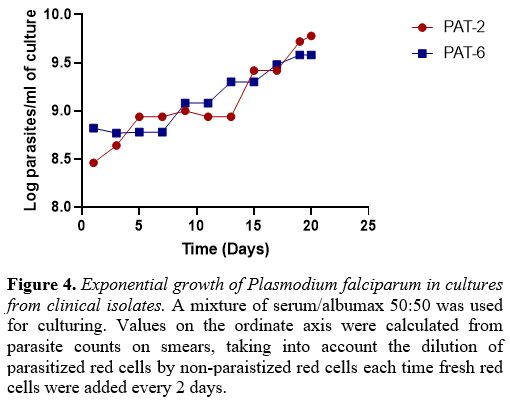 |
Figure 4. Exponential growth of Plasmodium falciparum in cultures from clinical isolates. A
mixture of serum/albumax 50:50 was used for culturing. Values on the
ordinate axis were calculated from parasite counts on smears, taking
into account the dilution of parasitized red cells by non-paraistized
red cells each time fresh red cells were added every 2 days. |
Composition of culture media.
Since the original notion of Trager & Jensen that a strong buffer
(HEPES) was required, and that 10-20% human serum would help to
optimize growth, attempts to improve culture media have not gone far:
except that human serum has been often replaced by bovine albumin
(Albumax). For a start we preferred human serum because it is easily
available and free of charge from generous donors; however, we were
aware that human serum in a malaria-endemic setting is likely to
contain antibodies that may inhibit P falciparum growth.
In several experiments we observed that a 1:1 mixture of human serum
with Albumax was either equivalent or superior to Albumax alone (Figure 5).
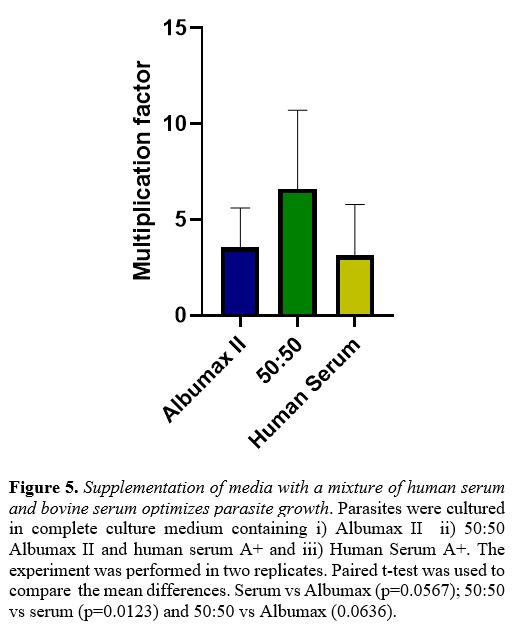 |
Figure 5. Supplementation of media with a mixture of human serum and bovine serum optimizes parasite growth.
Parasites were cultured in complete culture medium containing i)
Albumax II ii) 50:50 Albumax II and human serum A+ and iii) Human
Serum A+. The experiment was performed in two replicates. Paired t-test
was used to compare the mean differences. Serum vs Albumax
(p=0.0567); 50:50 vs serum (p=0.0123) and 50:50 vs Albumax (0.0636).
|
Synchronization. We have used the sorbitol technique[19] and the refrigeration technique.[21]
Starting from a culture with a parasite count of 8.6%, of which 65%
rings, 25% trophozoites and 20% schizonts, we obtained a culture that
had 77% rings after one round of sorbitol treatment, and 92% rings
after two rounds of sorbitol.
Recovery of frozen parasites.
Ideally parasitized red cells should be stored frozen in liquid
nitrogen (i.e. at -195°C). However, since this was not available, we
have stored parasitized red cells in a -80°C deep-freezer and recovered
them successfully after up to 120 days. The freezing solution consisted
of 28% Glycerol; 3% Sorbitol; 0.65% NaCl in distilled water; the
thawing solution was 3.5% NaCl.
