Guo Li1,*, Guang-Liang Chen2,3,*, Yong Zhou4, Gui-Qin Yao5, Shun’e Yang1 and Dong-Mei Ji1,2,6.
1Department of Lymphoma, Xinjiang Medical University Cancer Hospital, Xinjiang, China.
2 Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.
3 Department of Oncology, Shanghai Medical College Fudan University, Shanghai, China.
4 Department of imaging, Xinjiang Medical University Cancer Hospital, Xinjiang, China.
5 Department of infectious disease, Xinjiang Medical University Cancer Hospital, Xinjiang, China.
6 Phase I Clinical Trial Center, Fudan University Shanghai Cancer Center, Shanghai, China.
* These authors have contributed equally to this work and share the first authorship.
Correspondence to: Prof Dong-Mei Ji; No. 270, Dong'an Road, Xuhui District, Shanghai, 200032. E-mail:
jidongmei2000@hotmail.com Prof. Yang; No.789, Suzhou East Street, Xincheng District, Urumqi, Xinjiang, 830000, China. E-mail:
yangshune@yeah.net
Published: September 1, 2021
Received: May 23, 2021
Accepted: August 11, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021053 DOI
10.4084/MJHID.2021.053
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Purpose: To identify factors associated with lymphoma in patients with prior Mycobacterium tuberculosis infection.
Methods:
A retrospective case-control analysis was performed in a highly
tuberculosis (TB)-endemic area. Patients with a history of TB before
the diagnosis of lymphoma were retrospectively identified. Inpatients
with lymphoma (n=1,057) and pathologically confirmed benign diseases
(n=12,916) were consecutively enrolled at Xinjiang Medical University
Cancer Hospital between January 2016 and December 2019.
Results:
The proportion of TB infection in patients with lymphoma (n=148, 14.0%)
was significantly higher than that in the control (benign diseases)
group (n=175, 1.4%) (p<0.0001). The frequencies of TB infection in
patients with Hodgkin lymphoma, B-cell non-Hodgkin lymphoma (NHL), and
T/NK-cell NHL were 13.6%, 14.6%, and 11.9%, respectively. Relatively
high proportions of TB infection were found in patients with chronic
lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL), marginal
zone B-cell lymphoma (MZBL), and diffuse large B-cell lymphoma (DLBCL),
at 20.6%, 18.6% and 15.3%, respectively, compared to other subtypes of
B-cell NHL. For T/NK-cell NHL, the proportions of TB infection in
patients with peripheral T-cell lymphoma, not otherwise specified
(PTCL, NOS), and anaplastic large cell lymphoma (ALCL) were 18.2% and
20%, respectively. The multivariate analysis revealed that male sex was
an adverse risk factor for lymphoma after tubercular infection. In
addition, male sex and older age (>60 years) were associated with
B-cell NHL.
Conclusion: A
high proportion of TB infection was found in patients with lymphoma. In
TB-infected patients, older age and male sex were associated with
susceptibility to lymphoma, suggesting that screening programmes might
be useful for the early detection of lymphoma.
|
Introduction
There is an intricate and dangerous association between tuberculosis (TB) infection and cancer, especially lymphoma.[1-3] Tubercular infection may complicate the diagnosis of lymphoma.[1-3] Given its clinical and morphological similarities to TB, lymphoma diagnosis is often delayed in TB-infected individuals.[2,4-11] Approximately 10% of cancer patients may have active TB,[12]
which may cause a delay in antitumour therapy. The toxicity of anti-TB
treatment may lead to the administration of an insufficient dose in
patients with lymphoma, increasing the risk for mortality due to a
curable disease.[12] Additionally, some deaths in cancer patients are caused by TB flares and not the tumour per se.[13]
However, the prevalence of TB among lymphoma patients remains unclear.
Few studies have been performed in countries with a high TB burden.[14]
TB
induces a chronic inflammatory state that which compromises the normal
immune system and is a significant risk factor for the development of
malignant haematological tumours.[5,15,14,16,17] TB may occur for decades before the onset of lymphoma.[15-18] A typical example of the association between TB infection and lymphoma is pyothorax-associated pleural lymphoma.[18-20]
Therefore, the establishment of a lymphoma screening strategy targeting
TB-infected patients may be an important strategy, particularly
considering population ageing.
