Federico Mercolini1 and Simone Cesaro2.
1 Pediatric Hematology and Oncology Unit, Department of Pediatrics, Bolzano Hospital, Italy.
2 Pediatric Hematology Oncology, Department of Mother and Child, Azienda Ospedaliera Universitaria Integrata Verona, Italy.
Correspondence to:
Federico Mercolini, M.D. Pediatric Hematology and Oncology Unit,
Department of Pediatrics, Bolzano Hospital, Italy. Tel: +39-0471909796.
E-mail:
federico.mercolini@sabes.it
Published: January 1, 2022
Received: October 10, 2021
Accepted: December 10, 2021
Mediterr J Hematol Infect Dis 2022, 14(1): e2022009 DOI
10.4084/MJHID.2022.009
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
SARS-CoV-2
pandemic affected fewer children and adolescents with lower morbidity
and mortality rates than those reported for adults. This review focused
on the clinical course, risk factors for severe COVID 19, mortality,
treatment options, and prevention measures in the pediatric and
adolescent setting with special attention to pediatric
oncohematological patients. SARS-CoV-2 infection was often asymptomatic
in these subgroups of patients, but 47 to 68% of them required
hospitalization, and 9-10% of those hospitalized needed intensive care
with a COVID 19 attributable mortality of about 4%. The multisystem
inflammatory syndrome associated with COVID 19 was less frequent than
that reported in the non-oncohematological pediatric population.
Noteworthy, the course of COVID 19 was more severe in low-middle income
countries. The key measures to prevent SARS-CoV-2 infection are
reducing patient exposure to the SARS-CoV-2 and vaccination, now
available for parents and caregivers and patients and siblings above 12
years of age. The treatment of COVID 19 in pediatric patients is mainly
based on supportive care with dexamethasone and heparin prophylaxis for
severely ill patients. Other measures, such as convalescent plasma,
remdesivir, and monoclonal antibodies, have been used in limited cases
or within experimental protocols. Further studies are needed regarding
the risks factors and outcomes of SARS-CoV-2 infection in pediatric
immunocompromised patients.
|
Introduction
Coronaviruses
(CoVs) are a family of enveloped positive-sense single-stranded RNA
viruses, which can infect humans, other mammals, or avian species.[1]
Severe acute respiratory syndrome coronavirus (SARS- CoV) and the
Middle East respiratory syndrome coronavirus (MERS- CoV) have been
described in the human species respectively in 2002 and 2012, causing a
respiratory illness with high mortality rates.[2] At the end of 2019, a
novel highly infective and pathogenic Coronavirus designated as severe
acute respiratory coronavirus 2 (SARS-CoV-2) was reported in the city
of Wuhan, China, causing an outbreak of unusual viral pneumonia and
rapidly spreading around the world.[3]
This new coronavirus
targets both upper and lower respiratory tract tissues, and an
efficient human-to-human transmission even before the onset of symptoms
has been observed.[4] It is mainly transmitted by droplets and aerosol
from symptomless and symptomatic infected subjects, with a median
incubation period of 5.7 days (range 2-14).[5]
Covid-19 in Adults and Adults with Cancer
The
spectrum of infection severity in symptomatic patients ranges from mild
disease (81%), severe disease (14%), critical disease (5%), to death
(2.3%).[6] On September 28, 2021, more than 200 million cases have been
reported worldwide, with more than 4 million 300 thousand deaths,[7]
but the numbers are increasing day by day. Since the pandemic
onset, age was documented as the major risk factor for
mortality.[8] In a recent systematic review and meta-analysis,[9] age
was confirmed as the most important risk factor for both severe
clinical course (Odds Ratio> 75 years of 1.93 (1.32-2.52)) and
mortality (Odds Ratio> 75 years 5.82 (1.86-9.79)). Other risk
factors were obesity and the presence of comorbidities, in particular
cardiovascular diseases, chronic pulmonary and chronic kidney diseases.
In the same study, adult patients with active cancer showed an
increased risk, with Odds ratios for the severe course and mortality
1.48 (1.26-1.69) and 2.15 (2.15-2.16), respectively.
Other reports
in the literature confirm increased risk of severe COVID-19 course in
adult cancer patients: a 3.61-fold higher risk of severe COVID-19 was
reported in cancer patients compared to patients without cancer;[10]
and among cancer patients, a 2.45-fold increased risk of death was
reported in COVID-19 adult patients compared to non-infected
adults.[11] In addition, 2-fold higher mortality due to COVID-19 has
been reported for patients with hematological malignancies compared to
the non-cancer population.[12,13] Moreover, the highest frequency of
severe COVID-19 events has been reported in patients with hematologic
cancer, lung cancer, or metastatic cancer (stage IV).[6]
Table 1 shows the most important risk factors for a severe course and mortality of SARS-CoV-2 infection.
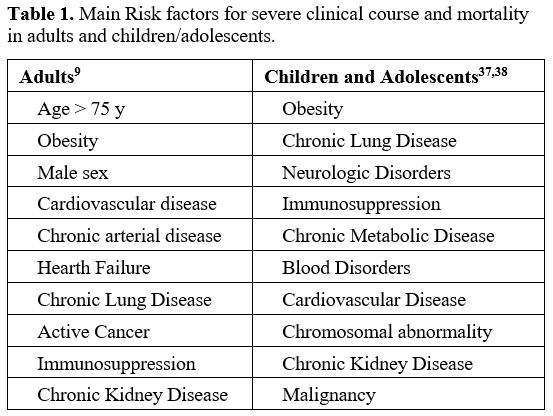 |
Table 1. Main Risk factors for severe clinical course and mortality in adults and children/adolescents. |
Late
sequelae related to COVID-19 infection, better known as post-acute
COVID-19 syndrome, are commonly reported in adults. The post-acute
COVID-19, defined as the persistence of symptoms and/or delayed or
long-term complications beyond 4 weeks from the onset of symptoms, is
characterized by pulmonary (dyspnea, decreased exercise capacity and
hypoxia, reduced diffusion capacity, restrictive pulmonary physiology,
and ground-glass opacities and fibrotic changes on imaging),
cardiovascular (palpitations, chest pain, myocardial fibrosis or
scarring, arrhythmias, tachycardia), hematological (thromboembolism),
renal (reduced eGFR), endocrine (new or worsening control of existing
diabetes mellitus, subacute thyroiditis and bone demineralization) and
neuropsychiatric (fatigue, myalgia, cephalea, dysautonomia, and
cognitive impairment, anxiety, depression, sleep disturbances)
involvement.[14]
In the largest series[15,16] at least one of these symptoms was reported in 30-87% of patients.
The
most frequently reported symptoms were: fatigue (35-64%), dyspnoea
(11-44%), sleep disturbances (24-26%), anxiety / depression (20-25%)
and chest pain (5-21%).
SARS-CoV-2 Infection and Covid-19 in Children and Adolescents
Due
to their developing immune system, children, compared to adults, are
more susceptible to infectious diseases. However, the susceptibility to
SARS-CoV-2 infection in children seems to be lower, with a low
incidence of severe COVID-19[17] and only rare fatality cases,
estimated between 2 and 5 cases per million for subjects below 18 years
of age.[18]
About 80-90% of infected children and adolescents
(80%)[19,20] present with symptoms, usually mild or moderate. Since the
first months after the start of the pandemic, children presented
clinically milder cases and a better prognosis than adults.[18] This
resulted in a lower hospitalization rate,[19] ranging from 2.5 to 4.1%.
Among hospitalized patients, 15% were admitted to the ICU.[21]
COVID-19
symptoms in children are similar to those in adults. The most frequent
are fever (46%), cough (37%), headache (15%), diarrhea (14%), sore
throat (13%), nausea/vomiting (10%), myalgia (10%), abdominal pain
(7%), rhinorrhea (7%) and shortness of breath (7%).[22,23]
Several
organ-specific involvements have been reported: heart failure,
myocarditis, pericarditis, arrhythmias, pulmonary embolism in the
cardiovascular system;[24-26] encephalopathy, stroke, Guillain-Barrè
syndrome, cerebral edema, status epilepticus, transient ischemic attack
in the nervous system;[27,28] urticarial, maculopapular, vesicular skin
rash, livedo reticularis, chilblain-like lesions as skin
manifestations.[29] The most fearful complication of COVID-19 infection
in pediatric age is the multisystem inflammatory syndrome in children
(MIS-C), described as early as April 2020. MIS-C is characterized by
fever, multisystem organ involvement, laboratory evidence of
inflammation, and severe course (Table 2).
Other features may include acute myocardial dysfunction, respiratory
failure, Kawasaki-like disease, and toxic shock syndrome.[30,31]
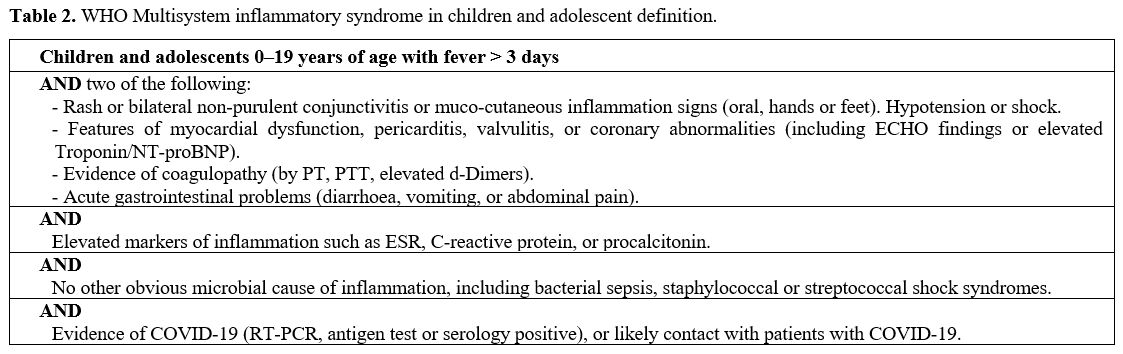 |
Table 2. WHO Multisystem inflammatory syndrome in children and adolescent definition.
|
MIS-C
appears to be relatively rare, occurring in <1% of confirmed
COVID-19 cases in children, corresponding to about 5-7 cases per
million people per month.[32,33] Among the hospitalized patients, the
MIS-C rate varies between 10 to 25%.[34] Currently, no long-term
follow-up studies that define with certainty the prognosis of patients
with MIS-C are reported. In a systematic review including 16 studies
and a total of 655 MIS-C patients, 10% of patients (68) required
critical care, and the mortality rate was 1.7% (11 deaths ).[35]
Treatment of MIS-C is mainly based on organ support, immunoglobulins, and steroids.[36-38]
Regarding
COVID-19 sequelae, these appear to be much less frequent than in
adults: in a cohort of 25 children, Denina et al.[39] no
COVID-19-related sequelae up to 4 months after the infection were
reported.
In a larger collection of cases,[40] out of 151 children
with COVID-19, whom 36% with asymptomatic course and 64% with mild,
moderate, or severe disease, 12 patients (8%) had post-acute COVID-19
symptoms. The most frequent documented symptoms were mild post-viral
cough (6 patients), fatigue (3 patients), or both post-viral cough and
fatigue (1 patient). Resolution of symptoms was seen in all cases in up
to 8 weeks.
Risk Factors for Severe Disease and Mortality.
Most pediatric patients affected by COVID-19 have a symptomless or
paucisymptomatic course that allows home management. In a review
including more than 7400 COVID-19 positive children, only 2% of cases
presented severe symptoms with dyspnea and hypoxemia, and critical
conditions in 0.7%. The reported fatality rate was 0.08% (6 patients
).[24] Similarly low (0.28%) is the mortality rate reported by Wang et
al.[41] in a meta-analysis that collects data from more than 11,000
COVID-19 positive children. However, some pediatric patients may
require hospitalization, particularly those with one or more
comorbidities. Kim et al.[42] reported the different clinical
characteristics on a total of 576 hospitalized patients, with a median
age of 8 years and equal male/female distribution: 222 patients (38.5%)
had one or more comorbidities, such as obesity (38%), chronic lung
disease (18%), prematurity defined as gestational age <37 weeks
(15.4%), neurologic disorder (14%), immunocompromised condition (5.4%).
