Elisabetta Schiaroli1, Giuseppe Vittorio De Socio1, Laura Martinelli2, Lisa Malincarne1, Martina Savoia3, Anna Laura Spinelli4 and Daniela Francisci1.
1 Unit of Infectious Diseases, Department of Medicine and Surgery, University of Perugia, Perugia, Italy.
2 Internal Medicine of Città di Castello Hospital, USL Umbria 1, Italy.
3 Hospital Pharmacy of Foligno Hospital, USL Umbria 2, Italy.
4 Internal Medicine of Spoleto Hospital, USL Umbria 2, Italy.
Correspondence to: Elisabetta
Schiaroli, MD. Unit of Infectious Diseases, Department of Medicine,
University of Perugia, Perugia, Italy. Hospital "Santa Maria della
Misericordia", Piazzale Menghini, 1 – 06156, Perugia, Italy. Tel:
+39-075-5784375 Fax: +39-075-5784346. E-mail:
elisabetta.schiaroli@unipg.it
Published: November 1, 2021
Received: July 1, 2021
Accepted: October 10, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021061 DOI
10.4084/MJHID.2021.061
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objective:
The use of monoclonal antibodies to the SARS-Cov-2 spike protein for
early treatment of COVID-19 disease is being evaluated, with only phase
2 studies available to date. The emergency authorization of
bamlanivimab monotherapy was obtained in November 2020 by the FDA and
in March 2021 by Italian agency AIFA. Its use was then revoked in April
2021 by both. This study reports the results of bamlanivimab
utilization in monotherapy in Umbria (Italian region) to verify
whether, in a population with multiple risk factors, comparable results
to the phase 2 BLAZE1 trial had been obtained.
Methods:
Between March and April 2021, a retrospective observational study was
performed on patients treated with bamlanivimab. Demographic and
clinical characteristics before and after infusion were
evaluated. Moreover, a telephone interview was conducted about 30
days after the infusion to evaluate the overall course.
Results: All patients had an early infection (mean 4±1.73 days), almost all by alpha variant (97%).
No
adverse events to treatment were observed. Altogether within 30 days,
the hospitalization rate was 20%, 15% for COVID-19 related pathologies,
versus 4% at 11 days in the BLAZE1 phase 2 study. In addition,
worsening of some symptoms observed at baseline such as asthenia (77
vs. 51.3%), shortness of breath (38 vs. 23%) was registered, as well as
the onset of non-restorative sleep (41%).
Conclusion:
The clinical outcome after bamlanivimab monotherapy was far below the
expectation despite the patients had been infected by a theoretically
sensitive viral variant.
|
Introduction
Bamlanivimab binds to SARS –CoV-2's spike protein and prevents viral attachment to human surface ACE2 receptors.[1]
Preliminary analysis of treatment with bamlanivimab alone in human
beings resulted in lower rates of COVID-19-related hospitalization
within 29 days compared to treatment with the placebo (1.6% vs. 6.3%),
and a post-hoc analysis demonstrated that the subgroup high risk
patients receiving bamlanivimab had a reduced rate of hospitalizations
compared to those receiving the placebo (4.2% v 14.6%).[2]
Based
on the preliminary Phase 2 study (BLAZE 1), in November 2020, the Food
and Drug Administration (FDA) authorized the monoclonal antibody
bamlanivimab (LY-CoV555, Lilly) as monotherapy for emergency use (EUA)
in outpatients for treating mild to moderate coronavirus disease 2019
(COVID-19) in patients with positive results of direct SARS-CoV-2 viral
testing, aged 12 or over, weighing at least 40 kg and at high risk of
progression towards severe COVID-19 and/or hospitalization.[2-3]
On
February 6, 2021, likewise in Italy, the drug was temporarily
authorized for the treatment of COVID- 19 by a Health Ministry Decree
similar to the FDA: the drug was to be given as soon as possible after
a SARS-CoV-2 positive test result and within 10 days of COVID-19
symptom onset.
Afterward, on April 16, the FDA revoked its
Emergency Use Authorization, based on its ongoing analysis of the
sustained increase of SARS CoV-2 viral variants that were resistant and
the risk of treatment failure.[4] Indeed, as of
mid-March 2021, approximately 20% of isolates sequenced in the U.S.
were reported as lineages expected to be resistant to bamlanivimab
alone, increasing from approximately 5% in mid-January 2021.[4]
In the meantime, in Umbria, from January to March, several P.1 (gamma) viral variant clusters were seen,[5] the "fragile" population was being vaccinated, and in March, bamlanivimab was first available.
