Antonio
Leone1, Silvia Macagnino2,
Giulia D’Ambra2, Giuseppe Veltri1
and Daniele Perla2.
1Department
of Radiological and Hematological Sciences, Fondazione Policlinico
Universitario A. Gemelli, IRCCS, Università Cattolica del Sacro Cuore,
Largo A. Gemelli 1, 00168 Rome, Italy.
2 Department
of Radiological and Hematological Sciences, Università Cattolica del
Sacro Cuore, Largo A. Gemelli 1, 00168 Rome, Italy.
Correspondence to:
Antonio Leone, MD. Department of Radiological and Hematological
Sciences, Fondazione Policlinico Universitario A. Gemelli, IRCCS.
Università Cattolica del Sacro Cuore, Largo A. Gemelli, 1,00168
Rome,Italy. Tel: +39-06-30156054, Fax: +39-06-35501928. E-mail:
a.leonemd@tiscali.it
http://orcid.org/0000-0003-3669-6321
Published: September 1, 2021
Received: July 23, 2021
Accepted: August 10, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021056 DOI
10.4084/MJHID.2021.056
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Radiological
diagnosis of systemic mastocytosis (SM) can be hard to establish. This
difficulty is mainly due to the variable radiological features
involving many organ systems (e.g., respiratory, cardiovascular,
lympho-reticular, digestive systems, and most commonly skin), and above
all, to the broad spectrum of skeletal findings. Skeletal involvement
is the most common and prominent imaging feature in patients with SM
and represents a prognostic factor as it may entail an aggressive
course of the disease. Diagnosis, largely established by histological
evaluation of a bone marrow trephine biopsy, supplemented by imaging
modalities such as radiography, CT, and magnetic resonance imaging,
requires a team approach between the hematologist, radiologist, and
pathologist. The general radiologist needs to be familiar with the
imaging findings because they may be the first to suggest the correct
diagnosis. The primary purpose of this review article was to equip
clinicians with pertinent radiological semiotics by presenting relevant
radiological features that assist early diagnosis and selection of an
effective treatment.
|
Introduction
In
systemic mastocytosis (SM), various organ systems, such as the
lympho-reticular, respiratory, cardiovascular, gastrointestinal, and
skeletal systems, may be involved, with a frequent localization in
extracutaneous organs such as the liver spleen, lymph nodes, and
gastrointestinal tract.[1] However,
skeletal involvement is one of the most important hallmarks of SM in
adults occurring in up to 90% of patients;[1,2]
bone marrow involvement occurs in virtually all patients with SM.[3,4]
Clinical manifestations such as organomegaly, signs of dysplasia, or
impaired organ function are due to the destructive accumulation of
abnormal mast cells, but mostly to the systemic effect of mast
cell-derived mediators.[5] Although
diagnosis is
mainly based on histological evaluation of a bone marrow biopsy,
radiography, CT, magnetic resonance [MR] imaging, and
hybrid
imaging techniques such as positron emission tomography [PET]/CT, it
may be valuable to suggest the diagnosis, to differentiate advanced
forms from indolent/smoldering subtypes of SM, and to define response
to treatment.[6-8] The prevalence
of osteoporosis was reported to range from 8% to 41%;[9,10]
thus, a dual-energy x-ray absorptiometry analyzing the lumbar spine and
hip should be assessed. The primary purpose of this review article was
to equip clinicians with pertinent radiological semiotics by presenting
relevant radiological features that assist early diagnosis and
selection of an effective treatment.
Imaging Findings
Musculoskeletal
System.
Radiological findings are valuable for detecting and characterizing
skeletal involvement, the most common radiological change reported in
SM.[1,2] There is considerable
heterogeneity in the
radiological features of SM-related bone involvement. The most common
types of skeletal abnormalities comprise: 1) multiple focal sclerotic
bone lesions affecting both the axial and appendicular skeleton (Figure 1, and 2)
diffuse, well-defined, roundish, sclerotic foci alternating with zones
with apparently normal or reduced bone density, predominating in the
axial skeleton, ribs, humerus, and femur (Figure 2).[11,12]
However, when such lesions are radiologically identified, final
diagnosis remains extremely challenging since they resemble
osteopoikilosis or metastases.[13,14]
Osteopoikilosis
is an asymptomatic bone dysplasia characterized by numerous bony
islands typically clustered around joints within the meta-epiphyseal
regions, carpal and tarsal bones, the pelvic ring, and scapulae. It is
usually asymptomatic, often discovered incidentally during radiographic
examinations made for other reasons, and normally does not demonstrate
radiotracer uptake on bone scintigraphy, contrary to what usually
occurs in metastasis.[15]
Diagnosis of SM is more
likely by considering clinical symptoms supported by laboratory
parameters (skin lesions, elevated serum tryptase levels, eosinophilia.