In a similar European study,[43] out of 582 patients with a median age
of 5 years, 25% of hospitalized patients had one or more comorbidities.
The latter group of patients had a 3.7 greater relative risk of
admission to ICU. About one-third of hospitalized patients required
intensive care and about 5% mechanical ventilation.[42,44]
Regarding
mortality, children and young people have a lower risk than adults.[45]
However, several authors reported case series of deceased pediatric
patients. McCormick et al.[46] reported 112 deaths, with a median age
of 17 years (range 0-21 years): 63% were male, and 86% of patients
presented with at least one of the following conditions: obesity (42%),
asthma (29%), and developmental disorders (22%). Similarly, Bixler et
al.[47] reported 121 deaths in patients under 21 years old: only 30
(25%) were patients otherwise healthy, whereas 91 (75%) patients had at
least one comorbidity, and 54 (45%) had two or more comorbidities:
asthma (28%), obesity (27%), neurologic and developmental conditions
(22%), cardiovascular diseases (18%), cancer or immune system disorder
(14%) and diabetes mellitus (9.1%). In a systematic review that
analyzed 9335 children with COVID-19, 27% of patients had underlying
comorbidity, and among them, the most frequent was
immunosuppression.[48] Conversely, other authors reported a similarly
favorable course, compared to healthy children, in patients undergoing
immunosuppressive treatment for inflammatory bowel diseases, rheumatic
diseases, and kidney diseases.[49-51] Table 1 summarizes the major risk factors for severe COVID-19 in pediatric patients.
Covid-19 in Children and Adolescents with Cancer
While
cancer is an established risk factor for severe COVID-19 in adults, it
has thus far not been considered so in children. In fact, the main risk
factors for severe COVID-19 course in children are medical complexity,
genetic, neurologic or metabolic conditions, congenital heart disease,
obesity, diabetes, asthma or other chronic lung diseases, sickle cell
disease, and immunosuppression.[52,53] Unlike the adult
oncohematological population, data regarding COVID-19 infection in
pediatric oncohematological patients are relatively scarce. The
incidence of COVID-19 is higher in patients with cancer than in the
general population, both in adults[54] and children/adolescents.[55]
This incidence could be explained by the increased susceptibility of
immunosuppressed patients towards viral respiratory community
infections and by the need for frequent hospital visits with higher
exposure to contagion. From the beginning of the
pandemic, recommendations for the prevention of infection have
been released by the scientific community of pediatric oncology[56]
that are still valid today: physical and social distancing of children
on active treatment for cancer, patient screening before chemotherapy,
limitation of hospital access for parents/caregivers, creation of
dedicated COVID-19 free wards, implementation of telemedicine and the
use of adequate personal protective equipment for health personnel,
patients and parents or caregivers. However, the adherence to these
measures has been variable during the pandemic, depending on country or
region socio-economic level and readiness to implement the plans to
prevent the diffusion of SARS-CoV-2 infection.
Cases
of COVID-19 in children and adolescents with cancer have been reported
worldwide. In the systematic review by Meena et al.,[57] collecting
data from 33 studies (18 case reports and 15 case series),[55,58-89]
clinical and outcome of 226 children with cancer and COVID-19 were
described: 53% of the patients were affected by hematological
malignancies and 47% by solid tumors. The median age was seven years
with a male to female ratio of 1.7:1; 34 patients were in intensive
chemotherapy and 17 post-HSCT. Sixty-three patients were symptomless,
47 had mild-moderate and 20 severe infections. Interestingly, out of
169 patients with data regarding chemotherapy, 123 (72.8%) had a
treatment delay, and 10 had a regimen modification. In this review,
morbidity and mortality related to SARS-CoV2 infection and the risk of
severe COVID-19 was higher compared with the general pediatric
population. Indeed, 96 of 226 patients (47%) required hospitalization,
and 21 needed ICU admission. Fifteen patients (11.5% of hospitalized
patients) died due to COVID-19. A meta-analysis of 15 studies,
including pediatric patients with hematological malignancies and solid
tumors, showed that the overall survival rate was 99.4%, with no
statistically significant differences in the risk of hospitalization,
ICU admission, and need for ventilation between patients with
hematological conditions malignancies and solid tumors.[90]
Nicastro
et al.,[91] in a review on COVID-19 in immunosuppressed children,
observed that pediatric cancer patients have overall good COVID-19
outcomes, though still slightly worse than the general population.
In
a European cohort of 582 hospitalized pediatric patients,[43] 27% were
affected by malignancy and presented a relative risk of ICU admission
2.7 times higher than the entire group; on the contrary, 29 patients on
immunosuppressive treatment and 3 affected by immunodeficiency did not
show an increased risk of ICU admission.
The largest collection of
COVID-19 infection in the pediatric oncology field has been recently
published:[92] this study included data of 1319 patients under
the age of 19 from 131 institutions of 45 countries who completed the
30-day follow-up. Deaths attributable to COVID 19 infection were 3.8%
(50 out of 1319), more than ten times higher than the general pediatric
population.
An important risk factor associated with severe or
critical illness was low-income or lower-middle-income country status,
with a relative risk 5.8 times greater than high-income country status.
Other risk factors were an age between 15 and 18 years, lymphocytes
<300/mmc, neutrophils <500/mmc, comorbidities, and being on
intensive chemotherapy. Oncological treatment was modified globally in
55.8% of patients, and, among them, chemotherapy was suspended in 80%
and reduced in 13.1%. In addition, radiation therapy was delayed in
6.6%, whereas surgery was postponed in 6.7% of patients.
Currently,
given the small number of fatal cases in pediatric oncology, the risk
factors for mortality are not known. On the contrary, in the adult
hematological oncology field, worse overall survival was associated
with advanced age, an uncontrolled or progressive disease status, the
diagnosis of acute myeloid leukemia, aggressive non-Hodgkin's lymphoma,
indolent non-Hodgkin lymphoma or plasma cell neoplasm, and the presence
of COVID-19 in severe or critical form.[13]
According to the
European Society for Blood and Marrow Transplantation (EBMT) data, the
mortality in 382 patients with COVID-19 after stem cell
transplant was 25% with a 6-week overall survival rate of 77.9% 72.1%
for allogeneic and autologous recipients, respectively.[88] In this
series, only 3 of 32 pediatric patients (29 allogeneic transplants and
3 autologous transplants) died, all after allogeneic stem cell
transplant, the 6-week overall survival being 93.4%. In multivariate
analysis, the risk factors for lower survival were older age, ICU
admission, and the moderate/high immunodeficiency index, whereas a
better performance status was protective.
The comparison between
the clinical course in the general pediatric/adolescent population and
the pediatric/adolescent cancer patients is shown in Table 3.
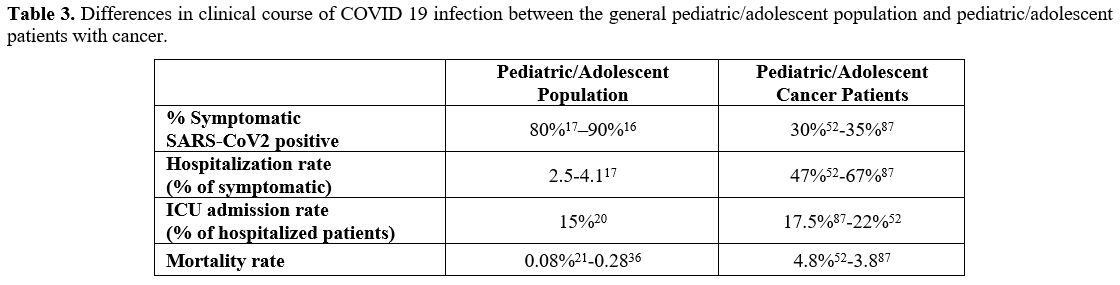 |
Table 3. Differences in
clinical course of COVID 19 infection between the general
pediatric/adolescent population and pediatric/adolescent patients with
cancer.
|
Treatment.
The treatment of pediatric cancer patients with COVID-19 is similar to
that of immunocompetent populations affected by COVID-19. Several
pediatric guidelines[48,90-92] stated that the cornerstone of treatment
is the supportive measures, such as the administration of fluids and
electrolytes, nutritional support, support of the respiratory function
with the administration of oxygen, or the use of non-invasive or
invasive ventilation systems, support of cardiac function with
inotropes, support of renal function, and antibiotic treatment in case
of bacterial superinfection.[96,97]
The underlying
immunosuppression of pediatric cancer patients can prolong the viral
phase of COVID-19 and reduce, delay or even nullify the inflammatory
phase of the disease.
Since the onset of the pandemic, several
drugs have been used in the treatment of pediatric cancer patients with
COVID-19:[55,61,63,66-68,73,74,76,81-83,85,87,88] the most used drug
was hydroxychloroquine, followed by steroids and oseltamivir. In
addition, the use of lopinavir/ritonavir, azithromycin, remdesivir,
tocilizumab, convalescent plasma, chloroquine, and IVIG has also been
reported in the literature.
The use of these drugs was based on
the protocols adopted for adults, but no treatment specific for the
pediatric age has been developed. Currently, some drugs initially used,
such as hydroxychloroquine/chloroquine (both in outpatients[98] and in
hospitalized patients),[99,100] lopinavir/ritonavir,[101-103] and
azithromycin)[104-107] are no longer recommended due to their
demonstrated ineffectiveness.
Instead, the following are the
currently used drugs for the treatment of COVID-19, concerning
the adult and pediatric literature.
Steroids.
Steroid therapy has shown conflicting results in adults hospitalized
due to SARS-CoV-2 infection.[108] In a systematic review and
meta-analysis, the use of systemic glucocorticoids was evaluated on a
total of 15.754 patients:[109] neither a reduction in mortality nor in
the duration of hospitalization and period of viral shedding was
demonstrated. Steroid therapy has not shown efficacy even in adult
oncology: Rivera et al.[110] reported a numerical (but not
statistically significant) increase in 30-day all-cause mortality in
109 patients treated with high-dose steroids compared to negative
controls.
However, the efficacy of dexamethasone has been
demonstrated in hospitalized patients receiving oxygen, noninvasive or
invasive mechanical ventilation, determining lower 28-day
mortality.[111] Unfortunately, the same benefit was not found in
patients not receiving respiratory support.
A Multidisciplinary
Guidance on the Use of Immunomodulatory Therapies for COVID-19 in
Pediatrics[112] published in December 2020 concluded that steroid
therapy is not recommended for mild/moderate disease course, while it
may be beneficial for severe or critical illnesses. Therefore the risk
and benefits should be evaluated on a case-by-case basis.
Currently,
there are no randomized trials that demonstrate the efficacy of steroid
therapy in patients with cancer or immunodeficiency, neither in the
adult nor in the pediatric population.
Remdesivir.
Remdesivir showed mixed results in the adult population: while in the
WHO solidarity trial[103] on 11,330 patients, of which 2750 treated
with Remdesivir, no improvements of mortality, of the need for invasive
ventilation and duration of hospitalization was found in patients
treated with remdesivir, Beigel et al.[113] reported a
significant reduction in mortality and days to recovery in a population
of 1062 patients (of which 80 with cancer) treated versus placebo; in
the analysis of subgroups based on respiratory support, efficacy was
demonstrated in patients not receiving oxygen or receiving oxygen, but
not in patients receiving high-flow oxygen, non-invasive ventilation,
or invasive ventilation. In a study conducted on 2186 adults with
cancer, including 470 with hematological malignancy,[110] 124 were
treated with remdesivir alone: its use was associated with a reduction
in 30-day all-cause mortality in comparison with positive controls
(Odds Ratio 0.41), however without statistical significance.
