Pouyan Kheirkhah1, Ana M. Avila-Rodriguez2, Bartlomiej Radzik1 and Carlos Murga-Zamalloa1.
1 Department of Pathology, University of Illinois at Chicago, USA.
2 Department of Internal Medicine, University of Illinois at Chicago, USA.
Correspondence to: Carlos
A. Murga-Zamalloa, M.D. Department of Pathology, University of Illinois
at Chicago, 840 S Wood Street, 260 CMET, Chicago, IL 60607. Tel:
312-413-3998. E-mail:
catto@uic.edu
Published: November 1, 2021
Received: September 7, 2021
Accepted: October 19, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021067 DOI
10.4084/MJHID.2021.067
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Myeloid
sarcomas can be detected in up to 30% of acute myeloid leukemia cases
or occur de-novo without bone marrow involvement. The most frequent
localization of myeloid sarcomas in the abdominal cavity is the small
intestine, and gastric presentations are infrequent, frequently
misdiagnosed, and a high level of suspicion should exist when the
characteristic histomorphology features are present. The current review
features a case report with gastric presentation of myeloid sarcoma in
a patient with a diagnosis of acute myeloid leukemia with trisomy 8. In
addition, a review of the literature of intestinal-type myeloid
sarcomas shows that less than 15% of these cases have been reported in
the stomach. The most common molecular aberrancy detected in intestinal
myeloid sarcomas is the fusion protein CBFB-MYH11. A review of several
large studies demonstrates that the presence of myeloid sarcoma does
not constitute an independent prognostic factor. The therapeutic
approach will be tailored to the specific genetic abnormalities
present, and systemic chemotherapy with hematopoietic stem cell
transplant is the most efficient strategy.
|
Case Presentation
A
60-year-old male with a relevant past medical history of hepatitis C
infection, methadone use, and heroin addiction was surgically managed
for a toe infection. Persistent leukocytosis was noted despite
antibiotic treatment, and a microscopic review of the peripheral blood
smear demonstrated circulating blasts with left shift in granulocytes.
Bone marrow biopsy evaluation demonstrated 70-80% myeloblasts, and the
concurrent flow cytometric analysis show myeloblasts with the
expression of CD34, CD117, CD33, CD11c, CD13, and negative for CD56. A
diagnosis of acute myeloid leukemia was rendered. Karyotype analysis of
the bone marrow aspirate demonstrated trisomy 8 (47, XY, +8[2]/46,
XY[18]). Molecular testing demonstrated mutations in SRF2, TET2, and
KRAS genes (SRSF2 P95H, TET2 F1901Lfs*4, TET2 S585*, KRAS A146T).
During admission, the patient complained of persistent abdominal pain,
with coffee-ground emesis and one episode of melena. Computed
tomography showed thickening of the gastric wall near the
gastroesophageal junction with multiple enlarged lymph nodes. An urgent
esophagogastroduodenoscopy revealed a large, firm, ulcerated, and
partially circumferential mass in the cardia extending into the fundus,
with multiple ulcers within the gastric fundus and proximal duodenum.
Histologic evaluation from biopsies of the cardia mass demonstrated
dense submucosal aggregates, composed of large cells, with lobated
nuclear contours and fine and dispersed chromatin with inconspicuous
nucleoli (Figure 1).
Immunohistochemical stains demonstrated that the infiltrates were
positive for MPO, CD43, with weak CD68 expression, and negative for
CD34, CD117, CD20, CD79a, CD5, CD3 and cyclin D-1 (Figure 1). A diagnosis of myeloid sarcomas was established. The following questions arise from this case: How
often is myeloid sarcoma identified in the stomach, and what are the
specific histological features that help establish the diagnosis? Do
intestinal myeloid sarcomas share common molecular features? What is
the prognosis and management of myeloid sarcomas?