The
aim of the study was to describe the clinical use of bamlanivimab in
Umbria (Italy) and compare the outcome according to the time of early
infusion (within 72 hours) vs. late (>72 hours).
Materials and Methods
This
study, carried out from March and early April 2021, was retrospective
observational. We describe the characteristics, clinical outcomes, and
safety of patients reported by their general practitioners as recently
infected, with mild/moderate COVID-19 and at high risk of developing
severe disease according to AIFA criteria (Table 1)
who were admitted to the Day Hospital of Infectious Diseases Clinic of
Perugia, to the COVID Hospitals of Spoleto and Città di Castello, in
order to receive a single 700 mg intravenous (IV) infusion of
bamlanivimab alone. Demographic, medical history, main comorbidities,
virological (nasopharyngeal swabs), vaccination, and clinical data were
collected from the medical records, and we calculated the timeliness of
the treatment (within 72 hours) from the onset of symptoms.
Temperature, blood pressure, respiratory rate, and oxygen saturation
(SpO2) in resting-state were measured before and one hour after the
infusion of bamlanivimab. Around thirty days after the infusion,
patients were interviewed about their health state, the presence of
mild adverse effects, the date and results of subsequent nasopharyngeal
swabs, and any changes in pre and post-treatment symptoms.
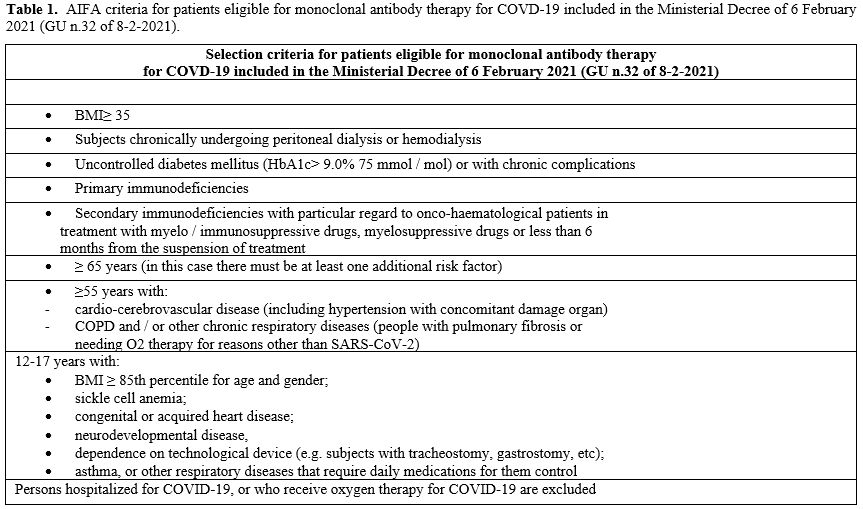 |
Table
1. AIFA criteria
for patients eligible for monoclonal antibody therapy for COVD-19
included in the Ministerial Decree of 6 February 2021 (GU n.32 of
8-2-2021). |
Statistical Analysis.
Standard descriptive statistics were used to summarize data, such as
mean, standard deviation (S.D.), and percentage. Pearson Chi-square
test was used to test differences between categorical variables. A
p-value < 0.05 was considered for statistical significance. The
student t-test for paired samples was used to test differences between
continuous variables. SPSS statistical package release 24.0 (SPSS Inc.,
Chicago, IL) was used for all statistical analyses.
Results
A
total of 39 patients received the infusion of bamlanivimab: 51.3% were
male, the mean age was 63±15.8 years (range 16-92), and cardiovascular
disease was the main risk factor (51.3%). Fever (Temperature ≥ 37.5°C)
was present in 18 patients, and the most represented COVID-19 related
symptoms were: cough (59%), myalgia (53.8%), and asthenia (51.3%). In
addition, genes N, S, and E were detected in 38/39, 1/39, and 29/39
patients, respectively, by a
reverse-transcriptase–polymerase-chain-reaction assay. (Table 2).