Diffuse osteosclerosis (Figure
3),
associated with focal sclerotic bone lesions, characterizes another
presentation of SM. Diffuse osteosclerosis, which predominates in the
axial skeleton, can simulate numerous other disorders such as
fluorosis, renal osteodystrophy, and idiopathic myelofibrosis
especially. The latter, however, is characterized by bone marrow
fibrosis and extramedullary hematopoiesis.[16]
Generalized osteoporosis is frequently encountered in SM; its
prevalence ranged from 8% to 41%, with a higher frequency in men than
women.[9,10,17,18]
Its prompt
diagnosis may prevent fragility fractures and decreases mortality and
morbidity. In their study of 82 patients with indolent SM, Rossini et
al.[18] found osteoporosis in 20%
of patients (7
women and 9 men) and vertebral fractures in 21.2 % of patients (5
postmenopausal women and 12 men). The high risk of vertebral fractures
in patients with indolent SM, as well as the higher prevalence of
osteoporosis in the male population, was confirmed by van der Veer et
al..[19] Thus, SM should be
considered in patients with unexplained osteoporosis and mast cell
mediators release symptoms;[9]
furthermore, bone turnover markers and bone mineral density should be
evaluated in such patients.[9,18] Single or multicentric osteolysis
is a rare radiological finding.[11,19]
This uncommon skeletal feature is often associated with osteosclerotic
foci or diffuse osteosclerosis in the spine, pelvis, and at the
meta-epiphysis of long bones.[12]
When skeletal
involvement presents as single osteolysis with a well or poorly defined
edge or surrounded by a sclerotic" halo" which has been reported as an
additional skeletal pattern in SM,[20]
its characterization may be challenging (Figure 4). It might
require a bone lesion biopsy.[21]
Furthermore, any sclerotic or lytic bone lesion may change its
appearance over time or by treatment; focal lesions may become diffuse
later on,[22] and any bony change
may be reversed because of treatment.[23]
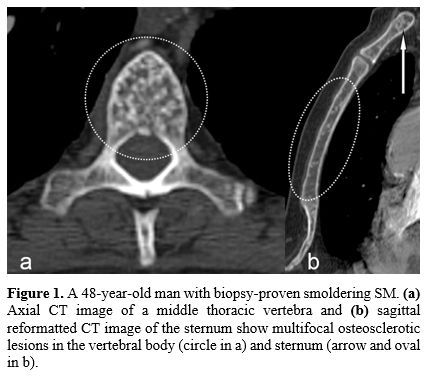 |
Figure
1. A 48-year-old man with biopsy-proven smoldering SM. (a) Axial CT image of
a middle thoracic vertebra and (b)
sagittal reformatted CT image of the sternum show multifocal
osteosclerotic lesions in the vertebral body (circle in a) and sternum
(arrow and oval in b). |
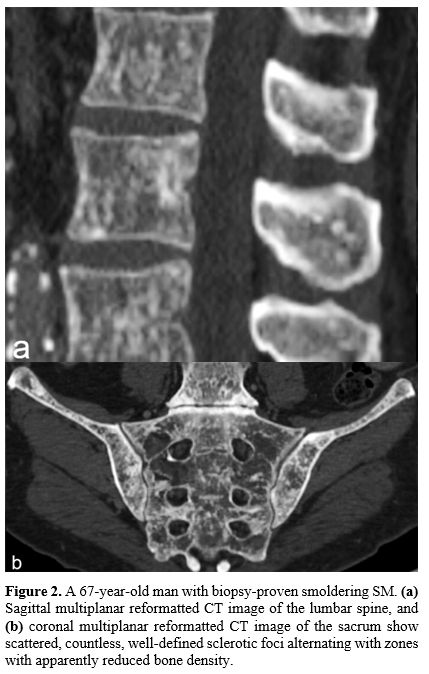 |
Figure
2. A 67-year-old man with biopsy-proven smoldering SM. (a) Sagittal
multiplanar reformatted CT image of the lumbar spine, and (b)
coronal multiplanar reformatted CT image of the sacrum show scattered,
countless, well-defined sclerotic foci alternating with zones with
apparently reduced bone density. |
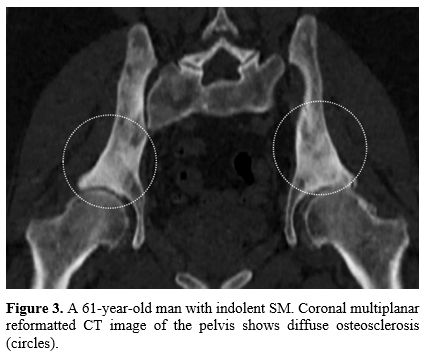 |
Figure
3. A 61-year-old
man with indolent SM. Coronal multiplanar reformatted CT image of the
pelvis shows diffuse osteosclerosis (circles). |
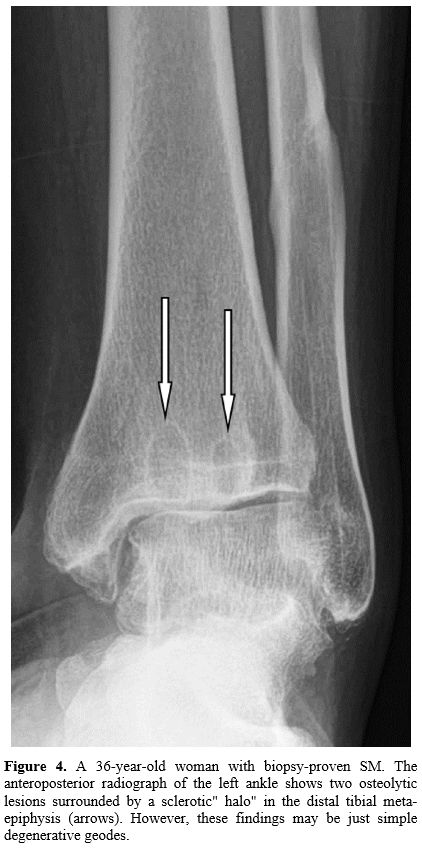 |
Figure
4. A 36-year-old
woman with biopsy-proven SM. The anteroposterior radiograph of the left
ankle shows two osteolytic lesions surrounded by a sclerotic" halo" in
the distal tibial meta-epiphysis (arrows). However, these findings may
be just simple degenerative geodes. |
Radiography and Dual-Energy
X-Ray Absorptiometry.