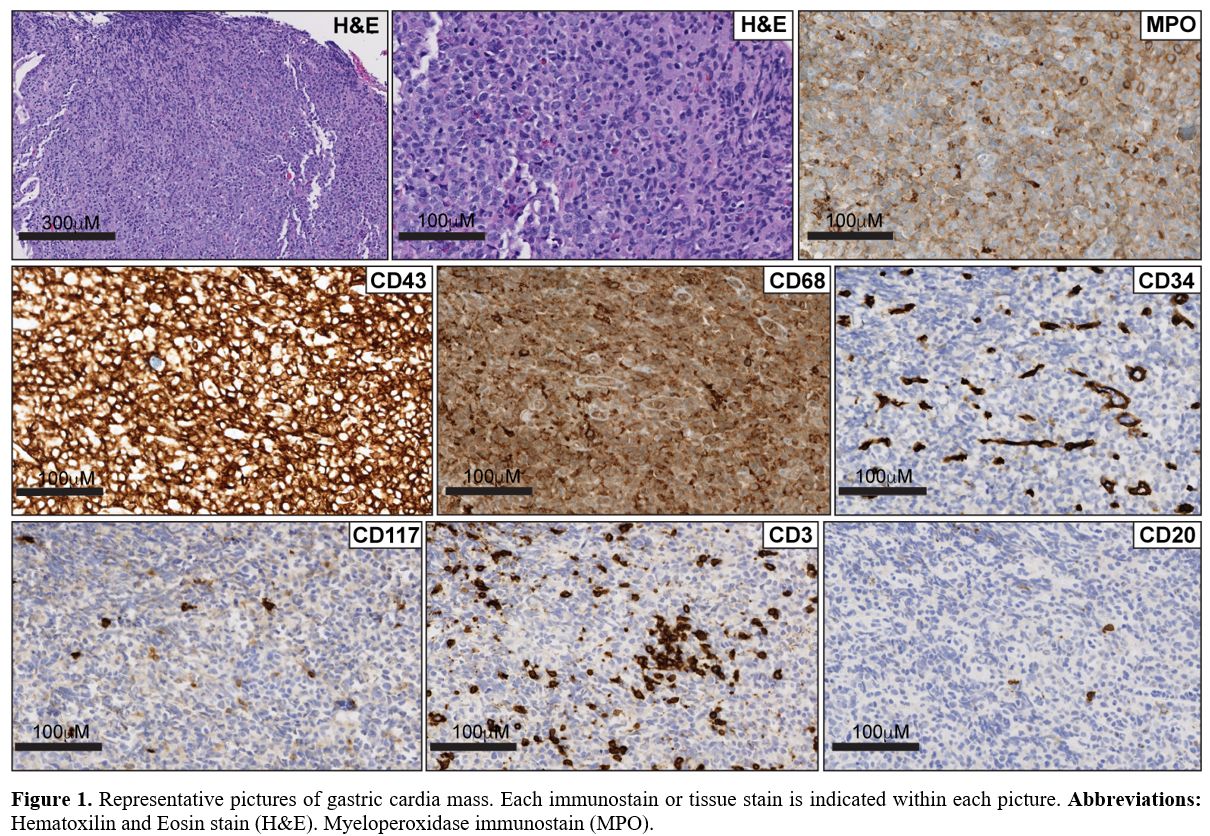 |
Figure
1. Representative
pictures of gastric cardia mass. Each immunostain or tissue stain is
indicated within each picture. Abbreviations: Hematoxilin and Eosin
stain (H&E). Myeloperoxidase immunostain (MPO). |
Definition and Frequency of Myeloid Sarcoma
Myeloid
sarcoma (MS) is defined by the presence of neoplastic extramedullary
infiltrates of myeloid precursors at a single or multiple sites, and
the term leukemia cutis is reserved for when those infiltrates are
present in the skin.[1] The incidence of myeloid sarcoma is variable, it has been reported in 7% to 30% of the cases of acute myeloid leukemia,[2]
and this variability will largely depend on the criteria utilized to
define extramedullary involvement. The majority of the studies that
identified myeloid sarcoma by physical examination and/or imaging
studies demonstrated a prevalence in the range of 20% to 30%, in
contrast to studies with the only biopsy-proven diagnosis that
identified a prevalence of 7-10%.[1-4]
Position-electron tomography (PET) has also been utilized, with
reported increased sensitivity compared to physical exam or other
imaging techniques.[5,6] A single prospective study
that compared PET-imaging, clinical examination, and histologic
analysis, reported an incidence of 22% with a sensitivity of 77% and
specificity of 97% for FDG-PET.[5]
The more
frequent localizations of myeloid sarcoma will also depend on the
methodology utilized for its detection. Detection by physical
examination identifies, as the most frequent sites, the gingiva,
spleen, and lymph nodes.[2] When imaging or biopsy
studies are utilized during the diagnosis, the most frequently involved
sites include the lymph nodes, testis, bone, and soft tissue.[1,7]
Some studies do not consider gingival, lymph node, liver, or splenic
involvement as myeloid sarcoma, as the hypertrophy identified during
physical examination may constitute migration/extravasation of blast
precursors and not a truly ‘tumor’ lesion.[5,8,9]
Myeloid
sarcoma can occur de-novo without simultaneous bone marrow involvement,
and in these instances, the diagnosis may be challenging, as it can be
misdiagnosed as lymphoma.[10,11] Moreover, a
diagnosis of myeloid sarcoma can precede the occurrence of acute
myeloid leukemia, or the occurrence of myeloid sarcoma can be the sole
manifestation of relapse. No specific risk factors for myeloid sarcoma
are identified; however, surface expression of CD56,[12] and CD11b,[2] and monocytic differentiation[4] are reported to be associated with an increased risk for the presence of extramedullary involvement by leukemia.
Intestinal Myeloid Sarcoma
Involvement
of the gastrointestinal tract by myeloid sarcoma is not a common
occurrence, and the most involved site is the small intestine. A PubMed
search using a combination of the terms’ intestinal’ ‘gastric’ ‘myeloid
sarcoma’ and ‘granulocytic sarcoma’ identified 58 individual clinical
case reports of myeloid sarcoma localized in the intestinal tract. From
the list, only 7 cases were reported to be localized in the gastric
compartment (Table 1). The
clinical presentation for gastric myeloid sarcoma was characterized by
non-specific symptoms, including persistent vague abdominal pain and
vomiting. Upper gastrointestinal endoscopy usually revealed thickening
of the stomach mucosa with hyperemic lesions and occasional nodularity
or tumor-forming lesions. Imaging studies, including computer
tomography (CT) scans, often revealed enlarged lymph nodes around the
stomach. In the current literature review series, 60% of the cases (n =
50) did not feature synchronous bone marrow involvement by acute
leukemia, making the diagnosis more challenging. Importantly, five
reported cases featured relapse of acute myeloid leukemia in the form
of gastrointestinal myeloid sarcoma, and three of those cases were post
stem cell transplantation.