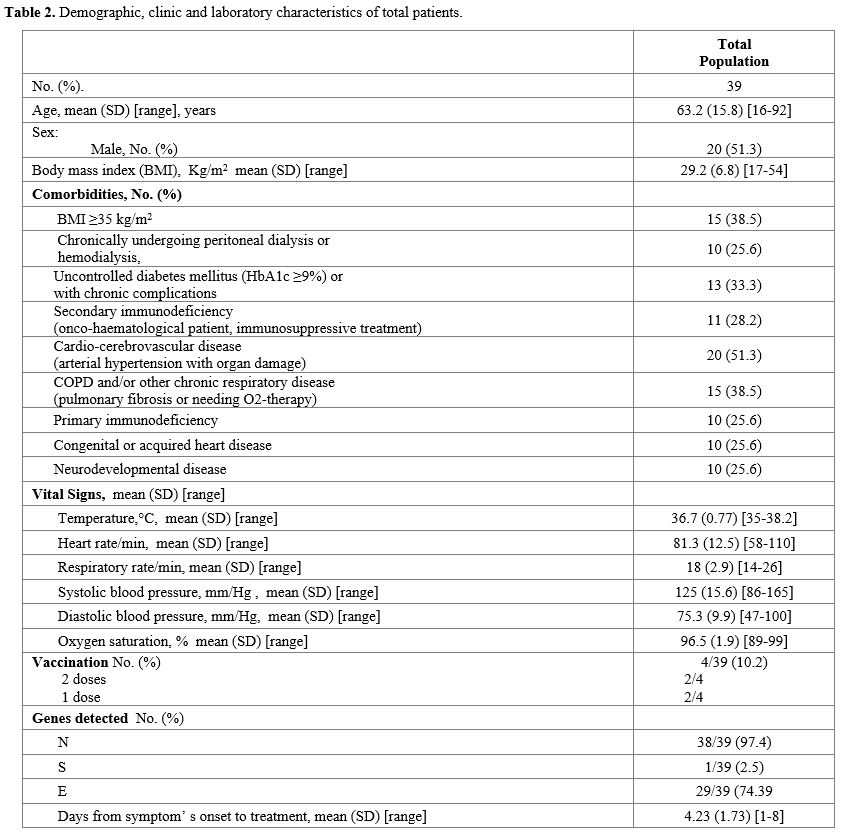 |
Table 2. Demographic, clinic and laboratory characteristics of total patients.
|
Four
patients had been vaccinated (3 Pfizer, 1 AstraZeneca): 2 patients had
received two doses (Pfizer), 2 only one (1 Pfizer and 1 AstraZeneca).
In patients who had completed the vaccination, COVID-19 arose > 15
days after the second dose, while in the patient who had received only
one dose of Pfizer, the disease arose after 8 days, and in the other
who had taken AstraZeneca after 2 days. In the latter, the aggravation
of an already known thrombocytopenia led to the patient's
hospitalization.
The average time between the onset of symptoms and treatment was 4.23±1.73 days (range 1-8).
Up
to one hour after the bamlanivimab infusion, vital signs remained
stable, and the patients were discharged in good health. No adverse
events were documented in the following days.
Eight patients
were hospitalized (one of them had received the first dose of
AstraZeneca): 4 for COVID-19 pneumonia, 4 for the worsening of
underlying diseases (thrombocytopenia, diabetic ketoacidosis, acute
renal failure in chronic kidney disease, bacterial pneumonia).
The hospitalization rate for any reason was 20%, for COVID-19
pneumoniae, 10%
Thirty days after the infusion, patients reported
an increase in symptoms such as asthenia (77 vs. 51%), dyspnea (38.5
vs. 23 %), tachypnea (44 vs. 33.3%) compared to baseline, and in 41% of
the patients, the onset of a new symptom: insomnia (Figure 1).
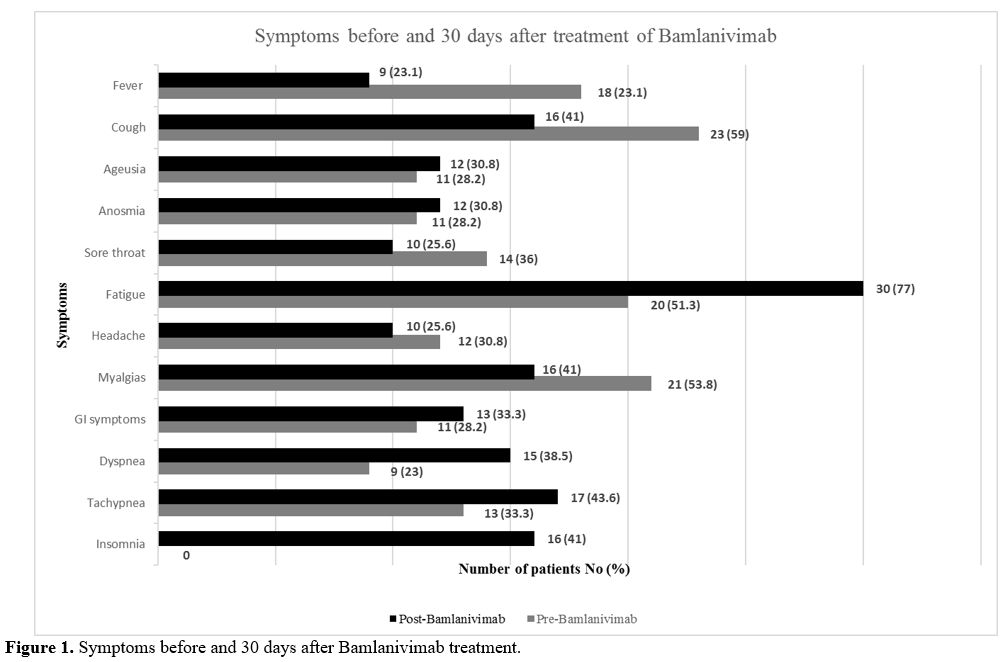 |
Figure 1. Symptoms before and 30 days after Bamlanivimab treatment.