Because of its simplicity, low expense, and wide availability,
radiography should be the first-line imaging modality in diagnosing and
assessing skeletal abnormalities. Although its sensitivity and
specificity are rather low,[24]
once a bone lesion is evident radiographically, the likelihood that it
truly exists is high (Figure
5).
Nevertheless, it should be kept in mind that radiography is not
suitable for detecting bone marrow changes. Furthermore, its role in
detecting and characterizing osteoporosis is limited as more than
30%-50% bone loss is required to appreciate decreased bone density
radiography. Nowadays, dual-energy x-ray absorptiometry (DEXA) at the
lumbar spine and hip is the reference standard for diagnosing
osteoporosis and predicting fracture risk.[19,25]
Thus, DEXA should be assessed in patients with idiopathic osteoporosis
and mast-cell mediator release symptoms and in all SM patients at
diagnosis and during follow-up to detect those who may benefit from an
anti-osteoporotic treatment.[26-29]
Meyer et al.,[27]
analyzing DEXA data, records, clinical data, and bone marrow biopsies
of 39 patients with SM, retrospectively, reported that DEXA findings
are positively associated with tryptase level and mast cell amount in
bone marrow biopsies. In their study of 61 patients with SM, Riffel et
al.[29] correlated the prevalence
of osteoporosis,
increased bone mineral density (BMD), and osteosclerosis with clinical
parameters, disease type, and prognosis. The authors found that an
increased BMD and osteosclerosis are frequently present in advanced SM
but not in indolent SM; furthermore, in advanced SM, a high BMD and
osteosclerosis are associated with a more aggressive phenotype,
high-risk molecular aberrations, and inferior survival.
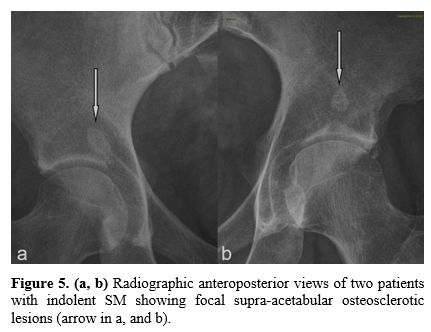 |
Figure
5. (a, b)
Radiographic anteroposterior views of two patients with indolent SM
showing focal supra-acetabular osteosclerotic lesions (arrow in a, and
b). |
CT.
CT is more sensitive and reliable than radiography in revealing and
providing a detailed view of small lesions, especially in areas that
may be poorly evaluated radiographically, because of their complex
anatomy, such as the craniocervical and cervicothoracic junctions,
anterior chest wall (Figure
1b), pelvic ring (Figure
2b), and acetabulum.[30]
CT is helpful in patients with SM and nonspecific radiographic findings
or patients with a clinically suspected diagnosis of SM and atypical
skin involvement. Furthermore, it has been reported that the assessment
of differences in attenuation values within the medullary cavity at CT
may be useful in identifying bone marrow infiltration, particularly in
the setting where MR imaging is contraindicated.[31]
Axial quantitative CT that can be conducted on conventional CT
examination allows establishing the true volumetric mineral density in
calcium hydroxyapatite milligrams per cubic centimeter of trabecular
and cortical bone. Quantitative CT has an excellent capability to
measure BMD, generally with better sensitivity than DEXA.[6]
MR Imaging.
MR imaging is the most sensitive imaging modality to assess bone marrow
cellular infiltration because of its high tissue contrast.[32,33]
Lesions with high cellularity are readily visible as decreased bone
marrow signal intensity on T1-weighted images and high marrow signal
intensity on fluid-sensitive fat-suppressed sequences (Figure 6).
However, these MR imaging findings are nonspecific, and the
differential diagnosis includes leukemia, myeloma, and Gaucher's
disease.[14] Osteosclerotic
lesions are constantly hypointense on both T1 and fluid-sensitive
images.[33]
Routine MR evaluation of bone marrow is not well suited for assessing
the effectiveness of the therapeutic agents; however, decreasing
fluid-sensitive and increasing T1-weighted signals usually indicate a
response to treatment.[34]
Whole-body (WB)-MR imaging
has become a modality that, allowing assessment of the entire skeleton
with high sensitivity for bone marrow changes, enhances diagnostic
performance and represents a valuable tool for screening, detecting the
extent of disease, and monitoring therapy in many oncologic disorders.[35] Riffel et al.,[11] analyzing
the bone marrow pattern of 115 patients with different forms of SM
through WB-MR imaging including T1-weighted, and turbo inversion
recovery magnitude (TIRM)-sequences, demonstrated the following five
distinct MR patterns:
1. Normal bone marrow.
2. Activated bone marrow (diffusely T1
hypointense, TIRM hyperintense).
3. Diffuse sclerotic bone marrow
(diffusely T1 hypointense, TIRM hypointense).
4. Small-spotted sclerotic bone marrow
(small-spotted T1 hypointense, TIRM hypointense).
5. Osteolytic lesions (sharply demarcated
T1 hypointense, TIRM hyperintense).
Furthermore,
these authors reported that sclerotic bone lesions were associated with
a high mast cells burden, organ damage, and adverse survival;
osteolytic lesions rarely resulted.