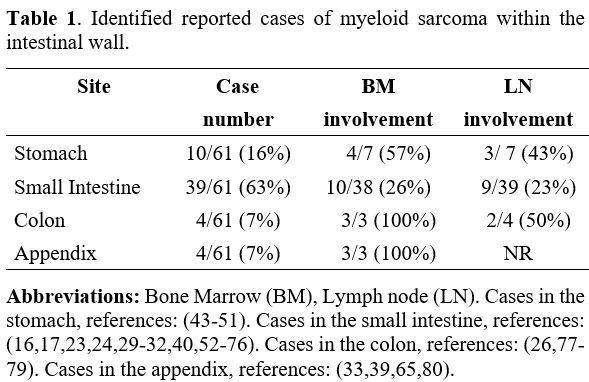 |
Table 1. Identified reported cases of myeloid sarcoma within the intestinal wall. |
Histologic Evaluation and Differential Diagnosis of Intestinal Myeloid Sarcomas
Myeloid
sarcomas are histologically characterized by dense submucosal
infiltrates, predominantly composed of large cells with cleaved or
slightly folded nuclear contours, variable amounts of cytoplasm, and
finely dispersed chromatin.[11] However, similar
features can be identified in lymphoid and non-lymphoid neoplasms in
the intestinal tract; so, the differential diagnosis includes mature
lymphoproliferative neoplasms like diffuse large B-cell lymphoma, the
blastoid variant of mantle cell lymphoma, and mature T-cell neoplasms
with secondary GI tract involvement or primary to the GI tract.
Non-hematolymphoid neoplasms that may show similar morphological
features includes poorly differentiated carcinoma and melanoma. The
possibility of benign extramedullary hematopoiesis (myeloid metaplasia)
should also be included in the differential diagnosis; however, those
are characterized by multilineage hematopoietic elements at progressive
stages of maturation.
Myeloid sarcoma localized within the
intestinal tract is not a frequent presentation and lacks specific
clinical symptoms; therefore, the diagnosis may remain elusive unless
clinical suspicion and a careful histological analysis exist. A
misdiagnosis of myeloid sarcoma is not uncommon,[11,13] one report indicated that 58% (n = 26) of cases were originally diagnosed as lymphoma,[14] and two independent reports demonstrated that 44% (n = 61) and 46% (n = 158) were initially mislabeled as lymphoma.[11,15]
Therefore, immunohistochemical stains are required to establish a
diagnosis, and a panel that includes myeloid-specific markers (MPO,
CD117), should be included with any suspicious infiltrate. The current
literature review demonstrates that the majority of the cases within
the intestinal tract featured positive expression of either CD34 (91%,
n = 36) or CD117 (93%, n = 29), and only a single report was identified
with negative expression of both of these markers (Table 1).[16] Importantly, expression of either CD43 (94%, n = 17) and MPO (97%, n = 32) is frequently detected in the tumor cells (Table 1).
Aberrant expression of associated lymphoid markers has been reported in
cases of myeloid sarcoma, including CD3 and CD79a. The current review
for the intestinal presentation of myeloid sarcoma shows that CD3 was
positive in a single case,[17] and expression of CD5,
CD20, and CD79a was not detected in any of the cases included. The
current case report also demonstrated divergent CD34 and CD117 between
the bone marrow biopsy and the stomach biopsies, a phenomenon
previously described in leukemia cutis.[18]
Recurrent Cytogenetic Abnormalities in Intestinal Myeloid Sarcomas
The previous series indicated that trisomy 8 is commonly detected in cases of myeloid sarcoma[19] and leukemia cutis,[20]
and the presence of chromosome 8 abnormalities are associated with
worse responses to initial therapies and worse overall clinical
outcomes.[21,22] Unlike the current case, the
literature review did not identify any previous report of abdominal
myeloid sarcoma with trisomy 8. Previous reports demonstrated a higher
prevalence for abdominal localization of myeloid sarcomas that are
positive for the CBFB-MYH11 fusion.[23] The largest
series to date included 13 cases of myeloid sarcoma with an abdominal
presentation, and 11 (85%) of those cases were positive for CBFB-MYH11
fusion protein.[23] The same report included an
additional number of 22 cases that were identified from reported cases
in the literature, and 20 (92%) of those cases were positive for
CBFB-MYH11 fusion protein.[23] The current review of the literature identified 11 additional cases of intestinal myeloid sarcoma[17,24-33]
that evaluated for genetic abnormalities by either cytogenetic or
molecular testing, and the most common chromosomal rearrangement was
inv(16) in 27% (3/11) of the cases (Table 2).
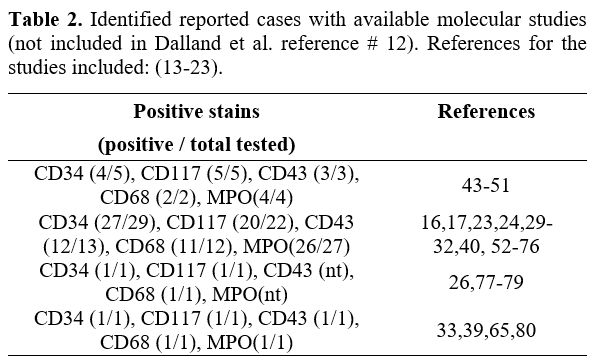 |
Table 2. Identified reported cases with available molecular studies (not included in Dalland et al. reference # 12). References for the studies included: (13-23).
