Fiorina Giona.
Hematology, Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy.
Correspondence to:
Fiorina Giona, MD. Hematology, Department of Translational and
Precision Medicine, Sapienza University of Rome, Via Benevento 6, 00161
Rome, Italy. Tel: +39-06-49974738. E-mail:
fiorina.giona@uniroma1.it
Published: November 1, 2021
Received: September 29, 2021
Accepted: October 23, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021069 DOI
10.4084/MJHID.2021.069
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Mastocytosis
is a rare clonal disorder characterized by excessive proliferation and
accumulation of mast cells (MC) in various organs and tissues.
Cutaneous mastocytosis (CM), the most common form in children, is
defined when MC infiltration is limited to the skin. In adults, the
most common form is systemic mastocytosis (SM), characterized by MC
proliferation and accumulation in organs, such as bone marrow, lymph
nodes, liver, and spleen.[1] Genetic aberrations, mainly the KIT D816V
mutation, play a crucial role in the pathogenesis of mastocytosis,
enhancing MC survival and subsequent accumulation in organs and
tissues.[2,3] CM includes three forms: solitary mastocytoma,
maculopapular cutaneous mastocytosis (MPCM), and diffuse cutaneous
mastocytosis (DCM). In most children with CM, skin lesions regress
spontaneously around puberty; unfortunately, it is not always a
self-limiting disease.[4] Even if SM occurs occasionally, all children
with mastocytosis require planned follow-up over time. Children with
mastocytosis often suffer from MC mediator-related symptoms, the most
common of which is itching, often triggered by rubbing the lesions.
Management of pediatric mastocytosis is mainly based on strict
avoidance of triggers. Treatment with H1 and H2 histamine receptor
blockers on demand and the availability of epinephrine auto-injectors
for the patients to use in case of severe anaphylactic reactions are
recommended.
|
Introduction
Mastocytosis
is a rare myeloproliferative disease characterized by an increase in
clonal morphologically and phenotypically abnormal mast cells (MC) that
accumulate in one or more organs and/or tissues. Most commonly, MCs
infiltrate the skin, the bone marrow (BM), liver, spleen, lymph nodes,
and gastrointestinal tract.[1-5] Disease manifestations can be due to
the release and activity of MC mediators and/or from the infiltration
of MC within affected organs.[5] Traditionally, the disease is divided
into cutaneous mastocytosis (CM) if MC infiltration is only localized
on the skin and systemic mastocytosis (SM) if MCs infiltrate various
organs, such as bone marrow, spleen, liver, and gastrointestinal tract,
and also the skin. According to the 2001 WHO classification, updated in
2008 and 2016, the diagnosis of SM can be established if at least 1
major and 1 minor, or 3 minor SM criteria, shown in table 1, are fulfilled.[6,7]
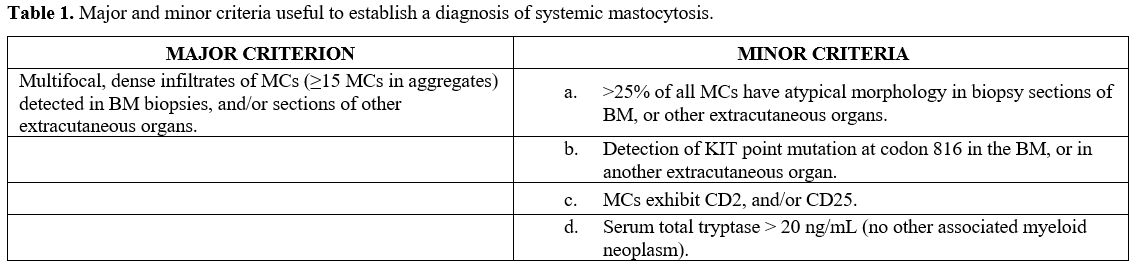 |
Table 1. Major and minor criteria useful to establish a diagnosis of systemic mastocytosis. |
CM is predominant in children, whereas the most common form of mastocytosis in adults is SM.
In
1869, the first pediatric case of mastocytosis localized in the skin
was described by Nettleship and Tay, but MC was first identified by
Elrich ten years later. The term urticaria pigmentosa was coined by Sangster in 1878 to describe skin lesions, while the term mastocytosis
was first used in 1936.[8] In 2007, an international Expert Working
Conference provided consensus statements on diagnostic criteria for
mastocytosis.[9] Three different forms of CM were identified: (1) solitary mastocytoma of the skin, (2) maculopapular cutaneous mastocytosis (MPCM), and (3)
diffuse cutaneous mastocytosis (DCM).[9] In addition, this Consensus
Group established diagnostic criteria to define CM: (a) the presence of
a typical skin lesion (major criterion), (b) increased numbers of MCs
in biopsy sections of the skin lesions (minor criterion), and (c) an activating KIT mutation at codon 816 in skin lesions (minor
criteria).[9,10,11] Recently, considering the different characteristics
of adulthood-onset and childhood-onset CM, an international task force
revised the classification and criteria for the diagnosis of cutaneous
manifestations (Table 2).[10]
In particular, the MPCM subgroup was subdivided into 2 variants, namely
a monomorphic variant with small maculopapular lesions, which is
predominant in adult patients, and a polymorphic variant with larger
lesions of variable size and shape, which is typically seen in children
(Figure 1).