In
the multicenter Interim Guidance on Use of Antivirals for Children With
Coronavirus,[114] experts suggested as a first choice the use of
remdesivir for children with severe illness, defined as a supplemental
oxygen requirement without the need for non-invasive or invasive
mechanical ventilation or extracorporeal membrane oxygenation
(ECMO). The evidence of good tolerance[113,114] and the efficacy
data deriving from the adult population suggest using remdesivir
instead of other antivirals. and. However, no efficacy and safety data
are currently available in pediatric cancer patients.
Monoclonal Antibodies.
The use of anti-Spike monoclonal antibodies to prevent severe COVID-19
has shown promising results in the adult population: several
studies[115-117] demonstrated a reduction of hospitalizations and
deaths among patients treated with banlanivimab + etesevimab and
casirivimab + imdevivab.
The best results were obtained with an
early administration of antibodies, and, therefore, their indication is
mainly in the early stages of the disease.[118,119] In 38 adult
patients with active cancer,[120] the use of neutralizing monoclonal
antibodies led to a lower hospitalization and mortality rate than those
previously described among active cancer patients.
Based on the
evidence available in December 2020, a panel of experts[121] expressed
an opinion against the routine use of monoclonal antibody therapy in
pediatric patients, including those at high risk of severe evolution.
Convalescent Plasma.
Several randomized trials demonstrated that convalescent plasma has no
significant impact on the main outcome indicators of COVID-19 in adult
patients.[122,123] However, the efficacy could be linked to the
anti-SARS-CoV-2 antibody titer: Joyner et al.[124] demonstrated a
reduction in the risk of death in hospitalized patients who were not
receiving mechanical ventilation by administration of convalescent
plasma with higher anti-SARS-CoV-2 IgG antibody levels, compared to
those treated with plasma with lower antibody levels.
Other
factors that could influence the effectiveness of this treatment are
the timing of administration and the severity of the infection: Libster
et al.[125] showed that early administration of high-titer convalescent
plasma against SARS-CoV-2 to mildly ill infected older adults reduced
disease progression.
Convalescent plasma with high neutralizing
antibody titers could find an indication in B-cell depleted
patients,[126] although there are currently no randomized studies that
can confirm benefits in this cohort.
In the adult cancer
population, convalescent plasma has shown efficacy in treating
COVID-19. In a retrospective study[127] conducted on 966 adult patients
with hematologic malignancy, hospitalized for COVID-19 infection, the
outcome of patients treated with plasma (n = 143) compared to those who
did not receive it (n = 823) was evaluated. In patients treated with
plasma, a favorable Hazard Ratio of 0.6 in 30-day all-cause mortality,
0.4 for ICU admission, and 0.32 for mechanical ventilation was found.
However, the efficacy of plasma in the adult cancer population remains unclear in the absence of randomized trials.[128]
Convalescent plasma was generally well tolerated in the adult population,[129] and no specific adverse reactions were reported.
In
a literature review,[130] in pediatrics, 8 case report studies with a
total of 14 children treated with plasma (age range 9 weeks-18 years)
were described: no adverse events related to plasma administration were
documented. All patients had a positive outcome, and 7 of the 8 studies
concluded that convalescent plasma could be a useful therapeutic
option. However, given the small number and heterogeneity of the
sample, more studies are needed.
Tocilizumab.
Although tocilizumab (anti-IL-6R monoclonal antibody) has been
emergently authorized in the USA in hospitalized patients over 2 years
of age on steroid therapy and in need of oxygen, mechanical
ventilation, or ECMO, there are currently no data on efficacy and
safety in the pediatric population.
Tocilizumab showed variable
efficacy in various retrospective and case-control studies in the adult
population.[131,132] Furthermore, being associated with an increase in
the rate of superinfection,[133] the risk/benefit ratio of its use is
to assess carefully in oncology[134] patients.
Several case
reports and case series[66,67,73,83] have shown that treatment with
Tocilizumab is feasible and well-tolerated in pediatric cancer
patients, but large studies are lacking.
Anticoagulation.
The risk of thrombotic complications in children with COVID19 is not
yet well defined, and thromboprophylaxis in these patients is limited
to cases at higher risk of thrombosis.
There are two main
pediatric consensus-based recommendations[135,136] suggesting the
administration of low-dose low molecular heparin subcutaneously twice
daily, targeting a 4-hour post-dose anti-Xa activity level of 0.2 to
< 0.5 U/ml, as prophylaxis in children hospitalized for COVID 19.
The indication to prophylaxis with heparin is the presence of an
elevated D-dimer value (> 5 times above the upper limit) or of risk
factors for hospital-related deep vein thrombosis (i.e., presence of a
central venous catheter, mechanical ventilation, prolonged length of
stay, complete immobility, obesity, active malignancy, cystic fibrosis
exacerbation, sickle cell disease vaso-occlusive crisis, congenital or
acquired cardiac disease with venous stasis or impaired venous return,
previous history of venous thromboembolism (VTE), first-degree family
history of VTE before 40 years of age, known thrombophilia,
post-pubertal age, estrogen-containing oral contraceptive pill therapy,
status-post splenectomy for underlying hemoglobinopathy).
Vaccination.
COVID-19 infection in the pediatric setting has other consequences than
health, such as social isolation and interruption of education.
Furthermore, the pediatric patient could act as a vector of the disease
within society and then pose risks for the adult population and certain
subsets of pediatric patients at risk of developing severe COVID
19.[137]
Therefore, vaccination against COVID 19 should be considered in the entire pediatric population.
To
date, safety, immunogenicity, and efficacy studies have only been
conducted in the population over 12 years of age. Frenck et al.[138]
reported the experience of administering the BNT162b2 Covid-19 vaccine
in the population aged 12 to 15 years in a multinational,
placebo-controlled, observer-blinded trial: 2600 adolescents were
enrolled, of whom half received the vaccine and half received placebo.
The vaccine showed a favorable safety and side-effect profile,
presenting mostly mild to moderate reactogenicity in the absence of
serious vaccine-related adverse events. The vaccine efficacy was 100%.
Similarly,
the mRNA-1273 vaccine showed a good safety profile and a serological
response in the population between 12 and 17 years, comparable to that
of young adults, with efficacy in preventing COVID 19.[139]
Walter
et al. reported recently the results of phase 2-3 study where 2268
children of 5-11 years of age were randomized (ratio 2:1) to receive 2
doses of 10 mg of BNT162b2 vaccine, 21 days apart, versus placebo.
After a median follow-up of 2.3 months from the second dose, the
vaccine efficacy against documented COVID-19 was 90.7%; moreover, no
vaccine-related serious adverse events were noted, and the serum
antibody level of neutralizing antibodies against SARS-CoV-2 was
comparable to that observed in a control group of subjects of 16-25
years vaccinated with the adult dose of 30 mg BNT162b2 vaccine.[140]
Although
mRNA vaccines' safety and tolerability profile is favorable,
myocarditis has been reported as a rare complication, especially in
adolescent or young adult males. A recent Israeli study[141] showed
that the incidence of myocarditis, albeit low, was increased in
16-19-year-old males who received the BNT162b2 mRNA vaccine (8.62
events / 100,000). The relative risk of developing myocarditis was 5.34
for the entire population and up to 13.6 in males between 16 and 19
years. It should be noted that after SARS-CoV-2 infection, the
myocarditis complication is greater (11.54 events/100,000). The
clinical presentation of myocarditis after vaccination was generally
mild with response to conservative or symptomatic treatment.
Data
on COVID 19 vaccines in patients with malignancy are limited since
these patients were largely excluded from the phase III vaccine trials.
However, the experience on 151 adult patients with cancer, of which 95
with solid tumors and 56 with hematological cancer, has recently been
reported.[142] The vaccine was well-tolerated, and no vaccine-related
deaths were reported. The serological response (IgG positivity) was
found after the first dose in 38% of patients with solid tumors, 18% of
hematological malignancies, 94% of healthy controls, while after the
second dose in 95%, 60%, and 100%, respectively.
Several reports
have been published in reference to specific cancers in the adult
population: after the second vaccine dose the antibody response was
45-65% for chronic lymphocytic leukemias,[143,144] 40-70% in
Non-Hodgkin lymphomas,[144,145] 94-100% in Hodgkin lymphomas,[144,146]
80-90% in acute lymphatic or myeloid leukemias,[144,146] 70-85% in
post-transplant patients.[147,148] Several observations showed
that, in the patients who have received anti-CD20 monoclonal
antibody therapy, B-cell directed immunotherapy or patients with
profound hypogammaglobulinemia or marked lymphopenia, the response to
vaccination is very poor.[149,150]
Revon-Liviere et al.
[151] reported the single-center vaccination experience of 10
patients between 16 and 21 years under treatment for solid tumors or
within 6 months after treatment conclusion. Vaccination was well
tolerated in all patients who presented exclusively mild local
reactivity symptoms; 7 out of 10 patients showed positive serology
after the first vaccine and 9 one month after the second. No patient
developed COVID 19 disease.
Vaccination has been shown to be safe
in adolescents and young adults (12-29 years) with a previous
PEG-asparaginase allergy, showing no vaccine reaction.[152]
In
Europe, the indication issued by National Authorities is to recommend
the full vaccination with a vaccine approved by the European Medical
Agency (EMA) in all people above 12 years of age, including frail
patients due to the presence of comorbidities, immunosuppression,
cancer treatment, chronic disease, and organ or stem cell
transplant.[151]
Considering that vaccination is not yet available
for patients under the age of 12, full vaccination of all eligible
family members of cancer patients is of paramount importance because it
reduces the viral transmission to these patients at high risk of severe
COVID 19 course.[153]
Conclusions
Pediatric
patients have a reduced incidence of severe COVID 19 compared to the
adult population. However, a subset of pediatric patients is at greater
risk for a severe course. This subset includes pediatric and adolescent
patients with active cancer and immunosuppression.
In pediatric
cancer patients, severity, morbidity, and mortality are higher than the
general pediatric population, particularly in low-middle income
countries.
The clinical course may be asymptomatic; however,
47-68% of patients require hospitalization and 9-10% admission to
intensive care. Mortality attributable to COVID 19 infection is about
4%.
A key measure for these patients is the prevention of COVID 19
infection by reducing the risk of exposure and vaccinating contacts.
Data
regarding the efficacy and safety of vaccination in adolescent cancer
patients are still very limited; however, based on data collected on
studies in adults, the safety profile and tolerability are reassuring.
In
the case of COVID 19 infection, the cornerstone of treatment is
supportive care. However, transferring the evidence gained from adults,
some medical treatments, such as the use of dexamethasone for severely
ill patients, the early adoption of convalescent plasma, the use of
remdesivir to reduce the viral shedding, and the anticoagulant
prophylaxis are reasonable in hospitalized patients. The use of
monoclonal antibodies must be assessed on the basis of the patient
clinical situation or within experimental protocols. Further studies
are needed to elucidate better the risk factors, treatment, and
outcomes of COVID 19 in pediatric cancer patients.
References
- V'kovski P, Kratzel A, Steiner S, Stalder H, Thiel
V. Coronavirus biology and replication: implications for SARS-CoV-2.
Nat Rev Microbiol 2021;19:155–170.
https://doi.org/10.1038/s41579-020-00468-6
- Hu
B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19.
Nat Rev Microbiol 2021;19:141–154.
https://doi.org/10.1038/s41579-020-00459-7
- Guan
W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L,
Hui DSC, Du B, Li L-J, Zeng G, Yuen K-Y, Chen R-C, Tang C-L, Wang T,
Chen P-Y, Xiang J, Li S-Y, Wang J-L, Liang Z-J, Peng Y-X, Wei L, Liu Y,
Hu Y-H, Peng P, Wang J-M, Liu J-Y, Chen Z, Li G, Zheng Z-J, Qiu S-Q,
Luo J, Ye C-J, Zhu S-Y, Zhong N-S, China Medical Treatment Expert Group
for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in
China. N Engl J Med 2020;382:1708–1720.
https://doi.org/10.1056/NEJMoa2002032
- Wölfel
R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D,
Jo-nes TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S,
Schneider J, Ehmann R, Zwirgl-maier K, Drosten C, Wendtner C.