|
Prognosis of Myeloid Sarcoma
Poor
outcomes with negative trends in the overall survival and event-free
survival sarcoma have been associated with a diagnosis of myeloid
sarcoma.[7,22] However, when
adjusted to age and cytogenetic risk, myeloid sarcoma is not identified
as an independent prognostic factor for overall survival,[2,4,34] and the overall 5-year survival rate for patients with concomitant myeloid sarcoma is no different from those without it.[1]
A retrospective study that evaluated the clinical outcomes of 84
patients with acute myeloid leukemia (AML) patients positive for
t(8;21)(AML1/ETO) included eight patients with extramedullary
involvement with significant worst responses to initial induction
chemotherapy. However, half of the patients with extramedullary
involvement featured CNS leukemic infiltration,[21] and additional studies did not identify myeloid sarcoma as an adverse prognostic factor in this subset of cases.[4]
Management of Myeloid Sarcoma
The
treatment of myeloid sarcoma will depend on the form of presentation
and whether this occurs at the initial diagnosis or at relapse.[1]
In the current era of targeted therapies, the consensus is to tailor
the therapeutic approach based on the tumor’s genetic profile, and
therefore molecular and cytogenetic testing are required to formulate
the treatment strategies.[35]
As a rare
condition, the lack of randomized controlled trials limits the
treatment strategies for isolated myeloid sarcoma without bone marrow
involvement. Retrospective series have demonstrated that delayed or
localized therapy alone will almost always progress to AML.[1,35]
Remission-type induction chemotherapy regimens have also been used in
isolated myeloid sarcoma, demonstrating a decrease in the rate of
progression and an increase in overall survival.[35,36]
The role of hematopoietic stem cell transplantation (HSCT) following
induction therapy is supported by retrospective studies, which indicate
that allogeneic HSCT is associated with a 5-year overall survival rate
of 48% and leukemia-free survival of 36%.[37] Localized radiation therapy (RT) has also been considered as consolidation treatment for isolated MS.[38]
Systemic
treatment is the first-line option for myeloid sarcoma with concurrent
marrow involvement at diagnosis. First-line treatments for
gastrointestinal myeloid sarcoma usually include conventional induction
chemotherapy with a combination of cytarabine and anthracycline agents.[39,40]
Unfortunately, there have been no randomized trials comparing the
different types of chemotherapy regimens and assessing the optimal AML
remission-induction chemotherapy regimens in the setting of myeloid
sarcoma with marrow involvement. However, autologous and allogeneic
HSCT are usually considered as treatment intensification based on the
superior outcomes with the use of HSCT in several retrospective
studies.[37,38]
Isolated myeloid sarcoma at
relapse is rare and usually precedes marrow relapse. For relapse after
chemotherapy alone, strategies such as relapse-type chemotherapy
regimens and localized radiation therapy (RT) are recommended.[1]
Isolated myeloid sarcoma after HSCT can present as the first
manifestation of relapse but has rarely been described, and currently,
there is no established standardized management. The therapeutic
strategies include donor lymphocyte infusion, tapering of
immunosuppression, or enrollment in clinical trials.[41]
Concomitant MS and marrow relapse are usually treated with reinduction
chemotherapy taking into account the possibility of HSCT or RT.[38]
In the setting of marrow and myeloid sarcoma relapse after HSCT, the
survival is poor and investigational agents or palliative care measures
must be considered.[1]
Finally, noncontrolled
anecdotal reports have described the use of highly targeted therapies
as a treatment strategy for myeloid sarcoma. For example, the humanized
anti-CD33 monoclonal antibody has been associated with good responses.[35,36]
In addition, tyrosine kinase inhibitors have also shown promising
results in a patient with myeloid sarcoma associated with FIP1L1-
PDGFRA fusion gene and eosinophilia.[42]
Conclusions
Myeloid
sarcoma can occur in up to 30% of newly diagnosed acute myeloid
leukemia. According to the site presentation, it is usually identified
by physical examination and imaging studies, and a confirmatory biopsy
will be required in a proportion of the cases. Histology analysis shows
overlapping morphology with mature lymphoproliferative neoplasms, and
immunohistochemical analysis is required to establish the diagnosis.
The most common site within the intestinal tract is the small
intestine, and the expression of CD34, MPO, and CD117 are the most
sensitive and specific markers. The aberrant fusion protein CBFB-MYH11
is the most predominant cytogenetic abnormality detected in intestinal
myeloid sarcoma. A diagnosis of myeloid sarcoma does not appear to
render a worse prognosis in comparison with patients that do not
feature extramedullary disease. The clinical management of myeloid
sarcoma will largely depend on the timing of the presentation and the
underlying genetic landscape of the tumor.
References
- Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118(14):3785-93. https://doi.org/10.1182/blood-2011-04-347229 PMid:21795742
- Ganzel
C, Manola J, Douer D, Rowe JM, Fernandez HF, Paietta EM, et al.
Extramedullary Disease in Adult Acute Myeloid Leukemia Is Common but
Lacks Independent Significance: Analysis of Patients in ECOG-ACRIN
Cancer Research Group Trials, 1980-2008. J Clin Oncol.
2016;34(29):3544-53. https://doi.org/10.1200/JCO.2016.67.5892 PMid:27573652 PMCid:PMC5074349
- Avni
B, Rund D, Levin M, Grisariu S, Ben-Yehuda D, Bar-Cohen S, et al.
Clinical implications of acute myeloid leukemia presenting as myeloid
sarcoma. Hematol Oncol. 2012;30(1):34-40. https://doi.org/10.1002/hon.994 PMid:21638303
- Fianchi
L, Quattrone M, Criscuolo M, Bellesi S, Dragonetti G, Maraglino AME, et
al. Extramedullary Involvement in Acute Myeloid Leukemia. A Single
Center Ten Years' Experience. Mediterr J Hematol Infect Dis.
2021;13(1):e2021030. https://doi.org/10.4084/MJHID.2021.030 PMid:34007418 PMCid:PMC8114885
- Stolzel
F, Luer T, Lock S, Parmentier S, Kuithan F, Kramer M, et al. The
prevalence of extramedullary acute myeloid leukemia detected by
(18)FDG-PET/CT: final results from the prospective PETAML trial.
Haematologica. 2020;105(6):1552-8. https://doi.org/10.3324/haematol.2019.223032 PMid:31467130 PMCid:PMC7271590
- Cribe
AS, Steenhof M, Marcher CW, Petersen H, Frederiksen H, Friis LS.
Extramedullary disease in patients with acute myeloid leukemia assessed
by 18F-FDG PET. Eur J Haematol. 2013;90(4):273-8. https://doi.org/10.1111/ejh.12085 PMid:23470093
- Paydas
S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of
the literature. Leuk Lymphoma. 2006;47(12):2527-41. https://doi.org/10.1080/10428190600967196 PMid:17169797
- Kobayashi
R, Tawa A, Hanada R, Horibe K, Tsuchida M, Tsukimoto I, et al.