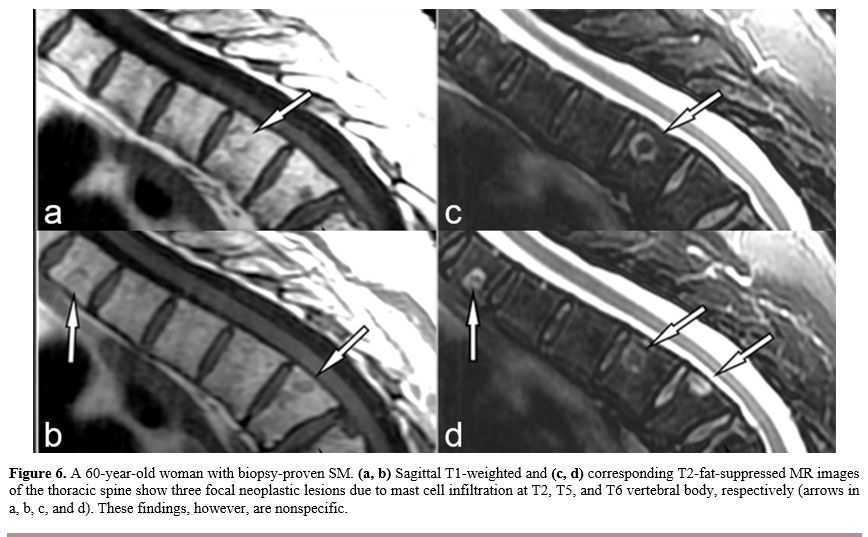 |
Figure
6. A 60-year-old woman with biopsy-proven SM. (a, b) Sagittal
T1-weighted and (c, d)
corresponding T2-fat-suppressed MR images of the thoracic spine show
three focal neoplastic lesions due to mast cell infiltration at T2, T5,
and T6 vertebral body, respectively (arrows in a, b, c, and d). These
findings, however, are nonspecific. |
18Fluorine-Fluorodeoxyglucose-Positron
Emission Tomography-CT.
Distinguishing malignant from a benign inflammatory process in cases
with multiple bony lesions with no skin disease is challenging because
both conditions can show increased 18fluorine-fluorodeoxyglucose
(18F-FDG) uptake.
In the study of Djelbani-Ahmed et al.,[36]
the retrospective analysis of 18F-FDG- positron
emission tomography
(PET)/CT examinations performed in 19 patients with an established
diagnosis of SM demonstrated pathological 18FDG uptake only
in the SM
with an associated hematologic neoplasm and in mast cell sarcoma cases,
suggesting a role of 18FDG-PET in the
assessment of these rare forms of
SM. However, the current data on the role of this imaging modality in
the evaluation of the different SM subtypes has not yet been
determined, and further studies are required before its true management
value can be determined.
Gastrointestinal System.
Among all manifestations of SM, symptoms related to gastrointestinal
involvement are common, being present in up to 80% of patients with SM
but are often nonspecific.[37,38]
Involvement of the
gastrointestinal system is mostly detected by endoscopic studies and
functional studies of absorption. The role of imaging modalities in SM
gastrointestinal involvement is limited. Radiological findings in
patients with SM include esophageal abnormalities (e.g., hiatus hernia,
esophagitis, stricture, varices, and motor incoordination) and peptic
ulcer disease. However, the most important imaging features are 1)
diffuse thickening and dilation of the stomach, small and large bowel
with nonocclusive strictures, 2) gastric, duodenal, and small-bowel
thickened folds, 3) mucosal nodular or polypoid lesions, and 4)
organomegaly.[39] Thickened folds
are due to mast
cell proliferation in the lamina propria. Several mucosal nodules are
"target" or "bull's-eye" lesions with a central collection of contrast
agents on barium examinations. These lesions, however, are nonspecific
since they may resemble lymphoma, primary bowel malignancies, and
carcinoid tumors.[40] Organomegaly
(hepatomegaly
and/or splenomegaly), which is a well-known manifestation of SM, may be
attributed to tissue infiltration by mast cells (Figure 7).[6]
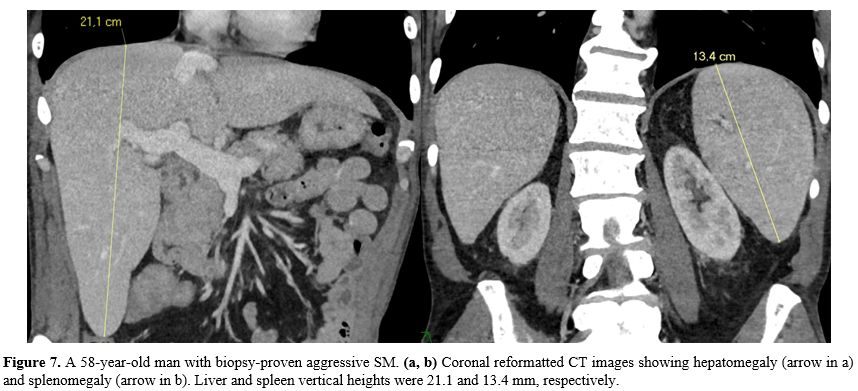 |
Figure
7. A 58-year-old man with biopsy-proven aggressive SM. (a, b)
Coronal reformatted CT images showing hepatomegaly (arrow in a) and
splenomegaly (arrow in b). Liver and spleen vertical heights were 21.1
and 13.4 mm, respectively. |
Imaging.
Ultrasound is the first-line imaging modality in patients with SM and
suspected gastrointestinal involvement. Ultrasound features are not
characteristic; differential diagnosis includes amyloidosis, neoplasms,
vasculitic disorders, inflammatory bowel disease, and mostly, lymphoma.