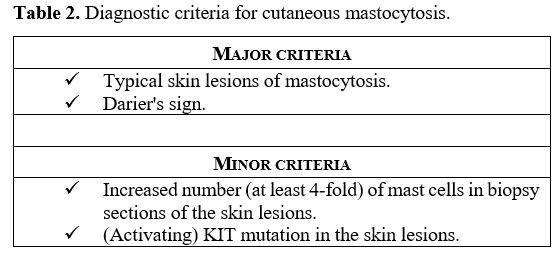 |
Table 2. Diagnostic criteria for cutaneous mastocytosis. |
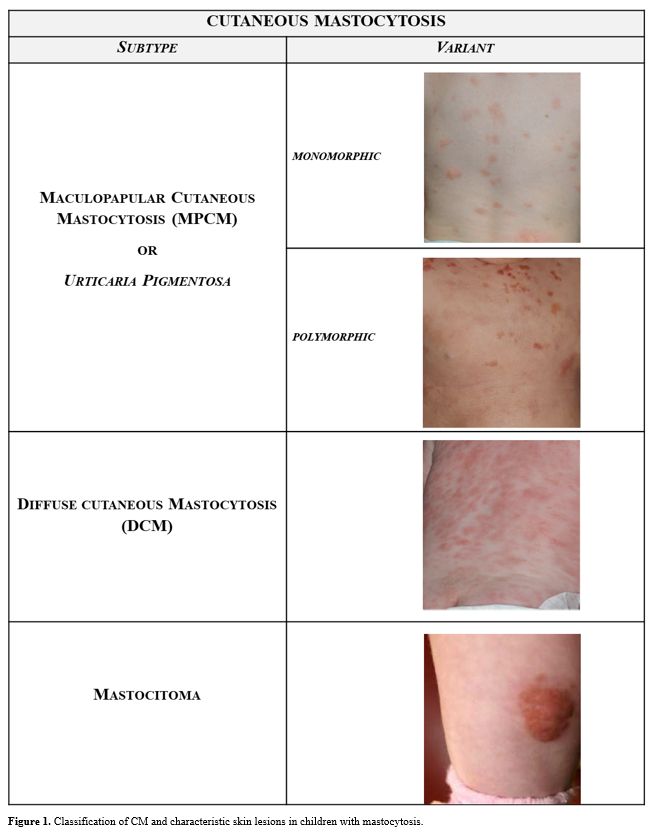 |
Figure 1. Classification of CM and characteristic skin lesions in children with mastocytosis. |
Regarding the
criteria for diagnosis, Darier's sign was established as a major
criterion to define CM, as it is always positive in children with CM (Table 2).
Darier's sign is a skin change observed, within 15-30 minutes, upon
mild rubbing of the lesions. In general, the skin becomes red, swollen,
and itchy due to the release of MC mediators.
Epidemiology and Natural History
Mastocytosis
in the pediatric age is characterized by an almost exclusively
cutaneous involvement and is considered a clonal expansion of benign
nature, with spontaneous regression by puberty in more than 80% of
cases. Skin lesions are located predominantly on the trunk, less
frequently on the limbs, and rarely on the head.[4,10,12] Even if some
rare familial cases are described, pediatric mastocytosis is considered
a sporadic disease, not hereditary.[1] Late-onset of the disease in
pediatric age correlates with an increased risk of developing SM,
whereas cases of aggressive neonatal mastocytosis are extremely
rare.[11] The disease course may be characterized by the complete
absence of symptoms, or alternatively by the appearance of the MC
mediator-related symptoms such as itching, flushing, blisters,
wheezing, abdominal pain, cramping, reflux, ulcers, diarrhea,
hypotension, headache, and depression. The risk of anaphylaxis in
children with mastocytosis is higher compared to the general
population; however, allergic hypersensitivity to Hymenoptera venoms is
less than in adults with mastocytosis. Regarding the onset age of
pediatric mastocytosis, 55% of cases are diagnosed between birth to 2
years, typically in the first six months of life, 35% over 15 years of
age, and the remaining 10% of cases under 15 years. In childhood
mastocytosis, the male/female ratio is reported to be 1.4.[11] Clinical Presentiations
In
pediatric patients, the most common symptom is itching, caused by MC
degranulation and often triggered by rubbing the lesions.[13] Among the
various cutaneous lesions shown in Figure 1,
the MPCM is the most frequent form, detectable in about 70% of children
with mastocytosis, generally younger than two years. Skin
manifestations are mainly macules and papules, brownish or reddish;
plaques and nodules may coexist. Lesions of the MPCM form may have
either a monomorphic or, more frequently, a polymorphic appearance, the
latter having a better prognosis. MPCM-large lesions generally occur
under seven months of age, and MPCM-small lesions over two years of
age. In children with MPCM, the disappearance of the small lesions
takes longer (≥8 years) than the large ones.[13] In MPCM, Darier's sign
is characteristic.[14] Cutaneous mastocytoma, congenital or developed
within the third month of life, is diagnosed in about 15% of children
with mastocytosis. The term cutaneous mastocytoma can be used in the
presence of up to three lesions that are brown/yellow and are usually
localized on the trunk and limbs. If lesions are more than three, a
diagnosis of MPCM is made. Mastocytoma can increase in size and change
in morphology, but it does not generally increase and usually regress
by puberty.[10,15] Darier's sign is positive.
DCM is less
common, but it is the most severe clinical presentation of CM, with MC
infiltration of the entire skin (5-13%). It usually appears in very
young children. The skin is typically thickened and has a typical
orange-peel appearance. Vesicles of various sizes, even giant, are
frequent. DCM has an excellent chance of remission at five years,
although a high mortality rate (24%), mainly due to anaphylactic shock
and digestive bleeding, is reported. It is important to avoid Darier's
sign in patients with DMC, in order to minimise the potential massive
release of mediators from MCs.
In most cases, marked and
persistent dermographism occurs after minimal mechanical
irritation.[10,15] Elevated tryptase levels are found in a minority of
children, and systemic symptoms are not always indicative of SM.[16]
However, it is important to consider the possibility of evolution in
the systemic form. In a recent review of literature, it emerged that
approximately 1/100 children with CM would develop a systemic form.[15]
There are little data available regarding the role of the
environment or epigenetic factors in children's disease development or
progression. In a study including 32 patients, it was demonstrated that
some drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs),
beta-lactams, nifedipine, and levothyroxine, and or/and tobacco mothers
chronic exposure during pregnancy, negatively influenced spontaneous
disease remission in children. Surprisingly, it was found that children
who achieved complete regression of the disease had a chronic
dysreactive disease, usually asthma or eczema, before or after the
diagnosis of mastocytosis.[17]
Pathophysiology
The
pathogenesis of CM in children is still unclear. In the surface
membrane, MCs express a receptor for a stem cell factor, CD117, also
known as KIT. It is known that activating mutations of the gene
encoding the KIT receptor (c-KIT), observed among 90% of adults with
SM, play a crucial role in the pathogenesis of mastocytosis, enhancing
MCs survival with subsequent accumulation of MCs in various organs and
tissues.[6,18,19] Pediatric mastocytosis is considered a clonal
disorder associated with mutations of the proto-oncogene KIT in varying
ratios, from 0 to 83%.[17] The mutation of Kit codon 816 (D816V) in
exon 17, found in 80% of cases with adult-onset disease, was found in
only 42% of pediatric patients. Among children without any mutation of
codon 816 (D816V, D816Y, and D816I), other mutations involving exons 8,
9, 11, and 13 were found in 44% of them by sequencing the entire KIT
gene.[20] For this reason, in children with CM without KIT D816V
mutation, sequencing the entire c-KIT gene is recommended (Figure 2).