|
The
comparison between patients treated early (within 3 days) vs. later
showed no differences regarding COVID-19 pneumonia hospitalization
(3/15 vs. 2/24 p=0.289) or the nasopharyngeal swabs time negativization
(mean 16.58±4.10 vs. 16.70±4.63 days p=0.942).
Discussion
The
study reports on the clinical use of Bamanivimab in Umbria (Italy),
which has to be read in a specific clinical context. An early treatment
within 72 hours was obtained in 38.8% of cases; the mean time between
the onset of symptoms and drug infusion was 4.23±1.73 days. Indeed, the
cycle threshold (Ct) of the
reverse-transcriptase–polymerase-chain-reaction assay was compatible
with an early onset: moreover, 38/39 (97%) did not express gene S
(alpha variant). The hospitalization rate was higher than expected; 20%
of our patients were hospitalized within 30 days with no differences
between early or late therapy. The number of hospitalizations for
SARS-Cov-2 infection and related complications was 6 (15%). Pneumonia
occurred in four patients with normal SpO2 at the time of bamlanivimab
infusion.
Bamlanivimab, a monoclonal antibody directed against a
spike protein epitope of SARS-Cov-2 and proposed for the treatment of
COVID-19, was authorized as monotherapy for emergency use (EUA) in
November 2020 by the FDA to treat outpatients with mild to moderate
COVID-19 at high risk of progression and/or hospitalization. It was
based on an interim analysis of the Phase 2 study BLAZE 1, concerning
patients randomized in the United States from June 17 to August 17,
2020: it regarded 143 controls and 309 treated outpatients subdivided
into three subgroups according to different doses of intravenous
infusion (iv) bamlanivimab (700, 2800, and 7000 mg).[2]
Subsequently, the BLAZE 1 study from August 22 to September 3
randomized another 114 patients who underwent the iv combination
therapy bamlanivimab-etesevimab.[6] The whole trial
was performed even before the diffusion of the several different
variants that, to date, are affecting the whole world.[7]
Therefore,
the final analysis concerned outpatients who had been randomized to
receive a single infusion of bamlanivimab alone (700 mg [n = 101], 2800
mg [n = 107], or 7000 mg [n = 101]), the combination treatment (2800 mg
of bamlanivimab and 2800mg of etesevimab [n = 112]), or the placebo (n
= 156).[6]
The inclusion criteria of the above
trials were the presence of at least one mild or moderate symptom of
COVID-19, the primary outcome was the change from base of the
SARS-CoV-2 viral load at 11 days and, in the overall context of the
secondary outcomes, the rate of hospitalizations within 4 weeks.
About
67% of patients had at least one risk factor for severe COVID-19
(aged ≥ 55 years, BMI ≥ 30, or ≥ 1 relevant comorbidity such as
hypertension), and treatment was started early (median 4 days from
onset of symptoms).
Compared with the placebo, there were no
significant differences in the viral load change at 11 days with the
three different doses of bamlanivimab alone. In contrast, the
combination bamlanivimab - etesevimab significantly decreased
SARS-CoV-2 log viral load at day 11 compared to the placebo
(between-group difference, –0.57 [95%CI, –1.00 to –0.14], P = .01).