Virological assessment of hospitalized patients with COVID-2019. Nature
2020;581:465–469. https://doi.org/10.1038/s41586-020-2196-x
- Salzberger
B, Buder F, Lampl B, Ehrenstein B, Hitzenbichler F, Holzmann T, Schmidt
B, Hanses F. Epidemiology of SARS-CoV-2. Infection 2021;49:233–239.
https://doi.org/10.1007/s15010-020-01531-3
- Dai
M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q,
Xiong Y, Xiong H, Wang C, Chen C, Xiong F, Zhang Y, Peng Y, Ge S, Zhen
B, Yu T, Wang L, Wang H, Liu Y, Chen Y, Mei J, Gao X, Li Z, Gan L, He
C, Li Z, Shi Y, Qi Y, Yang J, Tenen DG, Chai L, Mucci LA,
Santillana M, Cai H. Patients with Cancer Appear More Vulnerable to
SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer
Discov 2020;10:783–791. https://doi.org/10.1158/2159-8290.CD-20-0422
- Weekly
epidemiological update on COVID-19 - September 28 2021.
https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---28-september-2021.
Accessed October 2 2021
- Onder G, Rezza G,
Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying
in Relation to COVID-19 in Italy. JAMA 2020;323:1775–1776.
https://doi.org/10.1001/jama.2020.4683
- Booth
A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, Labrique A, Mohan
D.Population risk factors for severe disease and mortality in COVID-19:
A global systematic review and meta-analysis. PloS One
2021;16:e0247461. https://doi.org/10.1371/journal.pone.0247461
- Tian
J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, Cai Y, Lu Z, Wang J, Wang Y,
Liu S, Cheng B, Wang J, Zhang M, Wang L, Niu S, Yao Z, Deng X, Zhou F,
Wei W, Li Q, Chen X, Chen W, Yang Q, Wu S, Fan J, Shu B, Hu Z, Wang S,
Yang X-P, Liu W, Miao X, Wang Z. Clinical char-acteristics and risk
factors associated with COVID-19 disease severity in patients with
cancer in Wuhan, China: a multicentre, retrospective, cohort study.
Lancet Oncol 2020;21:893–903.
https://doi.org/10.1016/S1470-2045(20)30309-0
- Mehta
P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across
Speci-ality Collaboration, UK. COVID-19: consider cytokine storm
syndromes and immunosuppression. Lancet Lond Engl 2020;395:1033–1034.
https://doi.org/10.1016/S0140-6736(20)30628-0
- El-Sharkawi
D, Iyengar S. Haematological cancers and the risk of severe COVID-19:
Explo-ration and critical evaluation of the evidence to date. Br J
Haematol 2020;190:336–345. https://doi.org/10.1111/bjh.16956
- Passamonti
F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, Angelucci E,
Krampera M, Cairoli R, Della Porta MG, Fracchiolla N, Ladetto M,
Gambacorti Passerini C, Salvini M, Marchetti M, Lemoli R, Molteni A,
Busca A, Cuneo A, Romano A, Giuliani N, Galimberti S, Corso A, Morot-ti
A, Falini B, Billio A, Gherlinzoni F, Visani G, Tisi MC, Tafuri A, Tosi
P, Lanza F, Massaia M, Turrini M, Ferrara F, Gurrieri C, Vallisa D,
Martelli M, Derenzini E, Guarini A, Conconi A, Cuccaro A, Cudillo L,
Russo D, Ciambelli F, Scattolin AM, Luppi M, Selleri C, Ortu La Barbera
E, Ferran-dina C, Di Renzo N, Olivieri A, Bocchia M, Gentile M,
Marchesi F, Musto P, Federici AB, Candoni A, Venditti A, Fava C, Pinto
A, Galieni P, Rigacci L, Armiento D, Pane F, Oberti M, Zappasodi P,
Visco C, Franchi M, Grossi PA, Bertù L, Corrao G, Pagano L, Corradini
P, ITA-HEMA-COV In-vestigators. Clinical characteristics and risk
factors associated with COVID-19 severity in patients with
haematological malignancies in Italy: a retrospective, multicentre,
cohort study. Lancet Haematol 2020;7:e737–e745.
https://doi.org/10.1016/S2352-3026(20)30251-9
- Nalbandian
A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR,
Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D,
Der-Nigoghossian C, Li-yanage-Don N, Rosner GF, Bernstein EJ, Mohan S,
Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D,
Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV,
Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19
syndrome. Nat Med. 2021 Apr;27(4):601-615.
https://doi.org/10.1038/s41591-021-01283-z. Epub 2021 March 22. PMID:
33753937.
- Carfì A, Bernabei R, Landi F;
Gemelli Against COVID-19 Post-Acute Care Study Group. Per-sistent
Symptoms in Patients After Acute COVID-19. JAMA. 2020 August
11;324(6):603-605. https://doi.org/10.1001/jama.2020.12603. PMID:
32644129; PMCID: PMC7349096.
- Huang C,
Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo
J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie
W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang
J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients
discharged from hospital: a cohort study. Lancet. 2021 January
16;397(10270):220-232. https://doi.org/10.1016/S0140-6736(20)32656-8.
Epub 2021 January 8. PMID: 33428867; PMCID: PMC7833295.
- Lee
P-I, Hu Y-L, Chen P-Y, Huang Y-C, Hsueh P-R. Are children less
susceptible to COVID-19? J Microbiol Immunol Infect Wei Mian Yu Gan Ran
Za Zhi 2020;53:371–372. https://doi.org/10.1016/j.jmii.2020.02.011
- Ledford H .Deaths from COVID "incredibly rare" among children. Nature 2021;595:639. https://doi.org/10.1038/d41586-021-01897-w
- Dong
Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19
Among Children in China. Pediatrics 2020;145:e20200702.
https://doi.org/10.1542/peds.2020-0702
- Cui
X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, Zhang J, Dong C, Na R, Zheng
L, Li W, Liu Z, Ma J, Wang J, He S, Xu Y, Si P, Shen Y, Cai C. A
systematic review and meta-analysis of children with coronavirus
disease 2019 (COVID-19). J Med Virol 2021;93:1057–1069.
https://doi.org/10.1002/jmv.26398
- Ludvigsson
JF. Systematic review of COVID-19 in children shows milder cases and a
better prognosis than adults. Acta Paediatr Oslo Nor 1992
2020;109:1088–1095. https://doi.org/10.1111/apa.15270
- Stokes
EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie
Y, Fullerton KE. Coronavirus Disease 2019 Case Surveillance - United
States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep
2020;69:759–765. https://doi.org/10.15585/mmwr.mm6924e2
- Swann
OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, Seth S,
Egan C, Hardwick HE, Halpin S, Girvan M, Donohue C, Pritchard M, Patel
LB, Ladhani S, Sigfrid L, Sinha IP, Olliaro PL, Nguyen-Van-Tam JS,
Horby PW, Merson L, Carson G, Dunning J, Openshaw PJM, Baillie JK,
Harrison EM, Docherty AB, Semple MG, ISARIC4C Investigators. Clinical
characteris-tics of children and young people admitted to hospital with
covid-19 in United Kingdom: prospec-tive multicentre observational
cohort study. BMJ 2020; 370:m3249. https://doi.org/10.1136/bmj.m3249
- Liguoro
I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, Vidal E,
Cogo P. Cor-rection to: SARS-COV-2 infection in children and newborns:
a systematic review. Eur J Pediatr 2021;180:2343.
https://doi.org/10.1007/s00431-021-03961-z
- Samuel
S, Friedman RA, Sharma C, Ganigara M, Mitchell E, Schleien C, Blaufox
AD. Inci-dence of arrhythmias and electrocardiographic abnormalities in
symptomatic pediatric patients with PCR-positive SARS-CoV-2 infection,
including drug-induced changes in the corrected QT interval. Heart
Rhythm 2020;17:1960–1966. https://doi.org/10.1016/j.hrthm.2020.06.033
- Wu
L, O'Kane AM, Peng H, Bi Y, Motriuk-Smith D, Ren J. SARS-CoV-2 and
cardiovascu-lar complications: From molecular mechanisms to
pharmaceutical management. Biochem Pharmacol 2020;178:114114.
https://doi.org/10.1016/j.bcp.2020.114114
- LaRovere
KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, Walker TC,
Singh AR, Dapul H, Hobbs CV, McLaughlin GE, Son MBF, Maddux AB, Clouser
KN, Rowan CM, McGuire JK, Fitzgerald JC, Gertz SJ, Shein SL, Munoz AC,
Thomas NJ, Irby K, Levy ER, Staat MA, Tenforde MW, Feldstein LR, Halasa
NB, Giuliano JS, Hall MW, Kong M, Carroll CL, Schuster JE, Doymaz S,
Loftis LL, Tarquinio KM, Babbitt CJ, Nofziger RA, Kleinman LC,
Keen-aghan MA, Cvijanovich NZ, Spinella PC, Hume JR, Wellnitz K, Mack
EH, Michelson KN, Flori HR, Patel MM, Randolph AG, Overcoming COVID-19
Investigators. Neurologic Involvement in Children and Adolescents
Hospitalized in the United States for COVID-19 or Multisystem
In-flammatory Syndrome. JAMA Neurol 2021;78:536–547.
https://doi.org/10.1001/jamaneurol.2021.0504
- Ray
STJ, Abdel-Mannan O, Sa M, Fuller C, Wood GK, Pysden K, Yoong M,
McCullagh H, Scott D, McMahon M, Thomas N, Taylor M, Illingworth M,
McCrea N, Davies V, Whitehouse W, Zuberi S, Guthrie K, Wassmer E, Shah
N, Baker MR, Tiwary S, Tan HJ, Varma U, Ram D, Avula S, Enright N,
Hassell J, Ross Russell AL, Kumar R, Mulholland RE, Pett S, Galea I,
Thomas RH, Lim M, Hacohen Y, Solomon T, Griffiths MJ, Michael BD, Kneen
R, CoroNerve study group. Neu-rological manifestations of SARS-CoV-2
infection in hospitalised children and adolescents in the UK: a
prospective national cohort study. Lancet Child Adolesc Health
2021;5:631–641. https://doi.org/10.1016/S2352-4642(21)00193-0
- Andina
D, Belloni-Fortina A, Bodemer C, Bonifazi E, Chiriac A, Colmenero I,
Diociaiuti A, El-Hachem M, Fertitta L, van Gysel D, Hernández-Martín A,
Hubiche T, Luca C, Martos-Cabrera L, Maruani A, Mazzotta F, Akkaya AD,
Casals M, Ferrando J, Grimalt R, Grozdev I, Kinsler V, Morren MA,
Munisami M, Nanda A, Novoa MP, Ott H, Pasmans S, Salavastru C, Zawar V,
Torrelo A, ESPD Group for the Skin Manifestations of COVID-19. Skin
manifestations of COVID-19 in children: Part 1. Clin Exp Dermatol
2021;46:444–450. https://doi.org/10.1111/ced.14481
- Levin
M. Childhood Multisystem Inflammatory Syndrome - A New Challenge in the
Pan-demic. N Engl J Med 2020;383:393–395.
https://doi.org/10.1056/NEJMe2023158
- Feldstein
LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW,
Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM,
Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald
JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS, Gupta
A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford
TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A,
Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM,
Randolph AG, Overcoming COVID-19 Investigators, CDC COVID-19 Response
Team. Multisystem Inflammatory Syndrome in U.S. Children and
Adolescents. N Engl J Med 2020;383:334–346.
https://doi.org/10.1056/NEJMoa2021680
- Payne
AB, Gilani Z, Godfred-Cato S, Belay ED, Feldstein LR, Patel MM,
Randolph AG, Newhams M, Thomas D, Magleby R, Hsu K, Burns M, Dufort E,
Maxted A, Pietrowski M, Longenberger A, Bidol S, Henderson J, Sosa L,
Edmundson A, Tobin-D'Angelo M, Edison L, Heidemann S, Singh AR,
Giuliano JS, Kleinman LC, Tarquinio KM, Walsh RF, Fitzgerald JC,
Clouser KN, Gertz SJ, Carroll RW, Carroll CL, Hoots BE, Reed C,
Dahlgren FS, Oster ME, Pierce TJ, Curns AT, Langley GE, Campbell AP,
MIS-C Incidence Authorship Group, Balachandran N, Murray TS, Burkholder
C, Brancard T, Lifshitz J, Leach D, Charpie I, Tice C, Coffin SE,
Perella D, Jones K, Marohn KL, Yager PH, Fernandes ND, Flori HR,
Koncicki ML, Walker KS, Di Pentima MC, Li S, Horwitz SM, Gaur S, Coffey
DC, Harwayne-Gidansky I, Hymes SR, Thomas NJ, Ackerman KG, Cholette JM.