Extramedullary infiltration at diagnosis and prognosis in children with
acute myelogenous leukemia. Pediatr Blood Cancer. 2007;48(4):393-8. https://doi.org/10.1002/pbc.20824 PMid:16550530
- Muss HB, Moloney WC. Chloroma and other myeloblastic tumors. Blood. 1973;42(5):721-8. https://doi.org/10.1182/blood.V42.5.721.721
- Yamauchi
K, Yasuda M. Comparison in treatments of nonleukemic granulocytic
sarcoma: report of two cases and a review of 72 cases in the
literature. Cancer. 2002;94(6):1739-46. https://doi.org/10.1002/cncr.10399 PMid:11920536
- Neiman
RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, et al.
Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases.
Cancer. 1981;48(6):1426-37. https://doi.org/10.1002/1097-0142(19810915)48:6<1426::AID-CNCR2820480626>3.0.CO;2-G
- Chang
H, Brandwein J, Yi QL, Chun K, Patterson B, Brien B. Extramedullary
infiltrates of AML are associated with CD56 expression, 11q23
abnormalities and inferior clinical outcome. Leuk Res.
2004;28(10):1007-11. https://doi.org/10.1016/j.leukres.2004.01.006 PMid:15289011
- Hagen
PA, Singh C, Hart M, Blaes AH. Differential Diagnosis of Isolated
Myeloid Sarcoma: A Case Report and Review of the Literature. Hematol
Rep. 2015;7(2):5709. https://doi.org/10.4081/hr.2015.5709 PMid:26330997 PMCid:PMC4508548
- Menasce
LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour
(granulocytic sarcoma) is often misdiagnosed: a study of 26 cases.
Histopathology. 1999;34(5):391-8. https://doi.org/10.1046/j.1365-2559.1999.00651.x PMid:10231412
- Byrd
JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell
tumors in acute nonlymphocytic leukemia: a clinical review. J Clin
Oncol. 1995;13(7):1800-16. https://doi.org/10.1200/JCO.1995.13.7.1800 PMid:7602369
- Kim
NR, Lee WK, Lee JI, Cho HY. Multiple Jejunal Myeloid Sarcomas
Presenting with Intestinal Obstruction in a Non-leukemic Patient: A
Case Report with Ultrastructural Observations. Korean J Pathol.
2012;46(6):590-4. https://doi.org/10.4132/KoreanJPathol.2012.46.6.590 PMid:23323112 PMCid:PMC3540339
- Gajendra
S, Gogia A, Das P, Gupta R, Tanwar P. Acute myeloid leukemia presenting
as "bowel upset": a case report. J Clin Diagn Res. 2014;8(7):FD09-10.
- Benet
C, Gomez A, Aguilar C, Delattre C, Vergier B, Beylot-Barry M, et al.
Histologic and immunohistologic characterization of skin localization
of myeloid disorders: a study of 173 cases. Am J Clin Pathol.
2011;135(2):278-90. https://doi.org/10.1309/AJCPFMNYCVPDEND0 PMid:21228369
- Al-Khateeb
H, Badheeb A, Haddad H, Marei L, Abbasi S. Myeloid sarcoma:
clinicopathologic, cytogenetic, and outcome analysis of 21 adult
patients. Leuk Res Treatment. 2011;2011:523168. https://doi.org/10.4061/2011/523168 PMid:23213544 PMCid:PMC3505919
- Sen
F, Zhang XX, Prieto VG, Shea CR, Qumsiyeh MB. Increased incidence of
trisomy 8 in acute myeloid leukemia with skin infiltration (leukemia
cutis). Diagn Mol Pathol. 2000;9(4):190-4. https://doi.org/10.1097/00019606-200012000-00003 PMid:11129442
- Byrd
JC, Weiss RB, Arthur DC, Lawrence D, Baer MR, Davey F, et al.
Extramedullary leukemia adversely affects hematologic complete
remission rate and overall survival in patients with t(8;21)(q22;q22):
results from Cancer and Leukemia Group B 8461. J Clin Oncol.
1997;15(2):466-75. https://doi.org/10.1200/JCO.1997.15.2.466 PMid:9053467
- Shan
M, Lu Y, Yang M, Wang P, Lu S, Zhang L, et al. Characteristics and
transplant outcome of myeloid sarcoma: a single-institute study. Int J
Hematol. 2021;113(5):682-92. https://doi.org/10.1007/s12185-021-03081-2 PMid:33511548
- Dalland
JC, Meyer R, Ketterling RP, Reichard KK. Myeloid Sarcoma With
CBFB-MYH11 Fusion (inv(16) or t(16;16)) Prevails in the Abdomen. Am J
Clin Pathol. 2020;153(3):333-41. https://doi.org/10.1093/ajcp/aqz168 PMid:31671434
- Antic
D, Elezovic I, Bogdanovic A, Vukovic NS, Pavlovic A, Jovanovic MP, et
al. Isolated myeloid sarcoma of the gastrointestinal tract. Intern Med.
2010;49(9):853-6. https://doi.org/10.2169/internalmedicine.49.2874 PMid:20453407
- Derenzini
E, Paolini S, Martinelli G, Campidelli C, Grazi GL, Calabrese C, et al.
Extramedullary myeloid tumour of the stomach and duodenum presenting
without acute myeloblastic leukemia: a diagnostic and therapeutic
challenge. Leuk Lymphoma. 2008;49(1):159-62. https://doi.org/10.1080/10428190701704621 PMid:18203027
- El
H, II, Easler JJ, Ceppa EP, Shahda S. Myeloid sarcoma of the sigmoid
colon: An unusual presentation of a rare condition. Dig Liver Dis.