As a proportion of children do not show any c-KIT mutations, it is most
likely that other gene mutations could be responsible for the
pathogenesis of the disease.[18,20] The various c-KIT gene status, wild
type, mutations in exon 17, and other mutations found in children do
not correlate with clinical phenotypes, MPCM, DCM, and mastocytoma and
do not predict the outcome of the disease.[18,21-23] The presence of
c-KIT mutations of the MCs in the skin confirms the diagnosis of CM,
but this is not a diagnostic criterion for SM nor a predictor of the
evolution of the disease.[2,24,25] On the other hand, the presence of
KIT 816V mutation in the peripheral blood (PB) of children with CM
should suggest performing a bone marrow biopsy, which is useful for
identifying children at risk of developing SM.[18,26,27]
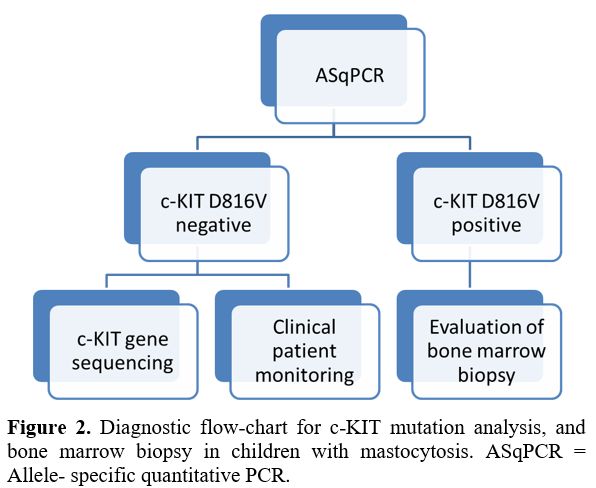 |
Figure
2. Diagnostic flow-chart for c-KIT mutation analysis, and bone marrow
biopsy in children with mastocytosis. ASqPCR = Allele- specific
quantitative PCR. |
Serum
tryptase levels (normal values <11.5 ng/ml), which include values of
protriptase, a molecule constitutively secreted by unstimulated MCs,
and mature tryptase, stored as secretory granules in cells, are
considered markers of MC activity.[28] In the pediatric age, increased
serum tryptase levels are more likely to indicate the presence of
active MC in the skin.[14] In a recent study, children with DCM showed
higher basal serum tryptase levels than those with mastocytoma and
MPCM.[29] It emerged from a previous study that children with MPCM with
smaller lesions had higher basal tryptase levels and presented a worse
outcome than those with larger ones.[30] High levels of basal serum
tryptase, combined with a skin lesion extension, in children with
mastocytosis were correlated with the possibility of developing very
serious symptoms due to the MC activation.[31] In the same study, the
tryptase cut-offs useful for better managing children with mastocytosis
were identified, as follows: a) 6.6 ng/ml: to start a daily
anti-mediator therapy; b) 15.5 ng/ml: to hospitalize the patient; c)
30.8 ng/ml: to admit the child to an intensive care unit. In case of
massive skin involvement and baseline serum tryptase values of 16
ng/ml, the initiation of intensive anti-mediator therapy is recommended
to reduce anaphylactic risk.[31] Tryptase levels were a good predictor
of pediatric anaphylactic events in a retrospective study including 102
children with CM.[32] In children with mastocytosis, over time, there
is a progressive decrease of tryptase levels, and it has been
speculated that this is probably due to pubertal hormones or
physiological reduced secretory activity by MCs during puberty.
Another
enzyme released from MCs is histamine (normal plasma levels in
children: 0.3–1.0 ng/mL). An increase in histamine levels has been
reported in children with DCM; however, an absolute correlation has not
been found between histamine levels and MC load in skin lesions.[33]
Serum histamine levels in DCM patients are higher during the first two
months of life but tend to decrease towards ages 9–12 months.[34] In
another study, children with mastocytosis and high histamine levels
showed more severe bone involvement and increased basal gastric acid
concentration.[34]
Some studies were carried out on the role of
some cytokines in pediatric mastocytosis. For example, increased levels
of Interleukin-31 (IL-31) were associated with the presence of
pruritus, and IL-6 was identified as a marker of mastocytosis
severity.[35]
Diagnostic Assessments
Pediatric
mastocytosis is generally limited to the skin without signs or symptoms
of SM or other hematologic diseases. In clinical practice, the
diagnosis of CM in children is based on the morphology of the skin
lesions and the Darier's sign, considered as major criteria. An
important tool to define CM subtypes is to perform a detailed
evaluation of the cutaneous lesion features, including number and type,
mono or polymorphic appearance, color, size, localization, and overall
skin aspect (Table 3). Among
laboratory tests, the baseline serum tryptase levels are useful to
define the disease extension in children with CM, and they represent an
indicator risk of anaphylaxis and other severe allergic reactions in
this category of patients. It is important to consider that increased
serum tryptase may occur in other diseases, such as hereditary
alphatriptasemia, chronic eosinophilic leukemia, and nephropathies.
High tryptase levels without organomegaly and/or abnormal cell count
are not indicative of performing a BM evaluation.