The
hospitalization rate at 29 days was 1.0% in the 700 mg group and 0.9%
in the combination therapy group, 5.8% in the placebo group.[6]
Thus, the difference from the placebo was significant for the
combination group (p=0.04), not for the 700 mg group (p=0.09). However,
in a post hoc analysis, only 143 subjects were over 65 years old or
with a BMI ≥ 35 (95 treated, 48 controls). Their hospitalization rate
was 2.7% in the 700 mg group, 0% in the combination therapy group,
13.5% in the placebo group. Disease-related symptoms were monitored for
the first 11 days, and the total symptoms score was assessed: an
improvement for all the treated patient groups was observed.
Our
case history refers to the use of bamlanivimab alone 700 mg iv from
March 16 to April 16, until the FDA revocation of EUA. Therefore, the
results of our experience must be contextualized to the local
epidemiology where a wide diffusion of the English variant (alpha),
which was not widespread at the time of the BLAZE 1 study, was being
observed and where, in Umbria, outbreaks of variant P.1, (the Brazilian
variant gamma), had first been observed two months before.
The
entry criteria were different compared to BLAZE 1. Our case series
consisted of patients who met the AIFA criteria for enrollment, apart
from mild to moderate symptoms, which included in addition to BMI and
age, other risk factors for potential disease progression such as: chronically undergoing peritoneal dialysis or hemodialysis, secondary immunodeficiency, cardio-cerebrovascular disease and so on (Table 1).
Furthermore, 33% of the patients had more than one risk factor, while
18% had a BMI ≤ 35, and 15.4% were ≤ 65 years old. Instead, BLAZE
1 entry criteria were only mild to moderate symptoms, without other
risk factors.
The hospitalization rate was much greater than that
observed in BLAZE 1, even considering the post hoc analysis of study
BLAZE 1 where, introducing the age limit and a BMI >35, the rate of
hospitalization of the controls more than doubled (13.5%). However, our
cases appeared to be even more severe, having to consider the AIFA
authorization criteria.
Our follow-up was 30 days, and we were able to document an average negative time of the nasopharyngeal swab of about 16.6 days.
However,
in the face of virological response, a worsening of some baseline
symptoms was seen: asthenia (77 vs. 51%), dyspnea (38 vs. 23%),
tachypnea (44 vs. 33%), and in 41% of patients, the onset of a new
symptom: a non-restorative sleep, thus configuring an evolution towards
a sub-acute form of COVID-19.[8]
Limitations of
our study were the small number of subjects and the absence of a
control group. A control group of patients with the same
characteristics (comorbidities) as those treated would have required a
comparative clinical trial. In addition, the patients we treated were
being followed by their general practitioners and only referred because
they met AIFA criteria for monoclonal treatment (Table 1). Moreover, a
control group would have needed randomization by general practitioners
and was not ethical once an authorization procedure had been approved.
Conclusions
Despite
several recent outbreaks of the gamma variant in Umbria, bamlanivimab
in monotherapy was taken by patients largely infected by the alpha
variant that seems to be susceptible to bamlanivimab,[9] missing E484K and L452R mutations which lead to resistance.[4]
However,
the clinical and virological outcome observed in the epidemiological
context was largely below the expected one of the phase 2 trial BLAZE
1, which had permitted its emergency use until April 2020, with a lower
virological efficacy monotherapy compared to the combination was
demonstrated. Furthermore, in a contemporary clinical setting with
several SARS-CoV2 variants, the bamlanivimab monotherapy was safe.
However, the hospitalization rate was 20%, much greater than that
observed on the registrative trial BLAZE 1. Therefore, our results are
in line with the grounds for withdrawing the use of bamlanivimab alone;
the association of monoclonal antibodies in clinical practice context
needs to confirm the expected antiviral efficacy.