Discussion
In vitro cultivation of continuous Plasmodium falciparum cultures was established more than 40 years ago, and it has been a tremendous booster for research.[2] Formerly P falciparum malaria could be investigated only in endemic countries [or experimentally in Aotus trivigatus, the owl monkey].[22]
With in vitro cultures available, there has been a reversal: research on P falciparum
has become easy in non-endemic areas; whereas it may be lagging where
cultures are not carried out. For this reason, set up of culture
facilities in endemic areas has become very important, and it has been
done in several countries in Africa, in order to conduct studies in
immunology, molecular biology, genetics, pharmacology and biochemistry.[23,24,25,26] In this paper, we have reported in detail how this can be done successfully with minimal resources.
Since
exponential growth is probably the best proof that the culture is doing
well, we find that for small scale experiments the candle jar method is
entirely satisfactory: it does not require customized gas mixtures, nor
a dedicated CO2 incubator. Since
maintaining cultures all the time is demanding in terms of labor,
media, and supply of fresh red cells, it is convenient to resort to
freezing whenever live parasites are not needed. From this point of
view, it is of practical importance that storage at -80°C is
satisfactory for 2-4 months.
In our initial experiments we have
supplemented media with human serum, in keeping with the original
formula of Trager & Jensen.[2] However, these
authors worked in a malaria-free setting in New York City. We have
observed, not surprisingly, that sera from different donors give
different results: it stands to reason that if donors who have been
exposed to malaria – the rule rather than the exception in Tanzania –
their serum may contain inhibitory antibodies.[27]
Dohutia et al found that the combination of fresh human serum and
Albumax might be superior, which is similar to our findings with strain
NF54.[28] Therefore, it may be expedient, though more
expensive, to use Albumax instead of human serum, or a 1:1 mixture of
both: the latter worked well in our hands.
P. falciparum
strains that are used worldwide, such as 3D7, FCR-3 are a great asset,
because they make it possible to compare results, no matter where an
experiment is carried out. On the other hand, it is abundantly clear
that laboratory-adapted strains are different from ‘wild’ parasites. In
this respect, a unique advantage of malaria cultures being carried out
in a malaria-endemic area is that one may obtain parasites that are
indeed wild, as previously shown by.[29,30,31,32] In
our small series this was successfully achieved in 4 out of 9 cases. In
all these cases we have observed low rates of multiplication in the
first 10 days followed by exponential growth (Figure 4),
even though the multiplication factor was still low compared to that of
well-established laboratory strains. This is similar to what was
observed in previous studies.[5,33]
It will be clearly interesting to determine why in some cases
adaptation to laboratory conditions is so prompt, whereas in other
cases it fails.
The main limitation of our study has been the
significant incidence of bacterial or fungal contamination, that on
several occasions has forced us to discard cultures. We have learnt
that one can never be too careful in this respect: for instance, to
keep the laminar flow cabinet free from clutter is imperative.
A
different kind of limitation is that of resources. We have already
enumerated the equipment needed. As for running costs, if we add up
maintenance of the laminar flow cabinet, media, plastic, and other
consumable materials, with a mean of 4-8 culture flasks in use our best
cost estimate is of approximately 490 US$ per month.
Despite the limitations, this study has highlighted some of the technical difficulties and solutions for setting up continuous in vitro cultures of malaria in an endemic region. Similar studies were conducted previously in Mali and Nigeria.[29,30]
Conclusions
Our first and foremost aim in establishing continuous in vitro cultures of P falciparum
was to make these available to our scientific community. In the
meantime, we have been also recently asked by the Tanzania Medicines
and Medical Devices Authority (TMDA) to provide cultures for quality
testing of malaria rapid diagnostic test kits. In addition, we plan to
investigate in greater depth the mechanisms whereby red cells with
different genotypes play host to P falciparum:
this endeavour is currently in progress. Most importantly, we believe
our malaria culture lab will enable malaria research into real life
clinical isolates and drug resistance.