In this study, we analysed the
proportion of TB infection in lymphoma patients and identified certain
characteristics of lymphoma in TB-infected patients. A lymphoma
screening strategy could help clinicians identify lymphoma early in the
high-risk TB patient population.
Materials and Methods
Case Selection Criteria.
Between January 2016 and December 2019, a total of 1,120 patients with
lymphoma were treated and followed in the Department of Lymphoma,
Xinjiang Medical University Cancer Hospital, a tertiary care hospital
in Xinjiang. Among this cohort, 42 patients with clinically suspected
lymphoma but without pathological findings as well as 21 patients with
comorbid lymphoma and TB after the initiation of lymphoma treatment
were excluded. Finally, a total of 1,057 patients with lymphoma, with
confirmed and clear pathological evidence according to the World Health
Organization (WHO) classification, were enrolled in the case group.
Patients with pathologically confirmed benign diseases (n=12,916)
treated at Xinjiang Medical University Cancer Hospital between January
2016 and December 2018 were enrolled in the control group.
Identification of TB Patients. TB cases were retrospectively identified as described in our previous report[21] according to the national guidelines for the diagnosis of tuberculosis in China.[22]
In brief, TB cases were identified in inpatients by past medical
history or radiologic findings, including chest X-ray and computed
tomography (CT) scans. Both active and inactive TB cases before the
diagnosis of lymphoma or benign diseases were included in the final
analysis of the risk of lymphoma after TB infection. Among the cohort
of 148 lymphoma patients with TB, most patients had inactive pulmonary
TB according to clinical and radiological indicators, such as evidence
of old pulmonary TB (n=133), while five patients had active pulmonary
TB according to radiographic abnormalities consistent with active
pulmonary TB or positive culture of Mycobacterium tuberculosis
from sputum. Ten patients had extrapulmonary TB. In the control group,
167 patients had inactive pulmonary TB. Only two patients had
radiographic abnormalities consistent with active pulmonary TB, while
six patients had a past medical history of pulmonary TB diagnosed by a
physician. For both groups, only the initial hospitalization was
included in the analysis. In addition, nine TB patients had B-cell
non-Hodgkin lymphoma (NHL), and three TB patients had T/NK-cell NHL,
while none of the TB patients in the control group had immunodeficiency
due to human immunodeficiency virus (HIV) infection.
Patient and Public Involvement.
Since our study was a retrospective study, study participants and
patient advisers were not involved in the recruitment or conduct of our
study. Participants have the right to access the results of the study
by contacting a member of the research team.
Statistical Analysis.
Data were analysed using SPSS (version 24.0; SPSS, Chicago, IL). A
two-tailed P value < 0.05 was considered statistically significant.
Logistic regression analysis was used to assess odds ratios (ORs) and
95% confidence intervals (95% CIs).
Results
We identified 1,057 patients with lymphoma; the demographic characteristics are described in Table 1.
Compared to that in control patients (1.4%), the proportions of TB
infection were significantly higher in patients with Hodgkin lymphoma
(13.6%), B-cell NHL (14.6%), and T/NK-cell NHL (11.9%). The results of
the subgroup analysis of the proportion of TB infection among lymphoma
patients are shown in Table 2.
The proportions of TB infection were 19.1%, 9.5%, and 8.3% in patients
with nodular sclerosis classical Hodgkin lymphoma (CHL),
lymphocyte-rich CHL, and mixed cellularity CHL, respectively. Among
B-cell NHL patients, patients with chronic lymphocytic leukaemia/small
lymphocytic lymphoma had the highest proportion of TB infection
(20.6%), followed by patients with marginal zone B-cell lymphoma
(18.6%), diffuse large B-cell lymphoma (DLBCL) (15.3%), and Burkitt
lymphoma (13.6%). Among the T/NK-cell NHL patients, the highest
proportion of TB infection was found in patients with anaplastic large
cell lymphoma (20.0%), followed by peripheral T-cell lymphoma,
nonspecified lymphoma (18.2%), and T-lymphoblastic lymphoma (12.5%).