Incidence of Multisystem Inflammatory Syndrome in Children Among US
Persons Infected With SARS-CoV-2. JAMA Netw Open 2021;4:e2116420 .
https://doi.org/10.1001/jamanetworkopen.2021.16420
- Castagnola
E, Mariani M, Sticchi C, Sticchi L, Spiazzi R, Caorsi R, Gattorno M,
Ravelli A. Incidence rate of MIS-C in paediatrics: A good reason to
vaccinate children against SARS-CoV-2. Acta Paediatr Oslo Nor 1992
2021. https://doi.org/10.1111/apa.16081
- Ben-Shimol
S, Livni G, Megged O, Greenberg D, Danino D, Youngster I,
Shachor-Meyouhas Y, Dabaja-Younis H, Scheuerman O, Mor M, Somekh E,
Yakub Hanna H, Givon-Lavi N, Guri A, Leibovitz E, Alkan Y, Grupel D,
Rubinstein U, Steinberg Ben Zeev Z, Bamberger E, Asher Kuperman A,
Grisaru-Soen G, Tasher D, Gottesman G, Glikman D, Stein M.COVID-19 in a
Subset of Hospitalized Children in Israel. J Pediatr Infect Dis Soc
2021;10:757–765. https://doi.org/10.1093/jpids/piab035
- Kaushik
A, Gupta S, Sood M, Sharma S, Verma S. A Systematic Review of
Multisystem In-flammatory Syndrome in Children Associated With
SARS-CoV-2 Infection. Pediatr Infect Dis J 2020;39:e340–e346.
https://doi.org/10.1097/INF.0000000000002888
- McArdle
AJ, Vito O, Patel H, Seaby EG, Shah P, Wilson C, Broderick C, Nijman R,
Tre-moulet AH, Munblit D, Ulloa-Gutierrez R, Carter MJ, De T, Hoggart
C, Whittaker E, Herberg JA, Kaforou M, Cunnington AJ, Levin M, BATS
Consortium. Treatment of Multisystem Inflammatory Syndrome in Children.
N Engl J Med 2021;385:11–22. https://doi.org/10.1056/NEJMoa2102968
- Son
MBF, Murray N, Friedman K, Young CC, Newhams MM, Feldstein LR, Loftis
LL, Tarquinio KM, Singh AR, Heidemann SM, Soma VL, Riggs BJ, Fitzgerald
JC, Kong M, Doymaz S, Giuliano JS, Keenaghan MA, Hume JR, Hobbs CV,
Schuster JE, Clouser KN, Hall MW, Smith LS, Horwitz SM, Schwartz SP,
Irby K, Bradford TT, Maddux AB, Babbitt CJ, Rowan CM, McLaugh-lin GE,
Yager PH, Maamari M, Mack EH, Carroll CL, Montgomery VL, Halasa NB,
Cvijanovich NZ, Coates BM, Rose CE, Newburger JW, Patel MM, Randolph
AG, Overcoming COVID-19 In-vestigators. Multisystem Inflammatory
Syndrome in Children - Initial Therapy and Outcomes. N Engl J Med
2021;385:23–34. https://doi.org/10.1056/NEJMoa2102605
- Ouldali
N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, Léger P-L,
Galeotti C, Claude C, Wiedemann A, Lachaume N, Ovaert C, Dumortier M,
Kahn J-E, Mandelcwajg A, Per-cheron L, Biot B, Bordet J, Girardin M-L,
Yang DD, Grimaud M, Oualha M, Allali S, Bajolle F, Beyler C, Meinzer U,
Levy M, Paulet A-M, Levy C, Cohen R, Belot A, Angoulvant F, French
Covid-19 Paediatric Inflammation Consortium.Association of Intravenous
Immunoglobulins Plus Methylprednisolone vs Immunoglobulins Alone With
Course of Fever in Multisystem Inflammatory Syndrome in Children. JAMA
2021;325:855–864. https://doi.org/10.1001/jama.2021.0694
- Denina
M, Pruccoli G, Scolfaro C, Mignone F, Zoppo M, Giraudo I, Silvestro E,
Bertolotti L, Rosati S, Ramenghi U, Garazzino S. Sequelae of COVID-19
in Hospitalized Children: A 4-Months Follow-Up. Pediatr Infect Dis J.
2020 Dec;39(12):e458-e459.
https://doi.org/10.1097/INF.0000000000002937. PMID: 33003103.
- Say
D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19
outcomes in children with mild and asymptomatic disease. Lancet Child
Adolesc Health. 2021;5(6):e22-e23.
https://doi.org/10.1016/S2352-4642(21)00124-3
- Wang
J-G, Zhong Z-J, Mo Y-F, Wang L-C, Chen R. Epidemiological features of
coronavirus disease 2019 in children: a meta-analysis. Eur Rev Med
Pharmacol Sci 2021;25:1146–1157.
https://doi.org/10.26355/eurrev_202101_24685
- Kim
L, Whitaker M, O'Halloran A, Kambhampati A, Chai SJ, Reingold A,
Armistead I, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Openo
KP, Weigel A, Ryan P, Monroe ML, Fox K, Kim S, Lynfield R, Bye E, Shrum
Davis S, Smelser C, Barney G, Spina NL, Bennett NM, Felsen CB, Billing
LM, Shiltz J, Sutton M, West N, Talbot HK, Schaffner W, Risk I, Price
A, Brammer L, Fry AM, Hall AJ, Langley GE, Garg S, COVID-NET
Surveillance Team (2020) Hospi-talization Rates and Characteristics of
Children Aged <18 Years Hospitalized with Laboratory-Confirmed
COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb
Mortal Wkly Rep 69:1081–1088. https://doi.org/10.15585/mmwr.mm6932e3
- Götzinger
F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò
Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U,
Lo Vecchio A, Shingadia D, So-riano-Arandes A, Melendo S, Lanari M,
Pierantoni L, Wagner N, L’Huillier AG, Heininger U, Ritz N, Bandi S,
Krajcar N, Roglić S, Santos M, Christiaens C, Creuven M, Buonsenso D,
Welch SB, Bogyi M, Brinkmann F, Tebruegge M, ptbnet COVID-19 Study
Group. COVID-19 in children and adolescents in Europe: a multinational,
multicentre cohort study. Lancet Child Adolesc Health 2020;4:653–661.
https://doi.org/10.1016/S2352-4642(20)30177-2
- Havers
FP, Whitaker M, Self JL, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Meek
J, Yousey-Hindes K, Anderson EJ, Openo KP, Weigel A, Teno K, Monroe ML,
Ryan PA, Reeg L, Kohrman A, Lynfield R, Como-Sabetti K, Poblete M,
McMullen C, Muse A, Spina N, Bennett NM, Gaitán M, Billing LM, Shiltz
J, Sutton M, Abdullah N, Schaffner W, Talbot HK, Crossland M, George A,
Patel K, Pham H, Milucky J, Anglin O, Ujamaa D, Hall AJ, Garg S, Taylor
CA, COVID-NET Surveillance Team. Hospitalization of Adolescents Aged
12-17 Years with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States,
March 1, 2020-April 24, 2021. MMWR Morb Mortal Wkly Rep
2021;70:851–857. https://doi.org/10.15585/mmwr.mm7023e1
- Bhopal
SS, Bagaria J, Olabi B, Bhopal R.Children and young people remain at
low risk of COVID-19 mortality. Lancet Child Adolesc Health
2021;5:e12–e13. https://doi.org/10.1016/S2352-4642(21)00066-3
- McCormick
DW, Richardson LC, Young PR, Viens LJ, Gould CV, Kimball A, Pindyck T,
Rosenblum HG, Siegel DA, Vu QM, Komatsu K, Venkat H, Openshaw JJ,
Kawasaki B, Siniscalchi AJ, Gumke M, Leapley A, Tobin-D'Angelo M,
Kauerauf J, Reid H, White K, Ahmed FS, Richard-son G, Hand J, Kirkey K,
Larson L, Byers P, Garcia A, Ojo M, Zamcheck A, Lash MK, Lee EH, Reilly
KH, Wilson E, de Fijter S, Naqvi OH, Harduar-Morano L, Burch A-K, Lewis
A, Kolsin J, Pont SJ, Barbeau B, Bixler D, Reagan-Steiner S, Koumans
EH, Pediatric Mortality Investigation Team. Deaths in Children and
Adolescents Associated With COVID-19 and MIS-C in the United States.
Pediatrics 2021;e2021052273 . https://doi.org/10.1542/peds.2021-052273
- Bixler
D, Miller AD, Mattison CP, Taylor B, Komatsu K, Peterson Pompa X, Moon
S, Kar-markar E, Liu CY, Openshaw JJ, Plotzker RE, Rosen HE, Alden N,
Kawasaki B, Siniscalchi A, Leapley A, Drenzek C, Tobin-D'Angelo M,
Kauerauf J, Reid H, Hawkins E, White K, Ahmed F, Hand J, Richardson G,
Sokol T, Eckel S, Collins J, Holzbauer S, Kollmann L, Larson L,
Schiffman E, Kittle TS, Hertin K, Kraushaar V, Raman D, LeGarde V,
Kinsinger L, Peek-Bullock M, Lifshitz J, Ojo M, Arciuolo RJ, Davidson
A, Huynh M, Lash MK, Latash J, Lee EH, Li L, McGibbon E,
McIntosh-Beckles N, Pouchet R, Ramachandran JS, Reilly KH, Dufort E,
Pulver W, Zamcheck A, Wilson E, de Fijter S, Naqvi O, Nalluswami K,
Waller K, Bell LJ, Burch A-K, Radcliffe R, Fiscus MD, Lewis A, Kolsin
J, Pont S, Salinas A, Sanders K, Barbeau B, Althomsons S, Atti S, Brown
JS, Chang A, Clarke KR, Datta SD, Iskander J, Leitgeb B, Pindyck T,
Priyamvada L, Reagan-Steiner S, Scott NA, Viens LJ, Zhong J, Koumans
EH, Pediatric Mortality Investigation Team. SARS-CoV-2-Associated
Deaths Among Persons Aged <21 Years - United States, February
12-July 31, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1324–1329.
https://doi.org/10.15585/mmwr.mm6937e4
- Irfan
O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical
characteristics, treatment and outcomes of paediatric COVID-19: a
systematic review and meta-analysis. Arch Dis Child archdischild
2021;2020-321385. https://doi.org/10.1136/archdischild-2020-321385
- Turner
D, Huang Y, Martín-de-Carpi J, Aloi M, Focht G, Kang B, Zhou Y, Sanchez
C, Kappelman MD, Uhlig HH, Pujol-Muncunill G, Ledder O, Lionetti P,
Dias JA, Ruemmele FM, Russell RK, Paediatric IBD Porto group of
ESPGHAN. Corona Virus Disease 2019 and Paediatric Inflammatory Bowel
Diseases: Global Experience and Provisional Guidance (March 2020) from
the Paediatric IBD Porto Group of European Society of Paediatric
Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol
Nutr 2020;70:727–733. https://doi.org/10.1097/MPG.0000000000002729
- Sengler
C, Eulert S, Minden K, Niewerth M, Horneff G, Kuemmerle-Deschner J,
Siemer C, Berendes R, Girschick H, Hühn R, Borte M, Hospach A, Emminger
W, Armann J, Klein A, Kal-linich T. Clinical manifestations and outcome
of SARS-CoV-2 infections in children and adoles-cents with rheumatic
musculoskeletal diseases: data from the National Paediatric
Rheumatology Da-tabase in Germany. RMD Open 2021;7:e001687.
https://doi.org/10.1136/rmdopen-2021-001687
- Marlais
M, Wlodkowski T, Vivarelli M, Pape L, Tönshoff B, Schaefer F, Tullus K.