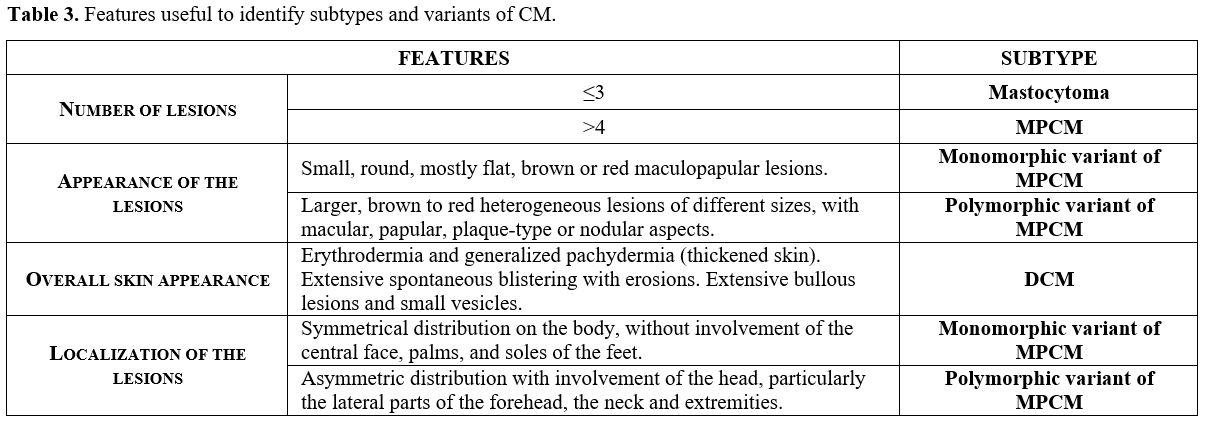 |
Table 3. Features useful to identify subtypes and variants of CM. |
A
complete diagnostic assessment of children with CM, above all for those
with severe symptoms or extensive disease, includes a careful clinical
examination, complete blood cell counts, hepatic functions, and an
abdominal ultrasound to evaluate organomegaly. Allele-specific
quantitative PCR (ASqPCR) for KIT D816V mutation analysis in PB has
been recently added to a diagnostic algorithm for pediatric
mastocytosis if organomegaly, elevated tryptase, or severe MC
mediator-related symptoms are present.[18,37] KIT D816V mutation in PB
identifies the subgroup of children who need a more in-depth follow-up,
as they are at risk of SM.
Gastroscopy and colonoscopy with
biopsies are indicated when gastrointestinal symptoms are present. Bone
marrow biopsy is rarely needed in children with CM except when very
high serum tryptase levels (>100 ng/mL) are combined with cytopenia
undue to other causes but associated with organomegaly and/or
gastrointestinal symptoms are present.[18,37,38] Once the diagnosis is
established, patients could be followed according to the abnormalities
detected, how recently suggested by some researchers who proposed a
specific workup algorithm for children with CM.[18,26,37] If systemic
MC mediator symptoms or extensive disease (widespread MPCM or DCM) are
present, a close follow-up including abdominal ultrasound, tryptase
levels, and KITD816V detection in PB are useful. The evaluation of KIT
D816V mutation in PB during follow-up is of great importance to
identify the subgroup of children at risk of SM who need BM
investigation. Patients with isolated organomegaly, and/or high
tryptase levels, and negative KIT D816V mutation should also be
evaluated for other mutations in KIT (Figure 2).
Management and Treatment
Management
of children with CM is aimed to prevent or control symptoms related to
MC degranulation. The triggers vary in patients and can include
mechanical and physical stimuli, infections, dietary, and
drugs.[11,18,31] Avoiding extreme physical exercise, sudden temperature
changes, and skin lesions friction are simple measures to prevent
exacerbation.[24,25] Regarding dietary, various histamine-containing
foods (e.g., cured meats, smoked fish, aged cheeses, fermented foods,
eggplant, spinach) and histamine-releasing foods (e.g., citrus fruits,
strawberries, pineapple, tomatoes, nuts, shellfish, chocolate,
additives) are considered as triggers. In a recent study, the rate of
vaccine reactions in children with mastocytosis is slightly
higher than that reported in the general population (3–6%). Therefore,
in children with CM, the authors recommend single-vaccine regimens and
postvaccination observation for 1–2 hours, whereas vaccines should be
administered on the recommended schedule in those with other cutaneous
forms of mastocytosis.[39]
Since medications commonly used in
general anesthesia can degranulate MCs, choosing anesthetic agents with
a low capacity to elicit MC degranulation and a regularly scheduled
antihistamine treatment are usually recommended before and after
procedures.[40]
Treatment
In
pediatric mastocytosis, the treatment approach is influenced by the
presence and severity of symptoms caused by the release of MC mediators
and by the forms of skin lesions. Considering that MPCM and mastocytoma
often regress spontaneously, the use of topical and systemic
medications should be avoided in these categories of otherwise
asymptomatic patients.[18,41]
The severity of symptoms due to MC degranulation has been graded in order to permit the choice of appropriate treatment (Table 4).
The Standard Consensus Guidelines have established a 4-grade scale for
adults with mastocytosis, and it is also useful for therapeutic choice
and for monitoring the risk of possible systemic evolution in
children.[10,42]
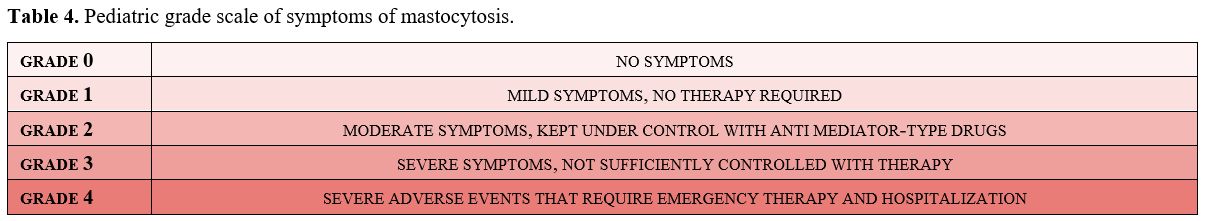 |
Table 4. Pediatric grade scale of symptoms of mastocytosis. |
Systemic treatment of symptoms.
Treatment of symptoms caused by the release of MC mediators is the main
goal in managing patients with CM, including children. The severity,
type, and frequency of symptoms must be considered to choose the
therapeutic approach. H1-antihistamines, continuously given orally, are
recommended as first-line treatment and can be used on-demand in
children with less frequent symptoms. The second generation,
non-sedating H1-antihistamines, are preferred for patients with
frequent and/or problematic manifestations. In case of persistence of
the symptoms, it is advisable to add an H2 antihistamine or
anti-leukotriene (Montelukast).[43] H2-antagonists (e.g., ranitidine),
or proton pump inhibitors (e.g., omeprazole, pantoprazole,
lansoprazole), are indicated in case of gastrointestinal symptoms as
cramps and diarrhea.
The oral use of sodium cromoglycate to
control gastrointestinal manifestations is currently controversial
because comprehensive data on its mechanism of action is lacking.