Notably, none of the patients with angioimmunoblastic T-cell lymphoma
had previous TB infection.
Although there was no significant
impact in HL and T/NK lymphoma patients, age was a notable effect
modifier in the association between TB infection and some subtypes of
B-cell NHL. The odds of DLBCL in patients with TB aged more than 60
years was 1.99 (95% CI, 1.16–3.42) (p=0.012) times higher than that in
patients aged less than or equal to 60 years. Patients with TB who were
older than 60 years also had an increased risk of MZBL compared to
patients in the younger age group (adjusted OR, 5.39; 95% CI,
1.23–23.58) (p=0.025). Notably, in contrast to that in younger
patients, the proportion of TB infection in patients with Burkitt
lymphoma aged over 60 years was 60% (p=0.006). Similarly, a significant
change in the effect was observed for the association between TB and
some B-cell NHL subtypes in all sex subgroups. For example, the
adjusted OR for the association between TB and DLBCL was higher in
males than in females (OR, 1.96; 95% CI, 1.14–3.35) (p=0.014). In
contrast, the proportion of TB infection was higher in female (42.9%,
3/7) than male (0.0%, 0/15) Burkitt lymphoma patients (p=0.023).
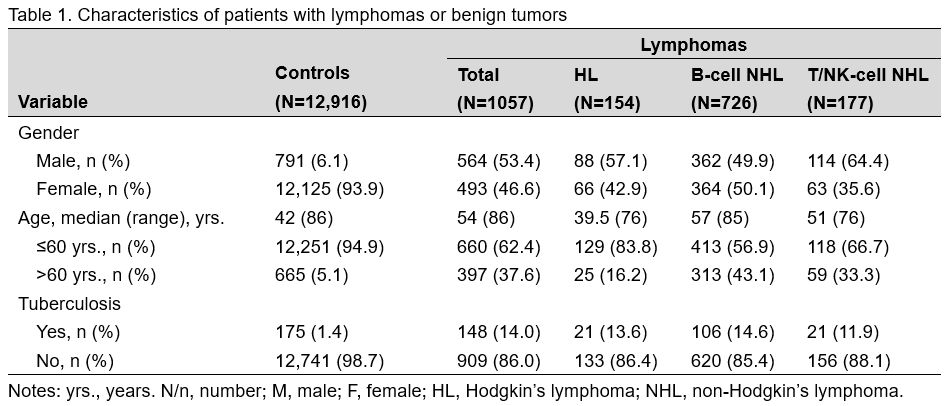 |