Nevertheless, the thickened gastric and bowel walls, as well as
abdominal lymph adenomegaly, and occasionally hypoechoic mucosa nodules
in the bowel wall can be revealed.[41,42]
When
carefully interpreted together with the clinical presentation and the
bone and skin status, these findings can lead to the suspicion of SM.[41] Furthermore, ultrasound, but mostly
CT and MR imaging, could be utilized to define hepatic and splenic
size.[43-47] Any decrease in
hepatic and splenic size in the treatment assessment setting indicates
treatment success in SM.[45]
Manual CT hepatic volumetry is time-consuming, laborious, and
software-dependent; therefore, simplified measuring methods are
extremely useful in clinical radiology practice. The longitudinal
dimension of the right lobe of the liver as measured in the
midclavicular plane is an easy and practical method for routine use.
The Hepatomegaly threshold for this parameter was up to 17 cm (Figure 7).[46] Verma et al.,[43]
correlating retrospectively hepatic measurements on MR imaging and
hepatic volume of 116 patients who had undergone post-contrast
abdominal MR imaging for conditions unrelated to the hepatobiliary
system, reported that simple linear hepatic measurements on MR imaging
are good indicators of hepatic volume and a reliable method for
monitoring the liver volume. There are complex methods of defining
splenomegaly;[47] however, in
their retrospective study of 264 abdominal CT examinations, Kucybala et
al.[44]
found that the strongest correlation with splenic volume, using a
single linear measurement, was the maximal height with a threshold for
this parameter of 12 cm (Figure
7). Epelboym et al.,[48]
analyzing 29 patients with confirmed mastocytosis, found that patients
with non-indolent mastocytosis were statistically more likely to have
hepatomegaly, splenomegaly, or lymphadenopathy on CT imaging as
compared to the indolent cohort. Hepatic and splenic involvements are
often characterized by prominent portal fibrosis, focal (perivascular)
or diffuse, respectively. Liver fibrosis, characterized by the
excessive accumulation of extracellular matrix proteins, leads to
portal hypertension and ultimately to cirrhosis. Conventional
ultrasound and cross-sectional imaging modalities have limited
capability to demonstrate liver fibrosis. Thus, diagnosis and staging
of hepatic fibrosis are currently performed by liver biopsy. However,
other imaging modalities such as ultrasonography-based transient
elastography, CT-based texture analysis, and diverse MR imaging-based
techniques have been proposed for noninvasive diagnosis and grading of
hepatic fibrosis.[49-51] MR
imaging-based techniques
include conventional post-contrast MR imaging, double contrast-enhanced
MR imaging, MR elastography, diffusion-weighted, and MR perfusion
imaging. Granted that a detailed discussion of MR imaging physical
phenomena is beyond the scope of this article, these MR techniques may
play a central role in treatment response monitoring and the clinical
management of patients with liver fibrosis.[51]
Lymphadenopathy. Another
central pathological feature is systemic infiltration and proliferation
of mast cells in lymph nodes (Figure
8).
Unfortunately, the radiological appearances of lymphadenomegaly (a
short axis measurement ≥ 1.0 cm) in mastocytosis cannot be
distinguished from those in lymphoma.[52,53]
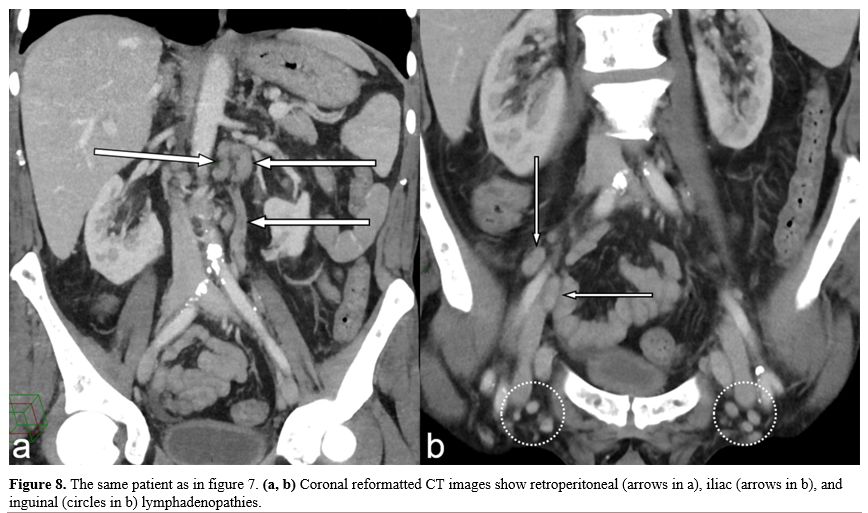 |
Figure
8. The same patient as in figure
7. (a, b)
Coronal reformatted CT images show retroperitoneal (arrows in a), iliac
(arrows in b), and inguinal (circles in b) lymphadenopathies. |
Respiratory
System.
Mast cells have been implicated in causing fibrosis since they could
stimulate fibroblasts proliferation, recruitment, and activity (e.g.,
transforming growth factor-β production).[54]
However, although the huge burden of mast cells within the lungs,
pulmonary involvement in SM and pulmonary fibrosis, in particular, are
rare.
Imaging.
Chest radiographic findings include perihilar or diffuse interstitial
fibrosis, cysts, lung nodules, and mediastinal lymphadenopathy.