2017;49(11):1280. https://doi.org/10.1016/j.dld.2017.05.012 PMid:28606757
- Goor
O, Goor Y, Konikoff F, Trejo L, Naparstak E. Common malignancies with
uncommon sites of presentation: case 3. Epigastric distress caused by a
duodenal polyp: a rare presentation of acute leukemia. J Clin Oncol.
2003;21(23):4458-9. https://doi.org/10.1200/JCO.2003.03.188 PMid:14645438
- Jeong
SH, Han JH, Jeong SY, Kang SY, Lee HW, Choi JH, et al. A case of
donor-derived granulocytic sarcoma after allogeneic hematopoietic stem
cell transplantation. Korean J Hematol. 2010;45(1):70-2. https://doi.org/10.5045/kjh.2010.45.1.70 PMid:21120167 PMCid:PMC2982999
- Le
Beau MM, Larson RA, Bitter MA, Vardiman JW, Golomb HM, Rowley JD.
Association of an inversion of chromosome 16 with abnormal marrow
eosinophils in acute myelomonocytic leukemia. A unique
cytogenetic-clinicopathological association. N Engl J Med.
1983;309(11):630-6. https://doi.org/10.1056/NEJM198309153091103 PMid:6577285
- Mrad
K, Abid L, Driss M, Ben Abid H, Ben Romdhane K. Granulocytic sarcoma of
the small intestine in a child without leukemia: report of a case with
cytologic findings and immunophenotyping pitfalls. Acta Cytol.
2004;48(5):641-4. https://doi.org/10.1159/000326435 PMid:15471256
- Narayan
P, Murthy V, Su M, Woel R, Grossman IR, Chamberlain RS. Primary myeloid
sarcoma masquerading as an obstructing duodenal carcinoma. Case Rep
Hematol. 2012;2012:490438. https://doi.org/10.1155/2012/490438 PMid:23243527 PMCid:PMC3517833
- Sun R, Samie AA, Theilmann L. A patient with a tumor of the ileocecal valve. Gastroenterology. 2011;141(6):1977, 2278. https://doi.org/10.1053/j.gastro.2010.11.046 PMid:22033185
- Toubai
T, Kondo Y, Ogawa T, Imai A, Kobayashi N, Ogasawara M, et al. A case of
leukemia of the appendix presenting as acute appendicitis. Acta
Haematol. 2003;109(4):199-201. https://doi.org/10.1159/000070971 PMid:12853694
- Shimizu
H, Saitoh T, Hatsumi N, Takada S, Yokohama A, Handa H, et al. Clinical
significance of granulocytic sarcoma in adult patients with acute
myeloid leukemia. Cancer Sci. 2012;103(8):1513-7. https://doi.org/10.1111/j.1349-7006.2012.02324.x PMid:22568487 PMCid:PMC7659365
- Almond
LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid Sarcoma:
Presentation, Diagnosis, and Treatment. Clin Lymphoma Myeloma Leuk.
2017;17(5):263-7. https://doi.org/10.1016/j.clml.2017.02.027 PMid:28342811
- Ibrahim
M, Chen R, Vegel A, Panse K, Bhyravabhotla K, Harris K, et al.
Treatment of myeloid sarcoma without bone marrow involvement with
gemtuzumab ozogamicin-containing regimen. Leuk Res. 2021;106:106583. https://doi.org/10.1016/j.leukres.2021.106583 PMid:33906021
- Chevallier
P, Labopin M, Cornelissen J, Socie G, Rocha V, Mohty M, et al.
Allogeneic hematopoietic stem cell transplantation for isolated and
leukemic myeloid sarcoma in adults: a report from the Acute Leukemia
Working Party of the European group for Blood and Marrow
Transplantation. Haematologica. 2011;96(9):1391-4. https://doi.org/10.3324/haematol.2011.041418 PMid:21685467 PMCid:PMC3166114
- Chen
WY, Wang CW, Chang CH, Liu HH, Lan KH, Tang JL, et al.
Clinicopathologic features and responses to radiotherapy of myeloid
sarcoma. Radiat Oncol. 2013;8:245. https://doi.org/10.1186/1748-717X-8-245 PMid:24148102 PMCid:PMC4016483
- Xavier
SG, Fagundes EM, Hassan R, Bacchi C, Conchon M, Tabak DG, et al.
Granulocytic sarcoma of the small intestine with CBFbeta/MYH11 fusion
gene: report of an aleukaemic case and review of the literature. Leuk
Res. 2003;27(11):1063-6. https://doi.org/10.1016/S0145-2126(03)00070-5
- Zhang
XH, Zhang R, Li Y. Granulocytic sarcoma of abdomen in acute myeloid
leukemia patient with inv(16) and t(6;17) abnormal chromosome: case
report and review of literature. Leuk Res. 2010;34(7):958-61. https://doi.org/10.1016/j.leukres.2010.01.009 PMid:20116851
- Wada
A, Kobayashi N, Asanuma S, Matsuoka S, Kosugi M, Fujii S, et al.
Repeated donor lymphocyte infusions overcome a myeloid sarcoma of the
stomach resulting from a relapse of acute myeloid leukemia after
allogeneic cell transplantation in long-term survival of more than 10
years. Int J Hematol. 2011;93(1):118-22. https://doi.org/10.1007/s12185-010-0737-z PMid:21181316
- Vedy
D, Muehlematter D, Rausch T, Stalder M, Jotterand M, Spertini O. Acute
myeloid leukemia with myeloid sarcoma and eosinophilia: prolonged
remission and molecular response to imatinib. J Clin Oncol.
2010;28(3):e33-5. https://doi.org/10.1200/JCO.2009.23.6976 PMid:19901109
- Gadage
V, Zutshi G, Menon S, Shet T, Gupta S. Gastric myeloid sarcoma--a
report of two cases addressing diagnostic issues. Indian J Pathol
Microbiol. 2011;54(4):832-5.