Acknowledgments
We
are grateful to the members of the Department of Biochemistry,
particularly the late Mr. Idrisa Mshanga for his tireless support in
setting up the laboratory; we also thank Dr. Francis Dida for kindly
making laboratory space available. We thank Mr. Ally Athuman Sule for
his great support in data collection at Muhimbili National Hospital. We
appreciate greatly the support from Dr. Daniel Minja and Mr. Gerson
Maro during data collection at NIMR-Korogwe and Dr. Lucas Matemba from
NIMR-Morogoro. We are grateful to the following, who provided frozen
parasites and live cultures of Plasmodium falciparum:
Mr. Mgeni Tambwe and Mr. Lorenz Hofer from Ifakara Health Institute,
Bagamoyo; Professor Jeffrey Dorfman from University of Capetown, South
Africa; Professor Maureen Coetzer University of Witwatersrand,
Johannesburg, South Africa; Dr. Gordon Awandare University of Ghana;
Dr. Donatella Taramelli, University of Milano, Italy; Dr. Pietro Alano
Istituto Superiore di Sanità, Rome, Italy. We thank Professor
Anastasios Karadimitris, University of London, UK, for the gift of a
vacuum dessicator. Lastly, we thank all staff of Muhimbili Sickle Cell
Programme, Muhimbili University of Health and Allied Sciences and
Muhimbili National Hospital.
References
- https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019
- Trager W, Jensen J. Human malaria parasites in continuous culture. Science (80- ) [Internet]. 1976 Aug 20;193(4254):673-5. https://doi.org/10.1126/science.781840 PMid:781840
- Bhasin
VK, Clayton C, Trager W, Cross GAM. Variations in the organization of
repetitive DNA sequences in the genomes of Plasmodium falciparum
clones. Mol Biochem Parasitol [Internet]. 1985 May;15(2):149-58. https://doi.org/10.1016/0166-6851(85)90116-1
- Corcoran
LM, Forsyth KP, Bianco AE, Brown G V., Kemp DJ. Chromosome size
polymorphisms in plasmodium falciparum can involve deletions and are
frequent in natural parasite populations. Cell [Internet]. 1986
Jan;44(1):87-95. https://doi.org/10.1016/0092-8674(86)90487-3
- Summary of discussions on in vitro cultivation of malaria parasites. Bull World Health Organ. 1977;55(2-3):411-9.
- Desai SA. Insights gained from P. falciparum cultivation in modified media. Sci World J. 2013;2013. https://doi.org/10.1155/2013/363505 PMid:23956690 PMCid:PMC3727134
- Ringwald
P, Meche FS, Bickii J, Basco LK. In vitro culture and drug sensitivity
assay of Plasmodium falciparum with nonserum substitute and acute-phase
sera. J Clin Microbiol. 1999;37(3):700-5. https://doi.org/10.1128/JCM.37.3.700-705.1999 PMid:9986835 PMCid:PMC84528
- Asahi
H, Kanazawa T. Continuous cultivation of intraerythrocytic Plasmodium
falciparum in a serum-free medium with the use of a growth-promoting
factor. Parasitology [Internet]. 1994 Nov 6;109(4):397-401. Available
from: https://doi.org/10.1017/S0031182000080641 PMid:7800407
- Divo
AA, Jensen JB. Studies on serum requirements for the cultivation of
Plasmodium falciparum. I. Animal sera. Bull World Health Organ.
1982;60(4):565-9.
- Ifediba T, Vanderberg
JP. Peptones and Calf Serum as a Replacement for Human Serum in the
Cultivation of Plasmodium falciparum. J Parasitol [Internet]. 1980
Apr;66(2):236 https://doi.org/10.2307/3280810
- Lingnau
A, Margos G, Maier WA, Seitz HM. Serum-free cultivation of
severalPlasmodium falciparum strains. Parasitol Res [Internet].