The
use of systemic corticosteroids, limited by numerous side effects, is
indicated as a short course in children with extremely severe
mediator-related symptoms and extensive and persistent
blistering.[18,41,44,45]
Treatment of anaphylaxis.
The risk of anaphylaxis in children with mastocytosis is 5-10%.
Therefore, preventing or eliminating triggers for anaphylaxis is the
main goal. The exact indications of self-injectable epinephrine have
been the subject of debate; however, its prescription is recommended in
children with extensive skin mastocytosis, blistering, previous
episodes of anaphylaxis, and/or elevated serum tryptase levels. The
dose of epinephrine should be prescribed according to children's body
weight: 0.1 mg, 0.15 mg, 0.3 mg to a child weighing 7.5–15 kg, 15–30
kg, >35 kg, respectively. If necessary, epinephrine could also be
used for infants at a dose of 0.15 mg or 0.1 mg to a child weighing
<15 kg or <7.5 kg, respectively.[46-48]
Topical treatment.
Topical corticosteroids have always been by far the most widely used,
especially in patients with frequent blistering. However, their
long-term use is contraindicated due to the side effects on the skin
(atrophy) and adrenocortical suppression.[49] Instead of
corticosteroids, local application of pimecrolimus is recommended in
pediatric CM due to its excellent results and safety
profiles.[18,50,51] Pimecrolimus is a calcineurin inhibitor with
significant anti-inflammatory activity by blocking T-cell activation,
inhibiting inflammatory cytokine synthesis, and immunomodulatory
effects with a low systemic immunosuppressive potential. Disodium
cromoglycate at a 1% to 4% concentration in an aqueous solution or
mixed with a water-based emollient cream may decrease
itching.[49,52,53] The use of phototherapy or photochemotherapy is not
recommended in children due to the potential carcinogenic effect. In
cases with diffuse localization, Narrow-band UVB rays (NB-UVB), UVA1,
UVA rays with psoralen (PUVA) therapy can be employed. The use of
lasers is very limited. Radical surgical excision is the extreme option
in solitary mastocytoma with high mediator release and a high risk of
anaphylaxis.[18,41]
Innovative therapies.
Several target drugs have been tested in pediatric mastocytosis.
Omalizumab, a recombinant humanized monoclonal antibody that blocks the
binding of IgE to the FceRI receptor on the surface of MCs, showed a
rapid and long-term efficacy to control severe MC-related symptoms in
an adolescent with frequent episodes of anaphylaxis.[54] Among kinase
inhibitors (KI) with activity against MC carrying D816V and other KIT
mutations, midostaurin, a multi-kinase inhibitor, is indicated to treat
aggressive SM in patients with D816V or wild type KIT. In childhood
mastocytosis, midostaurin was successfully used in an infant with
indolent SM associated with severe blistering who previously failed
conventional treatments.[55]
Tyrosine kinase inhibitors (TKI),
imatinib and masatinib, have different therapeutic indications.
However, the FDA approved imatinib's use in patients with SM and KIT
mutations outside of the exon 17, in the regulatory site (extracellular
and juxtamembrane portions of the gene), or with unknown mutations.[49]
Masatinib, a new highly selective TKI, targets wild-type KIT, LYN, and
FYN kinases that play a crucial role in the pathogenesis of
mastocytosis and could be effective in refractory cases with mutations
on the regulatory site of c-KIT. In addition, miltefosine is a
promising modulator of lipid rafts for topical use.[41]
Closing Remarks
Pediatric
forms of mastocytosis generally have a cutaneous presentation and
regress spontaneously with age. Acquired KIT receptor mutations, less
frequent in children than in adults, do not seem to be the only factor
responsible for triggering MC neoplastic transformation and do not
influence the development of the different clinical forms. New
molecular investigation techniques, including the Next Generation
Sequencing (NGS) method, may help identify other specific gene
aberrations, potentially useful to define alternative therapeutic
targets in forms of mastocytosis that require treatment.
Acknowledgments
I
thank Gianluca Signoretta, a graduating student who performed the
literature review for his dissertation; Martina Rousseau MD, who
reviewed the dissertation and Simona Bianchi MD, who reviewed the
references.
References
- Valent P, Horny HP, Escribano L, Longley BJ, Li CY,
Schwartz LB, Marone G, Nuñez R, Akin C, Sotlar K, Sperr WR, K Wolff, R
D Brunning, R M Parwaresch, K F Austen, K Lennert, D D Metcalfe, J W
Vardiman, J M Bennett. Diagnostic criteria and classification of
mastocytosis: A con-sensus proposal. Leuk Res. 2001; 25:603-25. https://doi.org/10.1016/S0145-2126(01)00038-8
- Verzijl
A, Heide R, Oranje AP, van Schaik RHN. C-kit Asp-816-Val mutation
analysis in patients with mastocytosis. Dermatology. 2007; 214:15-20. https://doi.org/10.1159/000096907 PMid:17191042
- Arock
M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L,
Kristensen TK, Kluin-Nelemans HC, Hermine O, Dubreuil P, Sperr WR,
Hartmann K, Gotlib J, Cross NC, Haferlach T, Garcia-Montero A, Orfao A,
Schwaab J, Triggiani M, Horny HP, Metcalfe DD, Reiter A, Valent P. KIT
mutation analysis in Mast Cell Neoplasms: Recommendations of European
Competence Net-work on Mastocytosis. Leukemia. 2015;29:1223-32. https://doi.org/10.1038/leu.2015.24 PMid:25650093 PMCid:PMC4522520
- Meni
C, Bruneau J, Gerogin-Lavialle S, Le Sachè de Peufeilhoux L, Damaj G,
Hadj-Rabis S, Fraitag S, Dubreuil P, Hermine O, Bodemer C. Pediatric
mastocytosis: A systematic review of 1747 cases. Dr J Dermatolog. 2015;
172:642-51. https://doi.org/10.1111/bjd.13567 PMid:25662299
- Lange
M, Hartmann K, Carter MC, Siebenhaar F, Alvarez-Twose I, Torrado I,
Brockow K, Ren-ke J, Irga-Jaworska N, Plata-Nazar K, Ługowska-Umer H,
Czarny J, Belloni Fortina A, Caroppo F, Nowicki RJ, Nedoszytko B,
Niedoszytko M, Valent P.. Molecular background, clinical features and
management of pediatric mastocytosis: status 2021. Internat J Molec
Sciences. 2021; 22:2586. https://doi.org/10.3390/ijms22052586 PMid:33806685 PMCid:PMC7961542
- Valent
P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification
and novel emerging treatment concepts. Blood. 2017; 129:1420-1427. https://doi.org/10.1182/blood-2016-09-731893 PMid:28031180 PMCid:PMC5356454
- Hartmann
K, Valent P, Horny HP. Cutaneous mastocytosis. In World Health
Organization (WHO) Classification of Skin Tumours, 4th ed.; Elder,
D.E., Massi, D., Scolyer, R., Willemze, R., Eds.; International Agency
for Research on Cancer: Lyon, France, 2018; pp. 275-278.