- Huang XL,
Tao J, Li JZ, Chen XL, Chen JN, Shao CK, et al. Gastric myeloid sarcoma
without acute myeloblastic leukemia. World J Gastroenterol.
2015;21(7):2242-8. https://doi.org/10.3748/wjg.v21.i7.2242 PMid:25717265 PMCid:PMC4326167
- Kini
S, Amarapurkar A, Balasubramanian M. Small Intestinal Obstruction with
Intussusception due to Acute Myeloid Leukemia: A Case Report. Case Rep
Gastrointest Med. 2012;2012:425358. https://doi.org/10.1155/2012/425358 PMid:22928122 PMCid:PMC3426187
- Koehler M. Granulocytic sarcoma of the stomach. Gastrointest Endosc. 1998;48(2):190. https://doi.org/10.1016/S0016-5107(98)70162-2
- Sekaran
A, Darisetty S, Lakhtakia S, Ramchandani M, Reddy DN. Granulocytic
Sarcoma of the Stomach Presenting as Dysphagia during Pregnancy. Case
Rep Gastrointest Med. 2011;2011:627549. https://doi.org/10.1155/2011/627549 PMid:22606423 PMCid:PMC3350040
- Yu
T, Xu G, Xu X, Yang J, Ding L. Myeloid sarcoma derived from the
gastrointestinal tract: A case report and review of the literature.
Oncol Lett. 2016;11(6):4155-9. https://doi.org/10.3892/ol.2016.4517 PMid:27313759 PMCid:PMC4888104
- Choi
ER, Ko YH, Kim SJ, Jang JH, Kim K, Kang WK, et al. Gastric recurrence
of extramedullary granulocytic sarcoma after allogeneic stem cell
transplantation for acute myeloid leukemia. J Clin Oncol.
2010;28(4):e54-5. https://doi.org/10.1200/JCO.2009.23.7560 PMid:19917867
- Jalil
Ur R, Shinawi M, Aslam M, Kelta M. Acute myeloid leukemia
post-allogeneic peripheral stem cell transplant with gastric chloroma.
Ann Saudi Med. 2011;31(6):658-60. https://doi.org/10.4103/0256-4947.81805 PMid:21727746 PMCid:PMC3221145
- Pasricha TS, Abraczinskas D. Gastrointestinal Myeloid Sarcoma. N Engl J Med. 2020;383(9):858. https://doi.org/10.1056/NEJMicm2001235 PMid:32846064
- Alvarez
P, Navascues CA, Ordieres C, Pipa M, Vega IF, Granero P, et al.
Granulocytic sarcoma of the small bowel, greater omentum and peritoneum
associated with a CBFbeta/MYH11 fusion and inv(16) (p13q22): a case
report. Int Arch Med. 2011;4(1):3. https://doi.org/10.1186/1755-7682-4-3 PMid:21255400 PMCid:PMC3032668
- Chen
YI, Paci P, Michel RP, Bessissow T. Myeloid sarcoma of the duodenum: a
rare cause of bowel obstruction and gastrointestinal bleeding.
Endoscopy. 2015;47 Suppl 1 UCTN:E181-2. https://doi.org/10.1055/s-0034-1391502
- Cicilet S, Tom FK, Philip B, Biswas A. Primary myeloid sarcoma of small bowel. BMJ Case Rep. 2017;2017. https://doi.org/10.1136/bcr-2017-220503 PMid:28596206 PMCid:PMC5535183
- Fukushima
M, Ono Y, Imai Y. Granulocytic sarcoma of the small intestine in a
patient with chronic myelomonocytic leukemia. Dig Endosc.
2014;26(6):757-8. https://doi.org/10.1111/den.12381 PMid:25251682
- Ghafoor
T, Zaidi A, Al Nassir I. Granulocytic sarcoma of the small intestine:
an unusual presentation of acute myelogenous leukaemia. J Pak Med
Assoc. 2010;60(2):133-5.
- He T, Guo Y,
Wang C, Yan J, Zhang M, Xu W, et al. A primary myeloid sarcoma
involving the small intestine and mesentery: case report and literature
review. Int J Clin Exp Pathol. 2018;11(8):4158-62.
- Hotta
K, Kunieda K. Granulocytic sarcoma of the jejunum diagnosed by biopsies
during double-balloon endoscopy before treatment (with video). Dig
Endosc. 2013;25(4):468. https://doi.org/10.1111/den.12107 PMid:23621465
- Huang
B, You P, Zhu P, Du Z, Wu B, Xu X, et al. Isolated duodenal myeloid
sarcoma associated with the CBFbeta/MYH11 fusion gene followed by acute
myeloid leukemia progression: A case report and literature review.
Oncol Lett. 2014;8(3):1261-4. https://doi.org/10.3892/ol.2014.2313 PMid:25120702 PMCid:PMC4114609
- Kitagawa
Y, Sameshima Y, Shiozaki H, Ogawa S, Masuda A, Mori SI, et al. Isolated
granulocytic sarcoma of the small intestine successfully treated with
chemotherapy and bone marrow transplantation. Int J Hematol.
2008;87(4):410-3. https://doi.org/10.1007/s12185-008-0067-6 PMid:18365139
- Kumar
B, Bommana V, Irani F, Kasmani R, Mian A, Mahajan K. An uncommon cause
of small bowel obstruction: isolated primary granulocytic sarcoma. QJM.