Pulmonary involvement occurs in less than 20% of patients. Travis et
al.,[2] evaluating 58 patients with
SM, found focal or
scattered areas of fibrosis, bilateral interstitial fibrosis, and
multiple pulmonary nodules in 16% of patients; however, none was with
biopsy-proven pulmonary mastocytosis. Hermans et al.[55]
described the case of a young Caucasian female with SM associated with
pulmonary interstitial disease. The latter was directly related to SM
because of the presence of mast cells in bronchoalveolar lavage.
Concerning chest CT findings in SM patients, only a few case reports
have been published in English literature. Schmidt et al.[56]
described a case of a 54-year- old man with biopsy-proven mast cell
infiltration of the lung. Corresponding chest CT showed multiple
lymphadenopathies of the mediastinum and nodular pulmonary lesions.
Central nervous system.
Central nervous system involvement is extremely rare in SM. Chronic
symptoms such as cognitive impairment and depression-anxiety-like
symptoms have been reported by Boddaert et al.;[57]
they may be related to tissue mast cell infiltration and mast cell
mediator release. Supratentorial and infratentorial ischemic lesions
and diffuse brain involvement may be demonstrated on MR imaging, but
these features are not characteristic.[58]
Imaging. In the
already mentioned prospective and monocentric comparative study of
Boddaert et al.,[57]
39 patients with mastocytosis and psycho-cognitive complaints were
compared with 33 healthy controls. The authors found a high prevalence
(49%) of morphological and functional abnormalities in the brains of
mastocytosis patients with neuropsychiatric complaints
(depression–anxiety-like symptoms and cognitive impairment). These
patients had mainly abnormally punctuated white matter abnormalities
and increased perfusion in the putamen demonstrated on MR examinations.
However, the specificity of these morphological and functional
abnormalities remains to be elucidated.
Conclusions
SM
involves many extracutaneous organs systems with a heterogeneous
clinical presentation and variable clinical course. For this reason, a
variety of imaging modalities such as radiography, CT of the bone,
thorax, and abdomen, DEXA, and MR imaging need to be performed to
supplement bone biopsy and determine the subtype and extent of disease.
Regardless
of the type of SM, bone involvement is the most common presentation and
a prognostic factor. The presence of bone lesions may help confirm
systemic involvement and, in advanced SM, an increased BMD and
osteosclerosis are associated with a more aggressive phenotype and
worse outcomes.
References
- Akin C, Metcalfe DD.
Systemic mastocytosis. Annu Rev Med. 2004;55:419-432.
- Travis
WD, Li CY, Bergstralh EJ, Yam LT, Swee RG. Systemic mast cell disease.
Analysis of 58 cases and literature review. Medicine (Baltimore)
1988;67:345-368. https://doi.org/10.1097/00005792-198811000-00001
- Pardanani
A, Akin C, Valent P. Pathogenesis, clinical features, and treatment
advances in mastocytosis. Best Pract Res Clin Haematol 2006;
19:595-615. https://doi.org/10.1016/j.beha.2005.07.010
- Horny
HP, Valent P. Diagnosis of mastocytosis: general histopathological
aspects, morphological criteria, and immunohistochemical findings. Leuk
Res. 2001;25(7):543-551. https://doi.org/10.1016/S0145-2126(01)00021-2
- Gülen
T, Hägglund H, Dahlén B, Nilsson G. Mastocytosis: the puzzling clinical
spectrum and challenging diagnostic aspects of an enigmatic disease. J
Intern Med. 2016;279(3):211-228. https://doi.org/10.1111/joim.12410
- Ozturk
K, Cayci Z, Gotlib J, Akin C, George TI, Ustun C. Non-hematologic
diagnosis of systemic mastocytosis: Collaboration of radiology and
pathology Blood Rev. 2021;45:100693. https://doi.org/10.1016/j.blre.2020.100693
PMid:32334853
- Valent
P, Akin C, Sperr WR, et al. diagnosis and treatment of systemic
mastocytosis: state of the art. Br J Haematol. 2003 Sep;122(5):695-717.
- Di
Leo C, Lodi A, Pozzato C, et al. Systemic mastocytosis: bone marrow
involvement assessed by Tc-99m MDP scintigraphy and magnetic resonance
imaging Haematologica. 2003 Jul;88(7): ECR26.
- Orsolini
G, Viapiana O, Rossini M, Bonifacio M, Zanotti R. Bone Disease in
Mastocytosis. Immunol Allergy Clin North Am. 2018;38(3):443-454. https://doi.org/10.1016/j.iac.2018.04.013
PMid:30007462
- Degboé
Y, Eischen M, Nigon D, et al. Prevalence and risk factors for fragility
fracture in systemic mastocytosis. Bone. 2017;105:219-225. https://doi.org/10.1016/j.bone.2017.09.005
- Riffel
P, Jawhar M, Gawlik K, et al. Magnetic resonance imaging reveals
distinct bone marrow patterns in indolent and advanced systemic
mastocytosis. Ann Hematol 2019;98(12):2693-2701. https://doi.org/10.1007/s00277-019-03826-4
- Leone
A, Criscuolo M, Gullì C, Petrosino A, Carlo Bianco N, Colosimo C.
Systemic mastocytosis revisited with an emphasis on skeletal
manifestations. Radiol Med. 2021;126(4):585-598. https://doi.org/10.1007/s11547-020-01306-8
- Harzy T, El Hajjaji A.