- Mutter RD, Tannembaum M, Ultmann JE. Systemic mast cell disease. Ann Intern Med. 1963; 59:887-906. https://doi.org/10.7326/0003-4819-59-6-887 PMid:14082741
- Valent,
P.; Akin, C.; Escribano, L.; Födinger, M.; Hartmann, K.; Brockow, K.;
Castells, M.; Sperr, W.R.; Kluin-Nelemans, H.C.; Hamdy, N.A.; et al.
Standards and standardization in mastocy-tosis: Consensus statements on
diagnostics, treatment recommendations and response criteria. Eur. J.
Clin. Investig. 2007, 37, 435-453. https://doi.org/10.1111/j.1365-2362.2007.01807.x PMid:17537151
- Hartmann
K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I,
Matito A, Broesby-Olsen S, Siebenhaar F, Lange M, Niedoszytko M,
Castells M, Oude Elberink JNG, Bo-nadonna P, Zanotti R, Hornick JL,
Torrelo A, Grabbe J, Rabenhorst A, Nedoszytko B, Butterfield JH, Gotlib
J, Reiter A, Radia D, Hermine O, Sotlar K, George TI, Kristensen TK,
Kluin-Nelemans HC, Yavuz S, Hägglund H, Sperr WR, Schwartz LB,
Triggiani M, Maurer M, Nilsson G, Horny HP, Arock M, Orfao A, Metcalfe
DD, Akin C, Valent P. Cutaneous manifestations in patients with
mastocytosis: Consensus report of the European Competence Network on
Mastocytosis; the Ameri-can Academy of Allergy, Asthma &
Immunology; and the European Academy of Allergology and Clinical
Immunology. J Allergy Clin Immunol. 2016; 137:35-45. https://doi.org/10.1016/j.jaci.2015.08.034 PMid:26476479
- Klaiber N, Kumar S, Irani AM. Mastocytosis in Children. Curr Allergy Asthma Reports. 2017; 17:80. https://doi.org/10.1007/s11882-017-0748-4 PMid:28988349
- Lange
M, Niedoszytko M, Renke J, Nedoszytko B. Clincal aspects of pediatric
mastocytosis: A review of 101 cases. J Eur Acad Dermatol Venereol.
2013; 27:97-102. https://doi.org/10.1111/j.1468-3083.2011.04365.x PMid:22126331
- Wiechers
T, Rabenhorst A, Schick T, Preussner M, Förster A, Valent P, Horny P,
Sotlar K, Hartmann K. Large maculopapular cutaneous lesions are
associated with favorable outcome in childhood-onset mastocytosis.
Journal of Allergy and Clinical Immunology. 2015; 136:1581-90. https://doi.org/10.1016/j.jaci.2015.05.034 PMid:26152315
- Matito
A, Azaña JM, Torrelo A, Alvarez-Twose I. Cutaneous Mastocytosis in
Adults and Chil-dren: New Classification and Prognostic Factors.
Immunol Allergy Clin N Am. 2018; 38:351-63. https://doi.org/10.1016/j.iac.2018.04.001 PMid:30007456
- Lange
M, Niedoszytko M, Nedoszytko B, Łata J, Trzeciak M, Biernat W. Diffuse
cutaneous mastocytosis: Analysis of 10 cases and a brief review of the
literature. J Eur Acad Dermatol Vene-reol. 2012; 26:1565-71. https://doi.org/10.1111/j.1468-3083.2011.04350.x PMid:22092511
- Sadaf H. Hussain: Pediatric mastocytosis, Curr Opin Pediatr 2020, 32:531-538 https://doi.org/10.1097/MOP.0000000000000922 PMid:32692050
- Ertugrul
A, Bostanci I, Kaymak AO, Gurkan A, Ozmen S. Pediatric cutaneous
mastocytosis and c-KIT mutation screening. Allergy Asthma Proc. 2019;
40:123-128. https://doi.org/10.2500/aap.2019.40.4201 PMid:30819282
- Lange
M, Hartmann K, Carter MC, Siebenhaar F, Alvarez-Twose I, Torrado I,
Brockow K, Renke J, Irga-Jaworska N, Plata-Nazar K, Ługowska-Umer H,
Czarny J, Belloni Fortina A, Caroppo F, Nowicki RJ, Nedoszytko B,
Niedoszytko M, Valent P.Molecular background, clinical features and
management of pediatric mastocytosis: status 2021, International
Journal of Molecular Sciences, 2021; 22, 2586. https://doi.org/10.3390/ijms22052586 PMid:33806685 PMCid:PMC7961542
- Pardoning,
A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk
stratification and management. Am. J. Hematol. 2018; 94, 363-377. https://doi.org/10.1002/ajh.25292 PMid:30328142
- Bodemer,
C.; Hermine, O.; Palmérini, F.; Yang, Y.; Grandpeix-Guyodo, C.;
Leventhal, P.S.; Hadj-Rabia, S.; Nasca, L.; Georgin- Lavialle, S.;
Cohen-Akenine, A.; et al. Pediatric mastocytosis is a clonal disease
associated with D816V and other activating c-KIT mutations. J.
Investig. Dermatol. 2010; 130, 804-815. https://doi.org/10.1038/jid.2009.281 PMid:19865100
- Méni,
C.; Georgin-Lavialle, S.; Le Saché de Peufeilhoux, L.; Jais, J.P.;
Hadj-Rabia, S.; Bruneau, J.; Fraitag, S.; Hanssens, K.; Dubreuil, P.;
Hermine, O.; et al. Paediatric mastocytosis: Long-term follow-up of 53
patients with whole sequencing of KIT. A prospective study. Br. J.