The se-verity of COVID-19 in children on immunosuppressive medication.
Lancet Child Adolesc Health 2020;4:e17–e18.
https://doi.org/10.1016/S2352-4642(20)30145-0
- CDC
Healthcare Workers. In: Cent. Dis. Control Prev. 2020.
https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
Accessed September 25 2021
- CDC
Coronavirus Disease 2019 (COVID-19). In: Cent. Dis. Control Prev. 2020.
https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
Accessed 25 Sep 2021
- Saini KS,
Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, Curigliano
G, de Azambuja E.Mortality in patients with cancer and coronavirus
disease 2019: A systematic review and pooled analysis of 52 studies.
Eur J Cancer Oxf Engl 1990 2020;139:43–50.
https://doi.org/10.1016/j.ejca.2020.08.011
- de
Rojas T, Pérez-Martínez A, Cela E, Baragaño M, Galán V, Mata C, Peretó
A, Madero L. COVID-19 infection in children and adolescents with cancer
in Madrid. Pediatr Blood Cancer 2020;67:e28397.
https://doi.org/10.1002/pbc.28397
- Bouffet
E, Challinor J, Sullivan M, Biondi A, Rodriguez-Galindo C,
Pritchard-Jones K. Ear-ly advice on managing children with cancer
during the COVID-19 pandemic and a call for sharing experiences.
Pediatr Blood Cancer 2020;67:e28327. https://doi.org/10.1002/pbc.28327
- Meena
JP, Kumar Gupta A, Tanwar P, Ram Jat K, Mohan Pandey R, Seth R.
Clinical presentations and outcomes of children with cancer and
COVID-19: A systematic review. Pediatr Blood Cancer 2021;68:e29005.
https://doi.org/10.1002/pbc.29005
- Boulad
F, Kamboj M, Bouvier N, Mauguen A, Kung AL. COVID-19 in Children With
Cancer in New York City. JAMA Oncol 2020;6:1459–1460.
https://doi.org/10.1001/jamaoncol.2020.2028
- Cesaro
S, Compagno F, Zama D, Meneghello L, Giurici N, Soncini E, Onofrillo D,
Mercolini F, Mura R, Perruccio K, De Santis R, Colombini A, Barone A,
Sainati L, Baretta V, Petris MG. Screening for SARS-CoV-2 infection in
pediatric oncology patients during the epidemic peak in Ita-ly. Pediatr
Blood Cancer 2020;67:e28466. https://doi.org/10.1002/pbc.28466
- Ferrari
A, Zecca M, Rizzari C, Porta F, Provenzi M, Marinoni M, Schumacher RF,
Luksch R, Terenziani M, Casanova M, Spreafico F, Chiaravalli S,
Compagno F, Bruni F, Piccolo C, Bettini L, D’Angiò M, Ferrari GM,
Biondi A, Massimino M, Balduzzi A. Children with cancer in the time of
COVID-19: An 8-week report from the six pediatric onco-hematology
centers in Lombardia, Italy. Pediatr Blood Cancer 2020;67:e28410.
https://doi.org/10.1002/pbc.28410
- Hamdy
R, El-Mahallawy H, Ebeid E. COVID-19 infection in febrile neutropenic
pediatric hematology oncology patients. Pediatr Blood Cancer
2021;68:e28765. https://doi.org/10.1002/pbc.28765
- López-Aguilar
E, Cárdenas-Navarrete R, Simental-Toba A, Pacheco-Rosas D, Thomé-Ortiz
P, Soto-Pérez G, Martín-Trejo J, Vázquez-Rosales G, Miranda-Novales G.
Children with cancer during COVID-19 pandemic: Early experience in
Mexico. Pediatr Blood Cancer 2021;68:e28660.
https://doi.org/10.1002/pbc.28660
- Sieni
E, Pegoraro F, Casini T, Tondo A, Bortone B, Moriondo M, Azzari C,
Galli L, Favre C. Favourable outcome of coronavirus disease 2019 in a
1-year-old girl with acute myeloid leukae-mia and severe
treatment-induced immunosuppression. Br J Haematol 2020;189:e222–e224.
https://doi.org/10.1111/bjh.16781
- Wang
S-M, Tao F, Hou Y, Zhang A, Xiong H, Sun J-J, Luo X-P, Hao Y, Li J-X,
Hu Q, Liu A-G. Screening of SARS-CoV-2 in 299 Hospitalized Children
with Hemato-oncological Diseases: A Multicenter Survey in Hubei, China.
Curr Med Sci 2020;40:642–645. https://doi.org/10.1007/s11596-020-2228-7
- André N, Rouger-Gaudichon J, Brethon B,
Phulpin A, Thébault É, Pertuisel S, Gandemer V. COVID-19 in pediatric
oncology from French pediatric oncology and hematology centers: High
risk of severe forms? Pediatr Blood Cancer 2020;67:e28392.
https://doi.org/10.1002/pbc.28392
- Bisogno
G, Provenzi M, Zama D, Tondo A, Meazza C, Colombini A, Galaverna F,
Com-pagno F, Carraro F, De Santis R, Meneghello L, Baretta V, Cesaro S.
Clinical Characteristics and Outcome of Severe Acute Respiratory
Syndrome Coronavirus 2 Infection in Italian Pediatric On-cology
Patients: A Study From the Infectious Diseases Working Group of the
Associazione Italiana di Oncologia e Ematologia Pediatrica. J Pediatr
Infect Dis Soc 2020;9:530–534. https://doi.org/10.1093/jpids/piaa088
- Pérez-Martinez
A, Guerra-García P, Melgosa M, Frauca E, Fernandez-Camblor C, Remesal
A, Calvo C. Clinical outcome of SARS-CoV-2 infection in
immunosuppressed children in Spain. Eur J Pediatr 2021;180:967–971.
https://doi.org/10.1007/s00431-020-03793-3
- Montoya
J, Ugaz C, Alarcon S, Maradiegue E, García J, Díaz R, Zapata A, Chávez
S, Mo-rales R, Ordoñez K, Hernandez E, Reaño R, Gutierrez C, Vargas MP,
Sanchez K, Valdiviezo C, Maza I, Rojas N, Moore C, León E, Vásquez L.
COVID-19 in pediatric cancer patients in a re-source-limited setting:
National data from Peru. Pediatr Blood Cancer 2021;68:e28610.
https://doi.org/10.1002/pbc.28610
- Almassi
N, Mulhall JP, Funt SA, Sheinfeld J. "Case of the Month" from Memorial
Sloan Kettering Cancer Center, New York, NY, USA: managing newly
diagnosed metastatic testicular germ cell tumour in a COVID-19-positive
patient. BJU Int 2020;126:333–335. https://doi.org/10.1111/bju.15157
- Bernar
B, Kropshofer G, Crazzolara R, Kapelari K, Griesmacher A, Müller T,
Scholl-Bürgi S. SARS-CoV-2 infection in a 7-year-old girl with
pancytopenia during acute lymphocytic leukemia maintenance therapy.
Pediatr Blood Cancer 2020;67:e28391 . https://doi.org/10.1002/pbc.28391
- Dantonello TM, Kartal-Kaess M, Aebi C,
Suter-Riniker F, Busch JD, Kubetzko S, Bourquin J-P, Roessler J.
SARS-CoV-2 Infection During Induction Chemotherapy in a Child With
High-risk T-Cell Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol
2021;43:e804–e807. https://doi.org/10.1097/MPH.0000000000001943
- Flores
V, Miranda R, Merino L, González C, Serrano C, Solano M, Herrera J,
González P, Ruiz G, Saldaña R, Cárdenas A, Chávez-Aguilar LA.
SARS-CoV-2 infection in children with fe-brile neutropenia. Ann Hematol
2020;99:1941–1942. https://doi.org/10.1007/s00277-020-04115-1
- Jarmoliński
T, Matkowska-Kocjan A, Rosa M, Olejnik I, Gorczyńska E, Kałwak K,
Us-sowicz M. SARS-CoV-2 viral clearance during bone marrow aplasia
after allogeneic hematopoietic stem cell transplantation-A case report.
Pediatr Transplant 2021;25:e13875. https://doi.org/10.1111/petr.13875
- Marcia
M, Vania B, Pruccoli G, Vallero SG, Barisone E, Scolfaro C, Fagioli F.
Acute lym-phoblastic leukemia onset in a 3-year-old child with
COVID-19. Pediatr Blood Cancer 2020;67:e28423.
https://doi.org/10.1002/pbc.28423
- Offenbacher
R, Fabish L, Baker A, Chou AJ, Loeb DM. Respiratory Failure in a Child
With Pulmonary Metastatic Osteosarcoma and COVID-19. J Pediatr Hematol
Oncol 2021;43:e859–e860. https://doi.org/10.1097/MPH.0000000000001897
- Orf
K, Rogosic S, Dexter D, Ancliff P, Badle S, Brierley J, Cheng D, Dalton
C, Dixon G, Du Pré P, Grandjean L, Ghorashian S, Mittal P, O'Connor D,
Pavasovic V, Rao A, Samarasinghe S, Vora A, Bamford A, Bartram J.
Remdesivir during induction chemotherapy for newly diagnosed paediatric
acute lymphoblastic leukaemia with concomitant SARS-CoV-2 infection. Br
J Haematol 2020;190:e274–e276. https://doi.org/10.1111/bjh.17014
- Pérez-Heras
I, Fernandez-Escobar V, Del Pozo-Carlavilla M, Díaz-Merchán R,
Valerio-Alonso ME, Domínguez-Pinilla N. Two Cases of SARS-CoV-2
Infection in Pediatric Oncohemato-logic Patients in Spain. Pediatr
Infect Dis J 2020;39:1040–1042.
https://doi.org/10.1097/INF.0000000000002841
- Velasco
Puyó P, Moreno L, Díaz de Heredia C, Rivière JG, Soler Palacín P.
Tocilizumab in a child with acute lymphoblastic leukaemia and
COVID-19-related cytokine release syndrome. An Pediatr 2020;93:132–133.
https://doi.org/10.1016/j.anpede.2020.05.002
- Radhakrishnan
V, Gangopadhyay D. Repeat-positive SARS-CoV-2 in a child with cancer.
Pediatr Blood Cancer 2021;68:e28744. https://doi.org/10.1002/pbc.28744
- Schied A, Trovillion E, Moodley A.
SARS-CoV-2 infection in a neutropenic pediatric pa-tient with leukemia:
Addressing the need for universal guidelines for treatment of
SARS-CoV-2-positive, immunocompromised patients. Pediatr Blood Cancer
2020;67:e28546. https://doi.org/10.1002/pbc.28546
- Shankar
R, Radhakrishnan N, Dua S, Arora S, Rana M, Sahu DK, Rai S, Gupta DK.