1994;80(1):84-6. https://doi.org/10.1007/BF00932631 PMid:8153133
- Desjardins
RE, Alexander BM, Weatherly NF, Bowdre JH, Oduola AMJ. Use of Non-Human
Plasma for in Vitro Cultivation and Antimalarial Drug Susceptibility
Testing of Plasmodium Falciparum. Am J Trop Med Hyg [Internet]. 1985
Mar 1;34(2):209-15. https://doi.org/10.4269/ajtmh.1985.34.209 PMid:3885768
- Sax
LJ, Rieckmann KH. Use of Rabbit Serum in the Cultivation of Plasmodium
falciparum. J Parasitol [Internet]. 1980 Aug;66(4):621. https://doi.org/10.2307/3280518
- Willet
GP, Canfield CJ. Plasmodium falciparum: Continuous cultivation of
erythrocyte stages in plasma-free culture medium. Exp Parasitol
[Internet]. 1984 Feb;57(1):76-80. https://doi.org/10.1016/0014-4894(84)90065-1
- Binh
VQ, Luty AJF, Kremsner PG. Differential Effects of Human Serum and
Cells on the Growth of Plasmodium falciparum Adapted to Serum-Free in
Vitro Culture Conditions. Am J Trop Med Hyg [Internet]. 1997 Nov
1;57(5):594-600. https://doi.org/10.4269/ajtmh.1997.57.594 PMid:9392601
- Cranmer
SL, Magowan C, Liang J, Coppel RL, Cooke BM. An alternative to serum
for cultivation of Plasmodium falciparum in vitro. Trans R Soc Trop Med
Hyg [Internet]. 1997 May;91(3):363-5. https://academic.oup.com/trstmh/article-lookup/doi/10.1016/S0035-9203(97)90110-3 https://doi.org/10.1016/S0035-9203(97)90110-3
- Flores
MVC, Berger-Eiszele SM, Stewart TS. Long-term cultivation of Plasmodium
falciparum in media with commercial non-serum supplements. Parasitol
Res [Internet]. 1997 Aug 1;83(7):734-6. https://doi.org/10.1007/s004360050330 PMid:9272569
- Wang
P, Read M, Sims PFG, Hyde JE. Sulfadoxine resistance in the human
malaria parasite Plasmodium falciparum is determined by mutations in
dihydropteroate synthetase and an additional factor associated with
folate utilization. Mol Microbiol [Internet]. 1997 Mar 31;23(5):979-86.
https://doi.org/10.1046/j.1365-2958.1997.2821646.x PMid:9076734
- http://ki.se/sites/default/files/methods_in_malaria_research
- Trager W, Jensen J.B. Cultivation of erythrocytic stages. Bulletin of the World Health Organisation. 1977; 55:363-365
- Yuan
L, Hao M, Wu L, Zhao Z, Rosenthal BM, Li X, et al. Refrigeration
provides a simple means to synchronize in vitro cultures of Plasmodium
falciparum. Exp Parasitol [Internet]. 2014 May;140:18-23. https://doi.org/10.1016/j.exppara.2014.03.010 PMid:24632190 PMCid:PMC4018460
- Trager
W. A New Method for Intraerythrocytic Cultivation of Malaria Parasites
(Plasmodium coatneyi and P. falciparum). J Protozool.
1971;18(2):239-42. https://doi.org/10.1111/j.1550-7408.1971.tb03314.x PMid:4997037
- Awandare
GA, Nyarko PB, Aniweh Y, Ayivor-Djanie R, Stoute JA. Plasmodium
falciparum strains spontaneously switch invasion phenotype in
suspension culture. Sci Rep. 2018;8(1):1-10. https://doi.org/10.1038/s41598-018-24218-0 PMid:29636510 PMCid:PMC5893586
- Amoah
LE, Kakaney C, Kwansa-Bentum B, Kusi KA. Activity of Herbal Medicines
on Plasmodium falciparum Gametocytes: Implications for Malaria
Transmission in Ghana. Lanz-Mendoza H, editor. PLoS One [Internet].