Table 1. Characteristics of patients with lymphomas or benign tumors. |
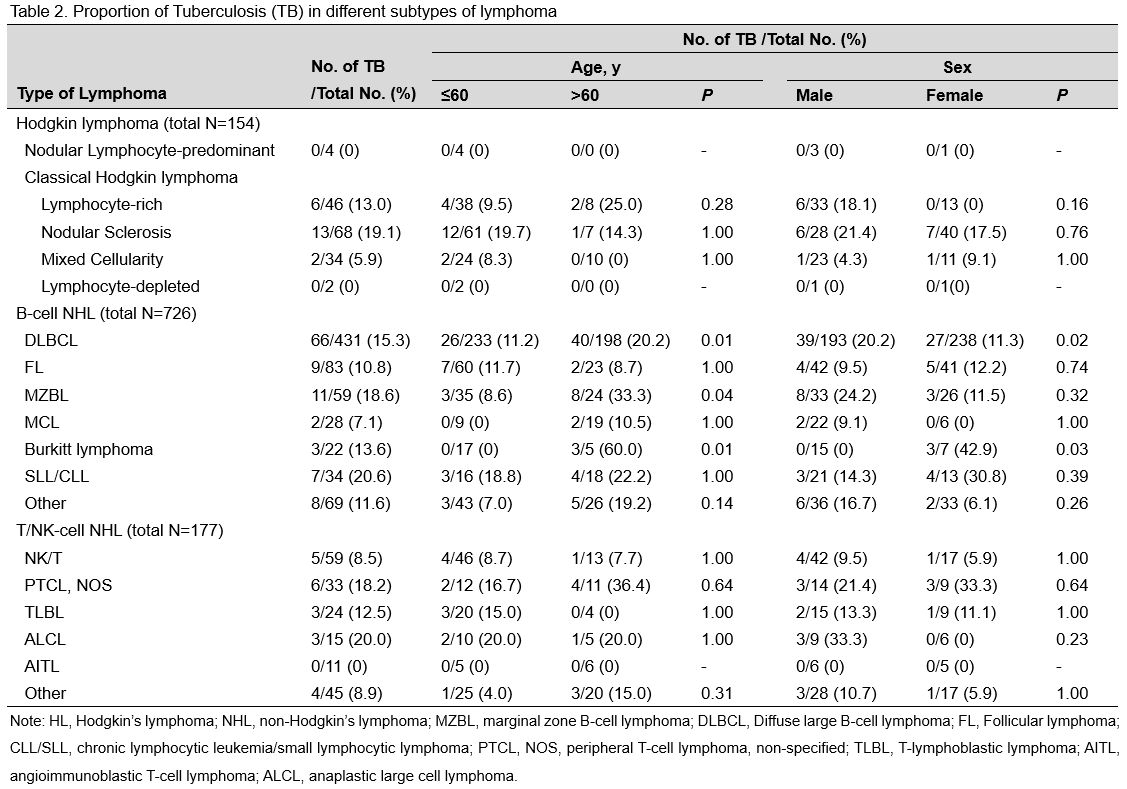 |
Table 2. Proportions of Tuberculosis (TB) in different subtypes of lymphoma. |
To
identify individuals at risk of lymphoma among the TB-infected
population, unconditional multivariate logistic regression analysis was
carried out. As shown in Table 3,
TB-infected men had higher risks of all types of lymphoma than
TB-infected women. Additionally, elderly patients (>60 years) were
susceptible to B-cell NHL, including DLBCL, but were protected against
HL.
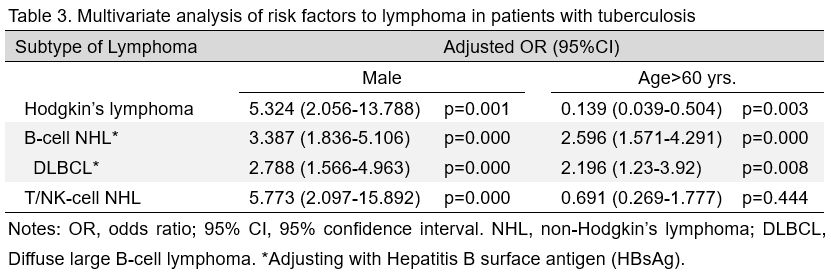 |
Table 3. Multivariate analysis of risk factors to lymphoma in patients with tuberculosis. |
Discussion
People infected with TB develop malignant lymphoma more frequently than the general population.[14,17,25,15]
In this study, we found that the proportion of TB infection in lymphoma
patients, ranging from 11.9% to 14.6%, was significantly higher than
that in control individuals (1.4%). Importantly, TB infection can have
a substantial impact on the older male population, predisposing them to
certain subtypes of lymphoma, particularly B-cell NHL. Screening for
lymphomas in men and/or older patients with a history of TB can prompt
timely diagnosis and treatment.