Conva-lescent plasma to aid in recovery of COVID-19 pneumonia in a
child with acute lymphoblastic leu-kemia. Transfus Apher Sci Off J
World Apher Assoc Off J Eur Soc Haemapheresis 2021;60:102956.
https://doi.org/10.1016/j.transci.2020.102956
- Smith
VR, Whittle SB, Coleman RD, Munoz FM, De Guzman MM, Foster JH, Navai
SA. Severe COVID-19 infection in a child receiving immunotherapy for
cancer. Pediatr Blood Cancer 2021;68:e28710 .
https://doi.org/10.1002/pbc.28710
- Stokes
CL, Patel PA, Sabnis HS, Mitchell SG, Yildirim IB, Pauly MG. Severe
COVID-19 disease in two pediatric oncology patients. Pediatr Blood
Cancer 2020;67:e28432. https://doi.org/10.1002/pbc.28432
- Vicent
MG, Martinez AP, Trabazo Del Castillo M, Molina B, Sisini L,
Morón-Cazalilla G, Díaz MÁ. COVID-19 in pediatric hematopoietic stem
cell transplantation: The experience of Span-ish Group of Transplant
(GETMON/GETH). Pediatr Blood Cancer 2020;67:e28514.
https://doi.org/10.1002/pbc.28514
- Zamperlini-Netto
G, Fernandes JF, Garcia JL, Ribeiro AAF, Camargo LFA, de Moraes Terra
C, Hamerschlak N. COVID-19 after hematopoietic stem cell
transplantation: report of two children. Bone Marrow Transplant
2021;56:713–715. https://doi.org/10.1038/s41409-020-01041-8
- Zhao
Y, Zhao W, Wang A, Qian F, Wang S, Zhuang L, Zhang F, Sun D, Gao G.
First Case of Coronavirus Disease 2019 in Childhood Leukemia in China.
Pediatr Infect Dis J 2020;39:e142–e145.
https://doi.org/10.1097/INF.0000000000002742
- Zhou
X, Wang G, Chen L, Meng F, Huang L, Huang L, Wang N, Li T, Cao Y, Zhou
J. Clin-ical characteristics of hematological patients concomitant with
COVID-19. Cancer Sci 2020;111:3379–3385.
https://doi.org/10.1111/cas.14544
- Sun
D, Li H, Lu X-X, Xiao H, Ren J, Zhang F-R, Liu Z-S. Clinical features
of severe pediat-ric patients with coronavirus disease 2019 in Wuhan: a
single center's observational study. World J Pediatr WJP
2020;16:251–259. https://doi.org/10.1007/s12519-020-00354-4
- Rossoff
J, Patel AB, Muscat E, Kociolek LK, Muller WJ. Benign course of
SARS-CoV-2 infection in a series of pediatric oncology patients.
Pediatr Blood Cancer 2020;67:e28504. https://doi.org/10.1002/pbc.28504
- Dorantes-Acosta
E, Ávila-Montiel D, Klünder-Klünder M, Juárez-Villegas L,
Márquez-González H. Survival and Complications in Pediatric Patients
With Cancer and COVID-19: A Meta-Analysis. Front Oncol 2020;10:608282.
https://doi.org/10.3389/fonc.2020.608282
- Nicastro
E, Verdoni L, Bettini LR, Zuin G, Balduzzi A, Montini G, Biondi A,
D’Antiga L. COVID-19 in Immunosuppressed Children. Front Pediatr
2021;9:629240. https://doi.org/10.3389/fped.2021.629240
- Mukkada
S, Bhakta N, Chantada GL, Chen Y, Vedaraju Y, Faughnan L, Homsi MR,
Mu-niz-Talavera H, Ranadive R, Metzger M, Friedrich P, Agulnik A, Jeha
S, Lam C, Dalvi R, Hessissen L, Moreira DC, Santana VM, Sullivan M,
Bouffet E, Caniza MA, Devidas M, Pritchard-Jones
K, Rodriguez-Galindo C, Global Registry of COVID-19 in Childhood
Cancer. Global characteristics and outcomes of SARS-CoV-2 infection in
children and adolescents with cancer (GRCCC): a co-hort study. Lancet
Oncol 2021;S1470-2045(21)00454-X.
https://doi.org/10.1016/S1470-2045(21)00454-X
- Ljungman
P, de la Camara R, Mikulska M, Tridello G, Aguado B, Zahrani MA,
Apperley J, Berceanu A, Bofarull RM, Calbacho M, Ciceri F, Lopez-Corral
L, Crippa C, Fox ML, Grassi A, Jimenez M-J, Demir SK, Kwon M, Llamas
CV, Lorenzo JLL, Mielke S, Orchard K, Porras RP, Val-lisa D, Xhaard A,
Knelange NS, Cedillo A, Kröger N, Piñana JL, Styczynski J. COVID-19 and
stem cell transplantation; results from an EBMT and GETH multicenter
prospective survey. Leuke-mia 2021.
https://doi.org/10.1038/s41375-021-01302-5
- COVID-19 resources. In: RCPCH. https://www.rcpch.ac.uk/key-topics/covid-19/all-resources Accessed September 25 2021
- Society
CP The acute management of COVID-19 in paediatrics (spring 2021 update)
| Ca-nadian Paediatric Society.
https://www.cps.ca/en/documents/position/the-acute-management-of-paediatric-coronavirus-disease-2019covid-19
Accessed September 25 2021
- Jiehao C, Jin
X, Daojiong L, Zhi Y, Lei X, Zhenghai Q, Yuehua Z, Hua Z, Ran J,
Pengcheng L, Xiangshi W, Yanling G, Aimei X, He T, Hailing C, Chuning
W, Jingjing L, Jianshe W, Mei Z. A Case Series of Children With 2019
Novel Coronavirus Infection: Clinical and Epidemiological Fea-tures.
Clin Infect Dis Off Publ Infect Dis Soc Am 2020;71:1547–1551.
https://doi.org/10.1093/cid/ciaa198
- Zimmermann
P, Curtis N. Coronavirus Infections in Children Including COVID-19: An
Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment
and Prevention Options in Children. Pediatr Infect Dis J
2020;39:355–368. https://doi.org/10.1097/INF.0000000000002660
- Reis
G, Moreira Silva EADS, Medeiros Silva DC, Thabane L, Singh G, Park JJH,
Forrest JI, Harari O, Quirino Dos Santos CV, Guimarães de Almeida APF,
Figueiredo Neto AD de, Savassi LCM, Milagres AC, Teixeira MM, Simplicio
MIC, Ribeiro LB, Oliveira R, Mills EJ, TOGETHER Investigators. Effect
of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir
on Risk of Hospitalization Among Patients With COVID-19: The TOGETHER
Randomized Clinical Trial. JAMA Netw Open 2021;4:e216468 .
https://doi.org/10.1001/jamanetworkopen.2021.6468
- RECOVERY
Collaborative Group, Horby P, Mafham M, Linsell L, Bell JL, Staplin N,
Em-berson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse
T, Felton T, Williams J, Faccenda J, Underwood J, Baillie JK, Chappell
LC, Faust SN, Jaki T, Jeffery K, Lim WS, Mont-gomery A, Rowan K,
Tarning J, Watson JA, White NJ, Juszczak E, Haynes R, Landray MJ.
Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N
Engl J Med 2020;383:2030–2040 . https://doi.org/10.1056/NEJMoa2022926
- Kashour
Z, Riaz M, Garbati MA, AlDosary O, Tlayjeh H, Gerberi D, Murad MH,
Sohail MR, Kashour T, Tleyjeh IM. Efficacy of chloroquine or
hydroxychloroquine in COVID-19 patients: a systematic review and
meta-analysis. J Antimicrob Chemother 2021;76:30–42.
https://doi.org/10.1093/jac/dkaa403
- Cao
B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M,
Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan
Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F,
Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong
X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou
J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo
L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of
Lopinavir-Ritonavir in Adults Hospitalized with Se-vere Covid-19. N
Engl J Med 2020;382:1787–1799. https://doi.org/10.1056/NEJMoa2001282
- RECOVERY
Collaborative Group. Lopinavir-ritonavir in patients admitted to
hospital with COVID-19 (RECOVERY): a randomised, controlled,
open-label, platform trial. Lancet Lond Engl 2020;396:1345–1352.
https://doi.org/10.1016/S0140-6736(20)32013-4
- WHO
Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo A-M,
Preziosi M-P, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM,
Hernández García C, Kieny M-P, Malekza-deh R, Murthy S, Reddy KS, Roses
Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E,
Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P,
Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow
TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L,
Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L,
Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T,
Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML,
Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR,
Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N,
Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid
H, Røttingen J-A, Swaminathan S. Repurposed Antiviral Drugs for
Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med
2021;384:497–511. https://doi.org/10.1056/NEJMoa2023184
- Cavalcanti
AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP,
Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E
Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT,
Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado
RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR,
Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A,
Falavigna M, Lopes RD, Machado FR, Berwanger O, Coalition Covid-19
Brazil I Investigators. Hydroxychloroquine with or without Azithromycin
in Mild-to-Moderate Covid-19. N Engl J Med 2020;383:2041–2052.
https://doi.org/10.1056/NEJMoa2019014
- Furtado
RHM, Berwanger O, Fonseca HA, Corrêa TD, Ferraz LR, Lapa MG, Zampieri
FG, Veiga VC, Azevedo LCP, Rosa RG, Lopes RD, Avezum A, Manoel ALO,
Piza FMT, Martins PA, Lisboa TC, Pereira AJ, Olivato GB, Dantas VCS,
Milan EP, Gebara OCE, Amazonas RB, Oliveira MB, Soares RVP, Moia DDF,
Piano LPA, Castilho K, Momesso RGRAP, Schettino GPP, Rizzo LV, Neto AS,
Machado FR, Cavalcanti AB, COALITION COVID-19 Brazil II Investigators.
Azithromycin in addition to standard of care versus standard of care
alone in the treatment of pa-tients admitted to the hospital with
severe COVID-19 in Brazil (COALITION II): a randomised clinical trial.
Lancet Lond Engl 2020;396:959–967.
https://doi.org/10.1016/S0140-6736(20)31862-6
- RECOVERY
Collaborative Group. Azithromycin in patients admitted to hospital with
COVID-19 (RECOVERY): a randomised, controlled, open-label, platform
trial. Lancet Lond Engl 2021;397:605–612.
https://doi.org/10.1016/S0140-6736(21)00149-5
- Rosenberg
ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, Weinberg
P, Kirkwood J, Muse A, DeHovitz J, Blog DS, Hutton B, Holtgrave DR,
Zucker HA. Association of Treatment With Hydroxychloroquine or
Azithromycin With In-Hospital Mortality in Patients With COVID-19 in
New York State. JAMA 2020;323:2493–2502.
https://doi.org/10.1001/jama.2020.8630
- Rochwerg
B, Agarwal A, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, Lytvyn
L, Leo Y-S, Macdonald H, Zeng L, Amin W, Burhan E, Bausch FJ, Calfee
CS, Cecconi M, Chanda D, Du B, Geduld H, Gee P, Harley N, Hashimi M,
Hunt B, Kabra SK, Kanda S, Kawano-Dourado L, Kim Y-J, Kissoon N,
Kwizera A, Mahaka I, Manai H, Mino G, Nsutebu E, Preller J,
Pshenichnaya N, Qadir N, Sabzwari S, Sarin R, Shankar-Hari M, Sharland
M, Shen Y, Ranganathan SS, Souza JP, Stegemann M, De Sutter A, Ugarte
S, Venkatapuram S, Dat VQ, Vuyiseka D, Wijewickrama A, Maguire B,
Zeraatkar D, Bartoszko JJ, Ge L, Brignardello-Petersen R, Owen A,
Guyatt G, Diaz J, Jacobs M, Vandvik PO. A living WHO guideline on drugs
for covid-19. BMJ 2020;370:m3379. https://doi.org/10.1136/bmj.m3379
- Sarkar
S, Khanna P, Soni KD. Are the steroids a blanket solution for COVID-19?