2015 Nov 12;10(11):e0142587. https://doi.org/10.1371/journal.pone.0142587 PMid:26562778 PMCid:PMC4642932
- Lusakibanza
M, Mesia G, Tona G, Karemere S, Lukuka A, Tits M, et al. In vitro and
in vivo antimalarial and cytotoxic activity of five plants used in
congolese traditional medicine. J Ethnopharmacol [Internet]. 2010
Jun;129(3):398-402. https://doi.org/10.1016/j.jep.2010.04.007 PMid:20430094
- Engelbrecht
D, Coetzer TL. Sunlight inhibits growth and induces markers of
programmed cell death in Plasmodium falciparum in vitro. Malar J
[Internet]. 2015 Dec 29;14(1):378. https://doi.org/10.1186/s12936-015-0867-0 PMid:26419629 PMCid:PMC4588498
- Khandros
E, Huang P, Peslak SA, Sharma M, Abdulmalik O, Giardine BM, et al.
Rapid emergence of clonal interference during malaria parasite
cultivation. Blood [Internet]. 2011;10(1):271. http://www.malariajournal.com/content/10/1/271
- Dohutia
C, Mohapatra PK, Bhattacharyya DR, Gogoi K, Bora K, Goswami BK. In
vitro adaptability of Plasmodium falciparum to different fresh serum
alternatives. J Parasit Dis [Internet]. 2017 Jun 30;41(2):371-4. https://doi.org/10.1007/s12639-016-0808-z PMid:28615843 PMCid:PMC5447585
- Sodeinde
O, Williams CK. Continuous in-vitro cultivation of Plasmodium
falciparum in Ibadan: solutions to scientific and logistical problems.
Afr J Med Med Sci [Internet]. 1990 Jun;19(2):71-6. http://www.ncbi.nlm.nih.gov/pubmed/2115731
- Djimde
AA, Kirkman L, Kassambara L, Diallo M, Plowe C V, Wellems TE, et al.
[In vitro cultivation of fields isolates of Plasmodium falciparum in
Mali]. Bull Soc Pathol Exot [Internet]. 2007 Feb;100(1):3-5. https://doi.org/10.3185/pathexo2883 PMid:17402683
- Held
J, Zanger P, Issifou S, Kremsner PG, Mordmüller B. In vitro activity of
tigecycline in Plasmodium falciparum culture-adapted strains and
clinical isolates from Gabon. Int J Antimicrob Agents. 2010;35(6). https://doi.org/10.1016/j.ijantimicag.2010.02.003 PMid:20227854
- Deans
AM, Nery S, Conway DJ, Kai O, Marsh K, Rowe JA. Invasion pathways and
malaria severity in Kenyan Plasmodium falciparum clinical isolates.
Infect Immun. 2007;75(6):3014-20. https://doi.org/10.1128/IAI.00249-07 PMid:17438038 PMCid:PMC1932858
- Murray
L, Stewart LB, Tarr SJ, Ahouidi AD, Diakite M, Amambua-ngwa A, et al.
Multiplication rate variation in the human malaria parasite Plasmodium
falciparum. Sci Rep [Internet]. 2017;(July):1-8. https://doi.org/10.1038/s41598-017-06295-9 PMid:28743888 PMCid:PMC5527095
Supplementary Data
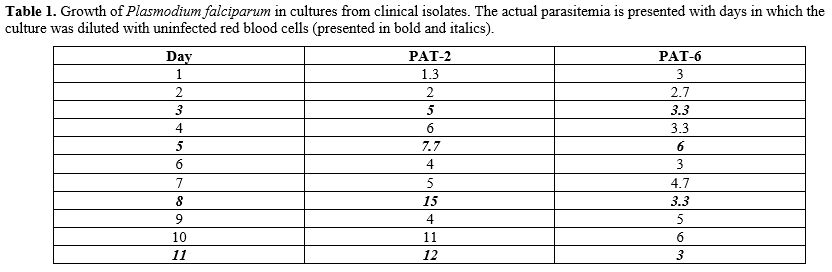 |
Table
1. Growth of Plasmodium falciparum
in cultures from clinical isolates. The actual parasitemia is presented
with days in which the culture was diluted with uninfected red blood
cells (presented in bold and italics).
|
[TOP]