In the present study, we confirmed
a positive association between HL and TB infection. This result
corroborates observations by Vineis P. et al., Kou et al., and Everatt
et al.[23-25] However, over the past two decades, the
association between NHL and TB has been controversial. Here, we suggest
that TB infection is positively related to NHL. Similarly, a systematic
review showed that TB infection was associated with haematological
malignancies, including HL, NHL, and leukaemia.[26]
Ageing is a risk factor for NHL transformation, particularly DLBCL,
MZBL, and Burkitt lymphoma, in TB patients. Case-control studies
support the indication that TB infection is a risk factor for the
incidence of DLBCL.[27] Therefore, TB may be a
pathogenic factor for DLBCL. This theory is extremely important in the
association between TB infection and pyothorax-associated pleural
lymphoma, a prototype of DLBCL-CI, which predominately occurs in older
male patients with DLBCL after a long history of TB infection.[28]
With ageing of the population, NHL lymphoma in individuals with prior
TB infection is becoming an increasingly important public health issue.
In this study, TB infection was particularly associated with Burkitt
lymphoma in older females. To the best of our knowledge, this may be
the first study to show that TB is a risk factor for Burkitt lymphoma
in older females.
We found a higher risk for B-cell NHL history in
older men with a history of TB infection than in women. Indeed, many
more males than females are infected with TB in low- and middle-income
countries.[29] Moreover, the incidence of NHL among males is significantly higher than that among females.[30] Several critical roles of oestrogens in the regulation of haematopoietic stem cells and immune cells[31,32]
may partly explain the lower risk of NHL in TB-infected females. Costas
et al. observed a significantly higher risk of B-cell NHL in women who
underwent hysterectomy and bilateral oophorectomy than in women who did
not, supporting the role of oestrogens.[33] In addition, more TB-infected men present clinical symptoms than women.[34] However, a large sample prospective cohort study is warranted.
A
definitive causal conclusion cannot be reached due to the small sample
size and the inherent nature of retrospective studies. In our study, we
adjusted for age and sex but did not examine the role of
immunodeficiency due to HIV infection, hereditary immunodeficiency
syndromes, immunosuppressive treatments, or other confounding factors
associated with both TB and lymphomas. In addition, without
confirmation of Epstein-Barr virus (EBV) infection, pathologists
sometimes could not determine if NHL belonged to the Burkitt lymphoma
or DLBCL subclassification due to extensive necrosis.[2]
Whether older women with TB have an increased risk of Burkitt lymphoma
warrants future research. Since we did not perform the HIV test in the
patients with Tuberculosis and Lymphoma, we cannot exclude a role of
this virus.
Conclusions
The
present study indicated that approximately 10%-20% of lymphoma patients
in a highly TB-endemic area in China had prior TB infection. Male
patients with previous TB infection are more likely to develop
lymphoma, especially elderly male patients.
Ethical Approval
All
procedures performed in studies involving human participants were in
accordance with the ethical standards of the institutional and/or
national research committee and with the 1964 Helsinki Declaration and
its later amendments or comparable ethical standards. This study was
reviewed and approved by the Xinjiang Medical University Institutional
Review Board (number K-2021003). Informed consent was obtained from all
individual participants included in the study.
References
- Omri HE, Hascsi Z, Taha R, Szabados L, Sabah HE,
Gamiel A, et al. Tubercular Meningitis and Lymphadenitis Mimicking a
Relapse of Burkitt's Lymphoma on (18)F-FDG-PET/CT: A Case Report. Case
Rep Oncol. 2015;8(2):226-32. https://doi.org/10.1159/000430768
- Barros
MH, Leite E, Chabay P, Morais V, Stefanoff G, Hassan R. Diagnosing
lymphoma in a setting with a high burden of infection: a pediatric case
of Epstein-Barr virus-associated aggressive B-cell lymphoma with
t(8;14) (q23;q32) and extensive necrosis mimicking tuberculosis. Rev
Soc Bras Med Trop. 2015;48(1):108-11. https://doi.org/10.1590/0037-8682-0153-2014
- Hou
S, Shen J, Tan J. Case report: multiple systemic disseminated
tuberculosis mimicking lymphoma on 18F-FDG PET/CT. Medicine
(Baltimore). 2017;96(29):e7248.
- Antel K,
Levetan C, Mohamed Z, Louw VJ, Oosthuizen J, Maartens G, et al. The
determinants and impact of diagnostic delay in lymphoma in a TB and HIV
endemic setting. BMC Cancer. 2019;19(1):384. https://doi.org/10.1186/s12885-019-5586-4
- Anibarro
L, Pena A. Tuberculosis in patients with haematological malignancies.