Osseous mastocytosis of the knee. Clin Rheumatol 2007;26(12):2171-2172.
https://doi.org/10.1007/s10067-007-0651-9
- Fritz
J, Fishman EK, Carrino JA, Horger MS. Advanced imaging of skeletal
manifestations of systemic mastocytosis. Skeletal Radiol
2012;41(8):887-897. https://doi.org/10.1007/s00256-012-1374-9
- Vanhoenacker
FM, De Beuckeleer LH, Van Hul W et al. Sclerosing bone dysplasias:
genetic and radioclinical features. Eur Radiol 2000;10(9):1423-1433. https://doi.org/10.1007/s003300000495
- Barosi G, Hoffman R.
Idiopathic myelofibrosis. Semin Hematol. 2005;42(4):248-258. https://doi.org/10.1053/j.seminhematol.2005.05.018
- Barete
S, Assous N, de Gennes C, et al. Systemic mastocytosis and bone
involvement in a cohort of 75 patients. Ann Rheum Dis.
2010;69(10):1838-1841. https://doi.org/10.1136/ard.2009.124511
- Rossini
M, Zanotti R, Bonadonna P, et al. Bone mineral density, bone turnover
markers and fractures in patients with indolent systemic mastocytosis.
Bone. 2011;49(4):880-885. https://doi.org/10.1016/j.bone.2011.07.004
- van
der Veer E, van der Goot W, de Monchy JG, Kluin-Nelemans HC, van
Doormaal JJ. High prevalence of fractures and osteoporosis in patients
with indolent systemic mastocytosis. Allergy 2012;67(3):431-438. https://doi.org/10.1111/j.1398-9995.2011.02780.x
- Barer
M, Peterson OF, Dublin DR, Winkelmann RK, Stewart JR. Mastocytosis with
osseous lesions resembling metastatic malignant lesions in bone. J Bone
Joint Surg Am. 1968;50(1):142-152. https://doi.org/10.2106/00004623-196850010-00009
- Desportes
E, Lincot J, Hess A,Descamps V, Dallaudière B. Axial osseous lesions
mimicking disseminated metastases, a report of osseous mastocytosis.
JBR-BTR. 2014;97(5):295-297. https://doi.org/10.5334/jbr-btr.1333
- Chen
CC1, Andrich MP, Mican JM, Metcalfe DD. A retrospective analysis of
bone scan abnormalities in mastocytosis: correlation with disease
category and prognosis. J Nucl Med 1994;35(9):1471-1475.
- Graves
L 3rd, Stechschulte DJ, Morris DC, Lukert BP. Inhibition of mediator
release in systemic mastocytosis is associated with reversal of bone
changes. J Bone Miner Res 1990;5(11):1113-1119.
- Avila
NA, Ling A, Metcalfe DD, Worobec AS. Mastocytosis: magnetic resonance
imaging patterns of marrow disease. Skeletal Radiol 1998;27(3):119-126.
https://doi.org/10.1007/s002560050350
- Ulivieri
FM, Rinaudo L. Beyond Bone Mineral Density: A New Dual X-Ray
Absorptiometry Index of Bone Strength to Predict Fragility Fractures,
the Bone Strain Index. Front Med (Lausanne). 2021 January 15;7:590139.
- Brumsen
C, Papapoulos SE, Lentjes EG, Kluin PM, Hamdy NA. A potential role for
the mast cell in the pathogenesis of idiopathic osteoporosis in men.
Bone. 2002;31(5):556-561. https://doi.org/10.1016/S8756-3282(02)00875-X
- Meyer
HJ, Pönisch W, Monecke A, Gundermann P, Surov A. Bone mineral density
in patients with systemic mastocytosis: correlations with clinical and
histopathological features. Clin Exp Rheumatol. 2021;39(1):52-57.
- Ulivieri
FM, Rinaudo L, Piodi LP, et al. Usefulness of Dual X-ray
Absorptiometry-Derived Bone Geometry and Structural Indexes in
Mastocytosis. Calcif Tissue Int. 2020;107(6):551-558. https://doi.org/10.1007/s00223-020-00749-5
- Riffel
P, Schwaab J, Lutz C, et al. An increased bone mineral density is an
adverse prognostic factor in patients with systemic mastocytosis. J
Cancer Res Clin Oncol. 2020 Apr;146(4):945-951.
- Kropil
P, Fenk R, Fritz LB, et al. Comparison of whole-body 64-slice
multidetector computed tomography and conventional radiography in
staging of multiple myeloma. Eur Radiol 2008;18:51-58. https://doi.org/10.1007/s00330-007-0738-3
- Meyer
HJ, Pönisch W, Monecke A, Gundermann P, Surov A. Can Diagnostic
Low-dose Whole-body CT Reflect Bone Marrow Findings in Systemic
Mastocytosis? Anticancer Res 2020;40(2):1015-1022.
- Swartz PG, Roberts CC.
Radiological reasoning: bone marrow changes on MRI. AJR Am J Roentgenol
2009;193(3 Suppl):S1-4.
- Roca
M, Mota J, Giraldo P, García Erce JA. Systemic mastocytosis: MRI of
bone marrow involvement. Eur Radiol 1999;9(6):1094-1097. https://doi.org/10.1007/s003300050796
- Daldrup-Link HE,
Henning T, Link TM. MR imaging of therapy-induced changes of bone
marrow. Eur Radiol 2007;17(3):743-761. https://doi.org/10.1007/s00330-006-0404-1
- Lecouvet FE. Whole-Body
MR Imaging: Musculoskeletal Applications. Radiology
2016;279(2):345-365. https://doi.org/10.1148/radiol.2016142084
- Djelbani-Ahmed
S, Chandesris MO, Mekinian A, et al. FDG-PET/CT findings in systemic
mastocytosis: a French multicentre study. Eur J Nucl Med Mol Imaging
2015;42(13):2013-2020. https://doi.org/10.1007/s00259-015-3117-3
- Jensen
RT. Gastrointestinal abnormalities and involvement in systemic
mastocytosis. Hematol Oncol Clin North Am. 2000 Jun;14(3):579-623.