Dermatol. 2018, 179, 925-932. https://doi.org/10.1111/bjd.16795 PMid:29787623
- Yang
Y, Létard S, Borge L, Chaix A, Hanssens K, Lopez S, Vita M, Finetti P,
Birnbaum D, Bertucci F, Sophie Gomez, Paulo de Sepulveda, Patrice
Dubreuil. Pediatric mastocytosis-associated KIT extracellular domain
mutations exhibit different functional and signaling properties
compared with KIT-phosphotransferase domain mutations. Blood. 2010;
116:1114-23. https://doi.org/10.1182/blood-2009-06-226027 PMid:20484085
- Li
Y, Li X, Liu X, Kang L, Liu X. Genotypic and phenotypic characteristics
of Chinese neo-nates with cutaneous mastocytosis: A case report and
literature review. J Int Med Res. 2020;48:300060520952621. https://doi.org/10.1177/0300060520952621 PMid:32883129 PMCid:PMC7479863
- Azan
JM, Torrelo A, Matito A. Update on mastocytosis (part 2): Categories,
Prognosis, and Treatment. Actas Dermo-Sifiliogr. 2016; 107:15-22. https://doi.org/10.1016/j.adengl.2015.11.002
- Castells
M, Butterfield J. Mast cell activation syndrome and mastocytosis:
Initial treatment op-tions and long-term management. J Allergy Clin
Immunol Pract. 2019; 7:1097-1106. https://doi.org/10.1016/j.jaip.2019.02.002 PMid:30961835
- Carter
MC, Bai Y, Ruiz-Esteves KN, Scott LM, Cantave D, Bolan H, Eisch R, Sun
X, Hahn J, Maric I, Metcalfe DD. Detection of KIT D816V in peripheral
blood of children with manifestations of cutaneous mastocytosis
suggests systemic disease. Br J Haematol. 2018; 183:775-82. https://doi.org/10.1111/bjh.15624 PMid:30488427
- Czarny
J, Zuk M, Zawrocki A, Plata-Nazar K, Biernat W, Niedoszytko M,
Ługowska-Umer H, Nedoszytko B, Wasag B, Nowicki RJ, Lange M. New
Approach to Paediatric Mastocytosis: Impli-cations of KIT D816V
Mutation Detection in Peripheral Blood. Acta Dermato-Venereol.
2020;100:adv00149. https://doi.org/10.2340/00015555-3504 PMid:32399575
- Álvarez-Twose,
I.; Jara-Acevedo, M.; Morgado, J.M.; García-Montero, A.; Sánchez-Muñoz,
L.; Teodósio, C.; Matito, A.; Mayado, A.; Caldas, C.; Mollejo, M.; et
al. Clinical, immunophenotypic, and molecular characteristics of
well-differentiated systemic mastocytosis. J. Allergy Clin. mmunol.
2016, 137, 168-178.e1. https://doi.org/10.1016/j.jaci.2015.05.008 PMid:26100086
- Seth
N, Tuano KT, Buheis M, Chan A, Chinen J. Serum tryptase levels in
pediatric mastocytosis and association with systemic symptoms. Ann
Allergy Asthma Immunol. 2020; 125:219-21. https://doi.org/10.1016/j.anai.2020.04.021 PMid:32371241
- Barnes
M, Van L, De Long L, Lawley LP. Severity of cutaneous findings predict
the presence of systemic symptoms in pediatric maculopapular cutaneous
mastocytosis. Pediatr Dermatol. 2014; 31:271-75. https://doi.org/10.1111/pde.12291 PMid:24612340
- Alvarez-Twose,
I.; Vañó-Galván, S.; Sánchez-Muñoz, L.; Morgado, J.M.; Matito, A.;
Torrelo, A.; Jaén, P.; Schwartz, L.B.; Orfao, A.; Escribano, L.
Increased serum baseline tryptase levels and extensive skin involvement
are predictors for the severity of mast cell activation episodes in
children with mastocytosis. Allergy 2012, 67, 813-818. https://doi.org/10.1111/j.1398-9995.2012.02812.x PMid:22458675 PMCid:PMC3349769
- Lange
M, Zawadzka A, Schrörs S, Słomka J, Ługowska-Umer H, Nedoszytko B,
Nowicki R. The role of serum tryptase in the diagnosis and monitoring
of pediatric mastocytosis: A single-center experience. AdvDermatol
Alergol. 2017; 34:306-12. https://doi.org/10.5114/ada.2017.69308 PMid:28951704 PMCid:PMC5560177
- Kettelhut, B.V.; Metcalfe, D.D. Mastocytosis. J. Invest. Dermatol. 1991, 96, 115S-118S. https://doi.org/10.1111/1523-1747.ep12468942 PMid:16799603
- Belhocine
W, Ibrahim Z, Grandné V, Buffat C, Robert P, Gras D, Cleach I, Bongrand
P, Carayon P, Vitte J. Total serum tryptase levels are higher in young
infants. Pediatr Allergy Immunol. 2011; 22(6):600-7. https://doi.org/10.1111/j.1399-3038.2011.01166.x PMid:21736626
- Brockow,
K.; Akin, C.; Huber, M.; Metcalfe, D.D. IL-6 levels predict disease
variant and extent of organ involvement in patients with mastocytosis.
Clin. Immunol. 2005; 115, 216-223. https://doi.org/10.1016/j.clim.2005.01.011 PMid:15885646
- Hartmann
K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients
with mastocy-tosis: consensus report of the European Competence Network
on Mastocytosis; the American Acad-emy of Allergy, Asthma &
Immunology; and the European Academy of Allergology and Clinical
Immunology. J Allergy Clin Immunol. 2016; 137:35-45. https://doi.org/10.1016/j.jaci.2015.08.034 PMid:26476479
- Hussain, S.H. Pediatric mastocytosis. Curr. Opin. Pediatr. 2020; 32, 531-538. https://doi.org/10.1097/MOP.0000000000000922 PMid:32692050
- Lange,
M.; Ługowska-Umer, H.; Niedoszytko, M.; Wasa˛g, B.; Limon, J.; Z˙
awrocki, A.; Ne-doszytko, B.; Sobjanek, M.; Plata-Nazar, K.; Nowicki,
R. Diagnosis of Mastocytosis in Children and Adults in Daily Clinical
Practice. Acta Dermato-Venereol. 2016; 96,292-297. https://doi.org/10.2340/00015555-2210 PMid:26270728
- Carter
MC, Metcalfe DD, Matito A, Escribano L, Butterfield JH, Schwartz LB,
Bonadonna P, Zanotti R, Triggiani M, Castells M, Brockow K. Adverse
reactions to drugs and biologics in patients with clonal mast cell
disorders: a group report of the Mast Cells Disorder Committee,
American Academy of Allergy and Immunology. J Allergy Clin Immunol.