Mediterr J Hematol Infect Dis. 2014;6(1):e2014026.
- Dres
M, Demoule A, Schmidt M, Similowski T. Tuberculosis hiding a
non-Hodgkin lymphoma "there may be more to this than meets the eye".
Respir Med Case Rep. 2012;7:15-6. https://doi.org/10.1016/j.rmcr.2012.10.002
- Collu
C, Fois A, Crivelli P, Tidore G, Fozza C, Sotgiu G, et al. A
case-report of a pulmonary tuberculosis with lymphadenopathy mimicking
a lymphoma. Int J Infect Dis. 2018;70:38-41. https://doi.org/10.1016/j.ijid.2018.02.011
- Soria
A, Foresti S, Fortuna P, Dolara A, Bandera A, Lapadula G, et al.
Unmasked tuberculosis or lymphoma in late AIDS presenters: a difficult
differential diagnosis. Eur Respir J. 2009;34(4):997-8. https://doi.org/10.1183/09031936.00062309
- Fanourgiakis
P, Mylona E, Androulakis, II, Eftychiou C, Vryonis E, Georgala A, et
al. Non-Hodgkin's lymphoma and tuberculosis coexistence in the same
organs: a report of two cases. Postgrad Med J. 2008;84(991):276-7. https://doi.org/10.1136/pgmj.2007.066183
- Ab
U. Tuberculosis: The Great Lymphoma Pretender. International Journal of
Cancer Research and Molecular Mechanisms (ISSN 2381-3318 ). 2016;2(1).
- Touré
SA, Seck M, Diallo AB, Niang EHD, Keita M, Dabo MF, et al. Diagnosis
Dilemma of Angioimmunoblastic T-Cell Lymphoma in Tuberculosis Endemic
Region. Case Reports in Hematology. 2020;2020:1-4. https://doi.org/10.1155/2020/8824843
- Cuellar
L, Castaneda CA, Rojas K, Flores C, Dolores-Cerna K, Castillo M, et al.
Clinical features and toxicity of tuberculosis treatment in patients
with cancer. Rev Peru Med Exp Salud Publica. 2015;32(2):272-7.
- Hashmi
HRT, Mishra R, Niazi M, Venkatram S, Diaz-Fuentes G. An Unusual Triad
of Hemophagocytic Syndrome, Lymphoma and Tuberculosis in a Non-HIV
Patient. Am J Case Rep. 2017;18:739-45. https://doi.org/10.12659/AJCR.903990
- Shu
CC, Liao KM, Chen YC, Wang JJ, Ho CH. The burdens of tuberculosis on
patients with malignancy: incidence, mortality and relapse. Sci Rep.
2019;9(1):11901. https://doi.org/10.1038/s41598-019-48395-8
- Ibrahim
EM, Uwaydah A, al-Mulhim FA, Ibrahim AM, el-Hassan AY. Tuberculosis in
patients with malignant disease. Indian J Cancer. 1989;26(2):53-7.
- del
Giglio A, Pinczowski H, Portugal G, Feher O. Tuberculous skeletal
muscle involvement in acute leukemia: report on two cases. Tumori.
1997;83(2):618-20. https://doi.org/10.1177/030089169708300229
- Murgoci G. [Tuberculosis in Hodgkin's disease in a child]. Pneumoftiziologia. 1993;42(4):49-51.
- Narimatsu
H, Ota Y, Kami M, Takeuchi K, Suzuki R, Matsuo K, et al.
Clinicopathological features of pyothorax-associated lymphoma; a
retrospective survey involving 98 patients. Ann Oncol.
2007;18(1):122-8. https://doi.org/10.1093/annonc/mdl349
- Yasuda
N, Ohmori S, Usui T. Spinal epidural involvement in pleural lymphomas
developing from long-standing tuberculous pyothorax or pleuritis. Int J
Hematol. 1993;58(3):177-82.