- Sokol
H, Georgin-Lavialle S,et al. Gastrointestinal involvement and
manifestations in systemic mastocytosis. Inflamm Bowel Dis. 2010
Jul;16(7):1247-1253.
- Quinn SF, Shaffer
HA Jr, Willard MR, Ross S. Bull's-eye lesions: a new gastrointestinal
presentation of mastocytosis. Gastrointest Radiol. 1984;9(1):13-15. https://doi.org/10.1007/BF01887793
- Ustun
C, Savage NM, Gotlib J, Bhalla K, Manaloor E, George TI. Systemic
mastocytosis with associated clonal hematological non-mast-cell lineage
disease: a case review. Am. J. Hematol. 2012;87:191-193.
- Rosignuolo
M, Muscianese M, Pranteda G. Systemic mastocytosis presenting with
gastrointestinal, bone and skin involvement. J. Ultrasound
2015;18:287-292. https://doi.org/10.1007/s40477-014-0090-9
- Avila
NA, Ling A, Worobec AS, Mican JM, Metcalfe DD. Systemic mastocytosis:
CT and US features of abdominal manifestations. Radiology.
1997;202:367-372. https://doi.org/10.1148/radiology.202.2.9015059
- Verma
SK, McClure K, Parker L, Mitchell DG, Verma M, Bergin D. Simple linear
measurements of the normal liver: interobserver agreement and
correlation with hepatic volume on MRI. Clin Radiol. 2010
Apr;65(4):315-318.
- Kucybała I, Ciuk S,
Tęczar J. Spleen enlargement assessment using computed tomography:
which coefficient correlates the strongest with the real volume of the
spleen? Abdom Radiol (NY). 2018 Sep;43(9):2455-2461.
- Surasi
DSS, Wang X, Bathala TK, et al. Utility of Longitudinal Measurement of
the Liver with Ultrasound in Comparison to Computed Tomography Liver.
Abdom Radiol (NY) 2021 Apr; 27:1-8.
- Kratzer
W, Fritz V, Mason RA, Haenle MM, Kaechele V; Roemerstein Study Group.
Factors affecting liver size: a sonographic survey of 2080 subjects. J
Ultrasound Med. 2003 Nov;22(11):1155-1161.
- Yetter
EM, Acosta KB, Olson MC, Blundell K. Estimating splenic volume:
sonographic measurements correlated with helical CT determination. AJR
Am. J. Roentgenol. 2003;181:1615-1620.
- Epelboym
Y, Keraliya AR, Tirumani SH, Hornick JL, Ramaiya NH, Shinagare AB.
Differences in the imaging features and distribution of non-indolent
and indolent mastocytosis: a single institution experience of 29
patients. Clin Imaging. 2017 Jul-Aug;44:111-116.
- Zhang
YN, Fowler KJ, Ozturk Aet al. Liver fibrosis imaging: A clinical review
of ultrasound and magnetic resonance elastography. J Magn Reson Imaging
2020;51(1):25-42. https://doi.org/10.1002/jmri.26716
- Petitclerc
L, Gilbert G, Nguyen BN, Tang A. Liver Fibrosis Quantification by
Magnetic Resonance Imaging. Top Magn Reson Imaging 2017;26(6):229-241. https://doi.org/10.1097/RMR.0000000000000149
- Faria
SC, Ganesan K, Mwangi I, et al. MR Imaging of Liver Fibrosis: Current
State of the Art. Radiographics 2009;29(6):1615-1635. https://doi.org/10.1148/rg.296095512
- Xu
Z, Jamison B, Bence-Bruckler I. Smoldering systemic mastocytosis with
lymph node involvement mimicking malignant lymphoma. Annals of
Hematology 2014;93:1603-1604. https://doi.org/10.1007/s00277-013-1993-9
- Sciumè
M, Serpenti F, Muratori S, et al. A case of aggressive systemic
mastocytosis with bulky lymphadenopathy showing response to
midostaurin. Clin Case Rep. 2020;9(2):978-982. https://doi.org/10.1002/ccr3.3717
- Hügle
T, Hogan V, White KE, van Laar JM. Mast cells are a source of
transforming growth factor β in systemic sclerosis. Arthritis Rheum.
2011;63(3):795-799.
https://doi.org/10.1002/art.30190
- Hermans
MA, Broijl A, van Daele PL. A unique presentation of pulmonary disease
in advanced systemic mastocytosis, proven by the presence of mast cells
in bronchoalveolar lavage: a case report. J Med Case Rep. 2016 October
13;10(1):283.
- Schmidt M, Dercken C,
Loke
O, Reimann S, Diederich S, Blasius S, et al. Pulmonary manifestation of
systemic mast cell disease. Eur. Respir. J. 2000;15:623-625.
- Boddaert
N, Salvador A, Chandesris MO, et al. Neuroimaging evidence of brain
abnormalities in mastocytosis. Transl. Psychiatry 2017;7:e1197.
- Pieri
L, Bonadonna P, Elena C, et al. Clinical presentation and management
practice of systemic mastocytosis. A survey on 460 Italian patients.
Am. J. Hematol. 2016;91:692-699.
[TOP]