Funding
This
research was funded by Fondazione Cassa di risparmio di Perugia,
project "Studio prospettico sulla durata della contagiosità e sul
monitoraggio dei pazienti con infezione da Sars -CoV-2 in isolamento:
chi è infettato è anche infettivo?". Grant number 19663 (2020.05.08)
References
- Bryan E Jones, Patricia L Brown-Augsburger,
Kizzmekia S Corbett, Kathryn Westendorf, Julian Davies, Thomas P Cujec,
Christopher M Wiethoff, Jamie L Blackbourne, Beverly A Heinz, Denisa
Foster, Richard E Higgs, Deepa Balasubramaniam , Lingshu Wang , Yi
Zhang, Eun Sung Yang, Roza Bidshahri, Lucas Kraft, Yuri Hwang, Stefanie
Žentelis, Kevin R Jepson, Rodrigo Goya, Maia A Smith, David W Collins,
Samuel J Hinshaw, Sean A Tycho, Davide Pellacani, Ping Xiang, Krithika
Muthuraman, Solmaz Sobhanifar, Marissa H Piper, Franz J Triana, Jorg
Hendle, Anna Pustilnik, Andrew C Adams, Shawn J Berens, Ralph S Baric,
David R Martinez, Robert W Cross, Thomas W Geisbert, Viktoriya
Borisevich, Olubukola Abiona, Hayley M Belli, Maren de Vries, Adil
Mohamed, Meike Dittmann, Marie I Samanovic, Mark J Mulligan, Jory A
Goldsmith, Ching-Lin Hsieh , Nicole V Johnson , Daniel Wrapp , Jason S
McLellan, Bryan C Barnhart , Barney S Graham, John R Mascola, Carl L
Hansen, Ester Falconer. The nutralizing antibody, LY-CoV555, protects
against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med. 2021
May 12;13(593):eabf1906. doi: 10.1126/scitranslmed.abf1906. Epub 2021
Apr 5. https://doi.org/10.1126/scitranslmed.abf1906 PMid:33820835 PMCid:PMC8284311
- Peter
Chen, Ajay Nirula , Barry Heller, Robert L Gottlieb, Joseph Boscia,
Jason Morris, Gregory Huhn, Jose Cardona, Bharat Mocherla, Valentina
Stosor, Imad Shawa, Andrew C Adams, Jacob Van Naarden, Kenneth L
Custer, Lei Shen, Michael Durante, Gerard Oakley, Andrew E Schade,
Janelle Sabo, Dipak R Patel, Paul Klekotka, Daniel M Skovronsky,
BLAZE-1 Investigators. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in
Outpatients with Covid-19. N Engl J Med 2021 Jan 21 https://doi.org/10.1056/NEJMoa2029849 PMid:33113295 PMCid:PMC7646625
- Food and Drug Administration. Letter to Eli Lilly and Company. 10 Nov 2020. https://www.fda.gov/media/143602/download
- Food and Drug Administration. April 16, 2021 https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
- Anthony
Ramos-Yataco, Kelly Meza,Reyna Cecilia Farfán-García, Solange
Ortega-Rojas, Isaac Salinas-Mamani, Ivonne Silva-Arrieta Ontaneda and
Ricardo Correa . DKA in patients with pre-existing type 2 diabetes
mellitus related to COVID-19: a case series Endocrinol Diabetes Metab
Case Rep. 2021; 2021: 20-0148. Published online 2021 Mar 5. https://doi.org/10.1530/EDM-20-0148 PMCid:PMC7983528
- Ahmet
Burak Dirim, Erol Demir, Serap Yadigar, Nurana Garayeva, Ergun
Parmaksiz, Seda Safak , Kubra Aydin Bahat, Ali Riza Ucar, Meric Oruc,
Ozgur Akin Oto, Alpay Medetalibeyoglu, Seniha Basaran, Gunseli Orhun,
Halil Yazici, Aydin Turkmen. COVID-19 in chronic kidney disease: a
retrospective, propensity score-matched cohort study. Int Urol Nephrol.
2021 Feb 6;1-9. doi: 10.1007/s11255-021-02783-0. Online ahead of print.
https://doi.org/10.1007/s11255-021-02783-0 PMid:33548044 PMCid:PMC7864795
- Nalbandian
A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR,
Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D,
Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S,
Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D,
Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV,
Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19
syndrome Nat Med. 2021 Apr;27(4):601-615. doi:
10.1038/s41591-021-01283-z. Epub 2021 Mar 22. https://doi.org/10.1038/s41591-021-01283-z PMid:33753937
- Ministero
della Salute, Circolare n. 6251 del 17 febbraio 2021 "Indagine rapida
per la valutazione della prevalenza delle varianti VOC 202012/01
(ovvero lineage B.1.1.7-Regno Unito), P1 (ovvero Brasiliana), e 501.V2
(ovvero lineage B.1.351-Sud Africana) in Italia."
- Marek
Widera, Alexander Wilhelm, Sebastian Hoehl, Christiane Pallas, Niko
Kohmer, Timo Wolf, Holger F Rabenau, Victor Corman, Christian Drosten,
Maria JGT Vehreschild, Udo Goetsch, Rene Gottschalk, Sandra
Ciesek, Bamlanivimab does not neutralize two SARS-CoV-2 variants
carrying E484K in vitro medRxiv preprint. https://doi.org/10.1101/2021.02.24.21252372
[TOP]