- Chen GL, Xia
ZG, Jin J, Yu BH, Cao J. Characterization of Artificial
Pneumothorax-Unrelated Pyothorax-Associated Lymphoma. J Oncol.
2021;2021:3869438. https://doi.org/10.1155/2021/3869438
- Chen GL, Guo L, Yang S, Ji DM. Cancer risk in tuberculosis patients in a high endemic area. BMC Cancer. 2021;21(1):679. https://doi.org/10.1186/s12885-021-08391-6
- Guidelines
for Implementing the National Tuberculosis Control Program in China
(2008) [press release]. Beijing: Press of Chinese Peking Union Medical
College2009.
- Vineis P, Crosignani P,
Sacerdote C, Fontana A, Masala G, Miligi L, et al. Haematopoietic
cancer and medical history: a multicentre case control study. J
Epidemiol Community Health. 2000;54(6):431-6. https://doi.org/10.1136/jech.54.6.431
- Everatt
R, Kuzmickiene I, Davidaviciene E, Cicenas S. Non-pulmonary cancer risk
following tuberculosis: a nationwide retrospective cohort study in
Lithuania. Infect Agent Cancer. 2017;12:33. https://doi.org/10.1186/s13027-017-0143-8
- Kuo
SC, Hu YW, Liu CJ, Lee YT, Chen YT, Chen TL, et al. Association between
tuberculosis infections and non-pulmonary malignancies: a nationwide
population-based study. Br J Cancer. 2013;109(1):229-34. https://doi.org/10.1038/bjc.2013.220
- Leung
CY, Huang HL, Rahman MM, Nomura S, Krull Abe S, Saito E, et al. Cancer
incidence attributable to tuberculosis in 2015: global, regional, and
national estimates. BMC Cancer. 2020;20(1):412. https://doi.org/10.1186/s12885-020-06891-5
- Fan
R, Zhang LY, Wang H, Yang B, Han T, Zhao XL, et al. Multicentre
hospital-based case-control study of diffuse large B-cell lymphoma in
Shanghai, China. Asian Pac J Cancer Prev. 2012;13(7):3329-34. https://doi.org/10.7314/APJCP.2012.13.7.3329
- Nakatsuka
S, Yao M, Hoshida Y, Yamamoto S, Iuchi K, Aozasa K.
Pyothorax-associated lymphoma: a review of 106 cases. J Clin Oncol.
2002;20(20):4255-60. https://doi.org/10.1200/JCO.2002.09.021
- Horton
KC, MacPherson P, Houben RM, White RG, Corbett EL. Sex Differences in
Tuberculosis Burden and Notifications in Low- and Middle-Income
Countries: A Systematic Review and Meta-analysis. PLoS Med.
2016;13(9):e1002119.
- Horesh N, Horowitz
NA. Does gender matter in non-hodgkin lymphoma? Differences in
epidemiology, clinical behavior, and therapy. Rambam Maimonides Med J.
2014;5(4):e0038.
- Kumar RS, Goyal N. Estrogens as regulator of hematopoietic stem cell, immune cells and bone biology. Life Sci. 2021;269:119091. https://doi.org/10.1016/j.lfs.2021.119091
- Taneja V. Sex Hormones Determine Immune Response. Front Immunol. 2018;9:1931. https://doi.org/10.3389/fimmu.2018.01931
- Costas
L, Lujan-Barroso L, Benavente Y, Allen NE, Amiano P, Ardanaz E, et al.
Reproductive Factors, Exogenous Hormone Use, and Risk of B-Cell
Non-Hodgkin Lymphoma in a Cohort of Women From the European Prospective
Investigation Into Cancer and Nutrition. Am J Epidemiol.
2019;188(2):274-81. https://doi.org/10.1093/aje/kwy259
- Purohit
MR, Mustafa T, Morkve O, Sviland L. Gender differences in the clinical
diagnosis of tuberculous lymphadenitis--a hospital-based study from
Central India. Int J Infect Dis. 2009;13(5):600-5. https://doi.org/10.1016/j.ijid.2008.06.046
[TOP]