2019; 143:880-93. https://doi.org/10.1016/j.jaci.2018.10.063 PMid:30528617
- Hermans
MAW, Arends NJT, Gerth van Wijk R, van Hagen PM, Kluin-Nelemans HC,
Oude Elberink HNG, Pasmans SGMA, van Daele PLA. Management around
invasive procedures in mas-tocytosis: an update. Ann Allergy Asthma
Immunol. 2017; 119:304-9. https://doi.org/10.1016/j.anai.2017.07.022 PMid:28866309
- Czarny,
J.; Lange, M.; Ługowska-Umer, H.; Nowicki, R.J. Cutaneous mastocytosis
treatment: Strategies, limitations, and perspectives. Adv. Dermatol.
Alergol. 2018; 6, 541-545. https://doi.org/10.5114/ada.2018.77605 PMid:30618520 PMCid:PMC6320483
- Torrello, A.; Alvarez-Twose, I.; Escribano, L. Childhood mastocytosis. Curr. Opin. Pediatr. 2012; 24, 480-486. https://doi.org/10.1097/MOP.0b013e328355b248 PMid:22790101
- Turner
PJ, Kemp AS, Rogers M, Mehr S. Refractory symptoms successfully treated
with leuko-triene inhibition in a child with systemic mastocytosis.
Pediatr Dermatol. 2012; 29:222-3. https://doi.org/10.1111/j.1525-1470.2011.01576.x PMid:22044360
- Broesby
Broesby-Olsen, S.; Dybedal, I.; Gülen, T.; Kristensen, T.K.; Møller,
M.B.; Ackermann, L.; Sääf, M.; Karlsson, M.A.; Agertoft, L.; Brixen,
K.; et al. Multidisciplinary Management of Mas-tocytosis: Nordic Expert
Group Consensus. Acta Dermato-Venereol. 2016; 96, 602-612. https://doi.org/10.2340/00015555-2325 PMid:26694951
- Siebenhaar,
F.; Akin, C.; Bindslev-Jensen, C.; Maurer, M.; Broesby-Olsen, S.
Treatment strate-gies in mastocytosis. Immunol. Allergy Clin. N. Am.
2014, 34; 433-447. https://doi.org/10.1016/j.iac.2014.01.012 PMid:24745685
- González
de Olano, D.; de la Hoz Caballer, B.; Núñez López, R.; Sánchez Muñoz,
L.; Cuevas Agustín, M.; Diéguez, M.C.; Alvarez Twose, I.; Castells,
M.C.; Escribano Mora, L. Prevalence of allergy and anaphylactic
symptoms in 210 adult and pediatric patients with mastocytosis in
Spain: A study of the Spanish network on mastocytosis (REMA). Clin.
Exp. Allergy 2007; 37, 1547-1555. https://doi.org/10.1111/j.1365-2222.2007.02804.x PMid:17883734
- Brockow,
K.; Jofer, C.; Behrendt, H.; Ring, J. Anaphylaxis in patients with
mastocytosis: A study on history, clinical features and risk factors in
120 patients. Allergy 2008; 63, 226-232. https://doi.org/10.1111/j.1398-9995.2007.01569.x PMid:18186813
- Matito,
A.; Carter, M. Cutaneous and systemic mastocytosis in children: A risk
factor for ana-phylaxis? Curr Allergy Asthma Rep. 2015; 15, 22. https://doi.org/10.1007/s11882-015-0525-1 PMid:26139333
- Klaiber,
N., Kumar, S., & Irani, A.-M. (2017). Mastocytosis in Children.
Current Allergy and Asthma Reports, 17(11). Doi
:10.1007/s11882-017-0748-4. https://doi.org/10.1007/s11882-017-0748-4 PMid:28988349
- Mashiah,
J.; Harel, A.; Bodemer, C.; Hadj-Rabia, S.; Goldberg, I.; Sprecher, E.;
Kutz, A. Topi-cal pimecrolimus for pediatric cutaneous mastocytosis.
Clin. Exp. Dermatol. 2018; 43, 559-565. https://doi.org/10.1111/ced.13391 PMid:29460435
- Correia,
O.; Duarte, A.; Quirino, P.; Azevedo, R.; Delgado, L. Cutaneous
mastocytosis: Two pediatric cases treated with topical pimecrolimus.
Dermatol. Online J. 2010; 16, 8. https://doi.org/10.5070/D30FK211ZH PMid:20492825
- Edwards,
A.M.; Capková, S. Oral and topical sodium cromoglicate in the treatment
of diffuse cutaneous mastocytosis in an infant. BMJ Case Rep. 2011;
2011, bcr0220113910. https://doi.org/10.1136/bcr.02.2011.3910 PMid:22693187 PMCid:PMC3128352
- Vieira
Dos Santos, R.; Magerl, M.; Martus, P.; Zuberbier, T.; Church, M.K.;
Escribano, L.; Maurer, M. Topical sodium cromoglicate relieves
allergen- and histamine-induced dermal pruritus. Br. J. Dermatol.
2010;162, 674-676. https://doi.org/10.1111/j.1365-2133.2009.09516.x PMid:19785618
- Matito,
A.; Blázquez-Goñi, C; Morgado, JM; Alvarez-Twose, I; Mollejo, M;
Sánchez-Muñoz, L; Escribano, L. Short-term omalizumab treatment in an
adolescent with cutaneous masto-cytosis. Ann Allergy Asthma Immunol.
2013; 111:425-6. https://doi.org/10.1016/j.anai.2013.08.014 PMid:24125156
- Liu
M, Kohn L, Roach G, et al. Treatment of systemic mastocytosis in an
infant with midostau-rin. J Allergy Clin Immunol Pract. 2019;
7(2929-31):e1. https://doi.org/10.1016/j.jaip.2019.05.032 PMid:31154031
[TOP]