2009;102(7):491-3. https://doi.org/10.1093/qjmed/hcp051 PMid:19433489
- Lee
EY, Leung AY, Anthony MP, Loong F, Khong PL. Utility of 18F-FDG PET/CT
in identifying terminal ileal myeloid sarcoma in an asymptomatic
patient. Am J Hematol. 2011;86(12):1036-7. https://doi.org/10.1002/ajh.22077 PMid:21800353
- Li X, Huang C, Li Y. Obstructive jaundice caused by myeloid sarcoma in duodenal ampulla. Dig Liver Dis. 2019;51(2):321. https://doi.org/10.1016/j.dld.2018.08.004 PMid:30253977
- Lim
SW, Lee HL, Lee KN, Jun DW, Kim IY, Kim E, et al. A Case of Myeloid
Sarcoma of Intestine. Korean J Gastroenterol. 2016;68(3):148-51. https://doi.org/10.4166/kjg.2016.68.3.148 PMid:27646584
- Martinelli
G, Ottaviani E, Testoni N, Visani G, Pagliani G, Tura S. Molecular
analysis of granulocytic sarcoma: a single center experience.
Haematologica. 1999;84(4):380-2.
- McKenna
M, Arnold C, Catherwood MA, Humphreys MW, Cuthbert RJ, Bueso-Ramos C,
et al. Myeloid sarcoma of the small bowel associated with a
CBFbeta/MYH11 fusion and inv(16)(p13q22): a case report. J Clin Pathol.
2009;62(8):757-9. https://doi.org/10.1136/jcp.2008.063669 PMid:19638550
- Meireles
LC, Lagos AC, Marques I, Serejo F, Velosa J. Myeloid sarcoma of
gastrointestinal tract: A rare cause of obstruction. Rev Esp Enferm
Dig. 2015;107(5):326-7.
- Mizumoto R,
Tsujie M, Wakasa T, Kitani K, Manabe H, Fukuda S, et al. Isolated
myeloid sarcoma presenting with small bowel obstruction: a case report.
Surg Case Rep. 2020;6(1):2. https://doi.org/10.1186/s40792-019-0759-6 PMid:31900687 PMCid:PMC6942080
- Morel
F, Herry A, Le Bris MJ, Le Calvez G, Marion V, Berthou C, et al.
Isolated granulocytic sarcoma followed by acute myelogenous leukemia
type FAB-M2 associated with inversion 16 and trisomies 9 and 22.
Leukemia. 2002;16(12):2458-9. https://doi.org/10.1038/sj.leu.2402593 PMid:12454755
- Nemesio
RA, Costa B, Abrantes C, Leite JS. Myeloid sarcoma of the small
intestine in a patient without overt acute myeloid leukaemia: a
challenging diagnosis of a rare condition. BMJ Case Rep. 2018;2018. https://doi.org/10.1136/bcr-2017-222718 PMid:29848520 PMCid:PMC5976126
- Ono
K, Mikami Y, Hosoe N. Primary granulocytic sarcoma of the small
intestine diagnosed by single-balloon enteroscopy: A case report. Dig
Endosc. 2020;32(3):436. https://doi.org/10.1111/den.13611 PMid:31858650
- Palanivelu
C, Rangarajan M, Senthilkumar R, Annapoorni S. Laparoscopic management
of an obstructing granulocytic sarcoma of the jejunum causing
intussusception in a nonleukemic patient: report of a case. Surg Today.
2009;39(7):606-9. https://doi.org/10.1007/s00595-007-3807-y PMid:19562450
- Russell
SJ, Giles FJ, Thompson DS, Scanlon DJ, Walker H, Richards JD.
Granulocytic sarcoma of the small intestine preceding acute
myelomonocytic leukemia with abnormal eosinophils and inv(16). Cancer
Genet Cytogenet. 1988;35(2):231-5. https://doi.org/10.1016/0165-4608(88)90245-2
- Wang
P, Li Q, Zhang L, Ji H, Zhang CZ, Wang B. A myeloid sarcoma involving
the small intestine, kidneys, mesentery, and mesenteric lymph nodes: A
case report and literature review. Medicine (Baltimore).
2017;96(42):e7934. https://doi.org/10.1097/MD.0000000000007934 PMid:29049187 PMCid:PMC5662353
- Wong
SW, Lai CK, Lee KF, Lai PB. Granulocytic sarcoma of the small bowel
causing intestinal obstruction. Hong Kong Med J. 2005;11(3):204-6.
- Yoldas
T, Erol V, Demir B, Hoscoskun C. A rare cause of mechanical
obstruction: Intestinal myeloid sarcoma. Ulus Cerrahi Derg.
2014;30(3):176-8. https://doi.org/10.5152/UCD.2013.31 PMid:25931908 PMCid:PMC4379854
- Evans
C, Rosenfeld CS, Winkelstein A, Shadduck RK, Pataki KI, Oldham FB.
Perforation of an unsuspected cecal granulocytic sarcoma during therapy
with granulocyte-macrophage colony-stimulating factor. N Engl J Med.
1990;322(5):337-8. https://doi.org/10.1056/NEJM199002013220517 PMid:2404207
- Gupta
S, Chawla I, Singh V, Singh K. Granulocytic sarcoma (chloroma)
presenting as colo-colic intussusception in a 16-year-old boy: an
unusual presentation. BMJ Case Rep. 2014;2014. https://doi.org/10.1136/bcr-2014-206138 PMid:25150243 PMCid:PMC4154025
- Kaila V, Desai A. An Unusual Cause of Postpolypectomy Bleeding. Gastroenterology. 2020;159(4):e3-e5. https://doi.org/10.1053/j.gastro.2020.02.063 PMid:32205169
- Rodriguez
EA, Lopez MA, Valluri K, Wang D, Fischer A, Perdomo T. Acute
appendicitis secondary to acute promyelocytic leukemia. Am J Case Rep.
2015;16:73-6. https://doi.org/10.12659/AJCR.892760 PMid:25666852 PMCid:PMC4327184
[TOP]