A system-atic review and meta-analysis. J Med Virol 2021;93:1538–1547.
https://doi.org/10.1002/jmv.26483
- Rivera
DR, Peters S, Panagiotou OA, Shah DP, Kuderer NM, Hsu C-Y, Rubinstein
SM, Lee BJ, Choueiri TK, de Lima Lopes G, Grivas P, Painter CA, Rini
BI, Thompson MA, Arcobello J, Bakouny Z, Doroshow DB, Egan PC,
Farmakiotis D, Fecher LA, Friese CR, Galsky MD, Goel S, Gupta S,
Halfdanarson TR, Halmos B, Hawley JE, Khaki AR, Lemmon CA, Mishra S,
Olszewski AJ, Pennell NA, Puc MM, Revankar SG, Schapira L, Schmidt A,
Schwartz GK, Shah SA, Wu JT, Xie Z, Yeh AC, Zhu H, Shyr Y, Lyman GH,
Warner JL, COVID-19 and Cancer Consortium. Utili-zation of COVID-19
Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19
and Cancer Consortium (CCC19) Cohort Study. Cancer Discov
2020;10:1514–1527. https://doi.org/10.1158/2159-8290.CD-20-0941
- Dexamethasone in Hospitalized Patients with Covid-19 - PubMed. https://pubmed.ncbi.nlm.nih.gov/32678530/ Accessed October 2 2021
- Dulek
DE, Fuhlbrigge RC, Tribble AC, Connelly JA, Loi MM, El Chebib H,
Chandrakasan S, Otto WR, Diorio C, Keim G, Walkovich K, Jaggi P,
Girotto JE, Yarbrough A, Behrens EM, Cron RQ, Bassiri H.
Multidisciplinary Guidance Regarding the Use of Immunomodulatory
Therapies for Acute Coronavirus Disease 2019 in Pediatric Patients. J
Pediatr Infect Dis Soc 2020;9:716–737.
https://doi.org/10.1093/jpids/piaa098
- Beigel
JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E,
Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW,
Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA,
Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M-D, Ruiz-Palacios GM,
Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren
J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M,
Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members.
Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med
2020;383:1813–1826. https://doi.org/10.1056/NEJMoa2007764
- Chiotos
K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, Yarbrough A,
Ab-zug MJ, MacBrayne CE, Soma VL, Dulek DE, Vora SB, Waghmare A, Wolf
J, Olivero R, Grapentine S, Wattier RL, Bio L, Cross SJ, Dillman NO,
Downes KJ, Oliveira CR, Timberlake K, Young J, Orscheln RC, Tamma PD,
Schwenk HT, Zachariah P, Aldrich ML, Goldman DL, Groves HE, Rajapakse
NS, Lamb GS, Tribble AC, Hersh AL, Thorell EA, Denison MR, Ratner AJ,
New-land JG, Nakamura MM. Multicenter Interim Guidance on Use of
Antivirals for Children With Coronavirus Disease 2019/Severe Acute
Respiratory Syndrome Coronavirus 2. J Pediatr Infect Dis Soc
2021;10:34–48. https://doi.org/10.1093/jpids/piaa115
- Chen
P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona
J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL,
Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P,
Skovronsky DM, BLAZE-1 Investigators. SARS-CoV-2 Neutralizing Antibody
LY-CoV555 in Outpatients with Covid-19. N Engl J Med 2021;384:229–237.
https://doi.org/10.1056/NEJMoa2029849
- Dougan
M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, Perry
R, Bos-cia J, Heller B, Morris J, Crystal C, Igbinadolor A, Huhn G,
Cardona J, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL,
Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE,
Kallewaard NL, Sabo J, Patel DR, Dabora MC, Klekotka P, Shen L,
Skovronsky DM, BLAZE-1 Investigators. Bamlanivimab plus Etesevimab in
Mild or Moderate Covid-19. N Engl J Med 2021.
https://doi.org/10.1056/NEJMoa2102685
- Weinreich
DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo
Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD,
Turner KC, Hooper AT, Hamil-ton JD, Baum A, Kyratsous CA, Kim Y, Cook
A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T,
Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD, Trial
Inves-tigators. REGN-COV2, a Neutralizing Antibody Cocktail, in
Outpatients with Covid-19. N Engl J Med 2021;384:238–251.
https://doi.org/10.1056/NEJMoa2035002
- PINHO
AC. EMA reviewing data on monoclonal antibody use for COVID-19. In:
Eur. Med. Agency.
https://www.ema.europa.eu/en/news/ema-reviewing-data-monoclonal-antibody-use-covid-19
Accessed September 25 2021
- Commissioner
O of the. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal
Antibody for Treatment of COVID-19. In: FDA.
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
Accessed September 25 2021
- Outcomes of
active cancer patients with COVID-19 infection treated with COVID-19
neu-tralizing monoclonal antibodies. | Journal of Clinical Oncology.
https://ascopubs.org/doi/abs/10.1200/JCO.2021.39.15_suppl.3137 Accessed
September 24 2021
- Wolf J, Abzug MJ,
Wattier RL, Sue PK, Vora SB, Zachariah P, Dulek DE, Waghmare A, Olivero
R, Downes KJ, James SH, Pinninti SG, Yarbrough A, Aldrich ML, MacBrayne
CE, Soma VL, Grapentine SP, Oliveira CR, Hayes M, Kimberlin DW, Jones
SB, Bio LL, Morton TH, Hankins JS, Maron GM, Timberlake K, Young JL,
Orscheln RC, Schwenk HT, Goldman DL, Groves HE, Huskins WC, Rajapakse
NS, Lamb GS, Tribble AC, Lloyd EC, Hersh AL, Thorell EA, Ratner AJ,
Chiotos K, Nakamura MM. Initial Guidance on Use of Monoclonal Antibody
Therapy for Treat-ment of Coronavirus Disease 2019 in Children and
Adolescents. J Pediatr Infect Dis Soc 2021;10:629–634.
https://doi.org/10.1093/jpids/piaa175
- Simonovich
VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, Savoy
N, Giunta DH, Pérez LG, Sánchez MDL, Gamarnik AV, Ojeda DS, Santoro DM,
Camino PJ, Ante-lo S, Rainero K, Vidiella GP, Miyazaki EA, Cornistein
W, Trabadelo OA, Ross FM, Spotti M, Fun-towicz G, Scordo WE, Losso MH,
Ferniot I, Pardo PE, Rodriguez E, Rucci P, Pasquali J, Fuentes NA,
Esperatti M, Speroni GA, Nannini EC, Matteaccio A, Michelangelo HG,
Follmann D, Lane HC, Belloso WH, PlasmAr Study Group. A Randomized
Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med
2021;384:619–629. https://doi.org/10.1056/NEJMoa2031304
- RECOVERY
Collaborative Group. Convalescent plasma in patients admitted to
hospital with COVID-19 (RECOVERY): a randomised controlled, open-label,
platform trial. Lancet Lond Engl 2021;397:2049–2059.
https://doi.org/10.1016/S0140-6736(21)00897-7
- Joyner
MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES,
Wiggins CC, Bruno KA, Klompas AM, Lesser ER, Kunze KL, Sexton MA, Diaz
Soto JC, Baker SE, Shep-herd JRA, van Helmond N, Verdun NC, Marks P,
van Buskirk CM, Winters JL, Stubbs JR, Rea RF, Hodge DO, Herasevich V,
Whelan ER, Clayburn AJ, Larson KF, Ripoll JG, Andersen KJ, Bu-ras MR,
Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Paneth NS,
Fairweather D, Wright RS, Casadevall A. Convalescent Plasma Antibody
Levels and the Risk of Death from Covid-19. N Engl J Med
2021;384:1015–1027. https://doi.org/10.1056/NEJMoa2031893
- Libster
R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I,
Caballero MT, Wood C, Berrueta M, Rondan A, Lescano G, Cruz P, Ritou Y,
Fernández Viña V, Álvarez Paggi D, Esperante S, Ferreti A, Ofman G,
Ciganda Á, Rodriguez R, Lantos J, Valentini R, Itcovici N, Hintze A,
Oyarvide ML, Etchegaray C, Neira A, Name I, Alfonso J, López Castelo R,
Caruso G, Rapelius S, Alvez F, Etchenique F, Dimase F, Alvarez D,
Aranda SS, Sánchez Yanotti C, De Luca J, Jares Baglivo S, Laudanno S,
Nowogrodzki F, Larrea R, Silveyra M, Leberzstein G, Debonis A, Molinos
J, González M, Perez E, Kreplak N, Pastor Argüello S, Gibbons L,
Althabe F, Bergel E, Po-lack FP, Fundación INFANT–COVID-19 Group. Early
High-Titer Plasma Therapy to Prevent Se-vere Covid-19 in Older Adults.
N Engl J Med 2021;384:610–618. https://doi.org/10.1056/NEJMoa2033700
- Gharbharan
A, GeurtsvanKessel CH, Jordans CCE, Blaauw M, van der Klift M, Hassing
R-J, Smits-Zwinkels M, Meertens M, van den Hout EC, de Man AM, Hageman
I, Bogers S, van der Schoot CE, Swaneveld F, Anas AA, Rokx C, Rijnders
BJA. Effects of treatment of COVID-19 with convalescent plasma in 25
B-cell depleted patients. Clin Infect Dis Off Publ Infect Dis Soc Am
ciab647 2021. https://doi.org/10.1093/cid/ciab647
- Thompson
MA, Henderson JP, Shah PK, Rubinstein SM, Joyner MJ, Choueiri TK, Flora
DB, Griffiths EA, Gulati AP, Hwang C, Koshkin VS, Papadopoulos EB,
Robilotti EV, Su CT, Wulff-Burchfield EM, Xie Z, Yu PP, Mishra S,
Senefeld JW, Shah DP, Warner JL, COVID-19 and Cancer Consortium.
Association of Convalescent Plasma Therapy With Survival in Patients
With Hematologic Cancers and COVID-19. JAMA Oncol 2021.
https://doi.org/10.1001/jamaoncol.2021.1799
- Lyman
GH, Desai A, Leyfman Y, Kuderer NM. Opportunities and Challenges of
Observa-tional Studies and Randomized Controlled Trials for Evaluating
the Therapeutic Efficacy of COVID-19 Convalescent Plasma. Cancer Invest
2021;39:449–456. https://doi.org/10.1080/07357907.2021.1942127
- Joyner
MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, Wiggins CC,
Sene-feld JW, Klompas AM, Hodge DO, Shepherd JRA, Rea RF, Whelan ER,
Clayburn AJ, Spiegel MR, Baker SE, Larson KF, Ripoll JG, Andersen KJ,
Buras MR, Vogt MNP, Herasevich V, Dennis JJ, Regimbal RJ, Bauer PR,
Blair JE, van Buskirk CM, Winters JL, Stubbs JR, van Helmond N,
Butterfield BP, Sexton MA, Diaz Soto JC, Paneth NS, Verdun NC, Marks P,
Casadevall A, Fair-weather D, Carter RE, Wright RS. Safety Update:
COVID-19 Convalescent Plasma in 20,000 Hos-pitalized Patients. Mayo
Clin Proc 2020;95:1888–1897.
https://doi.org/10.1016/j.mayocp.2020.06.028
- Zaffanello
M, Piacentini G, Nosetti L, Franchini M. The use of convalescent plasma
for pe-diatric patients with SARS-CoV-2: A systematic literature
review. Transfus Apher Sci Off J World Apher Assoc Off J Eur Soc
Haemapheresis 2021;60:103043.
https://doi.org/10.1016/j.transci.2020.103043
- Campochiaro
C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, Tomelleri A,
Bal-dissera E, Rovere-Querini P, Ruggeri A, Monti G, De Cobelli F,
Zangrillo A, Tresoldi M, Castagna A, Dagna L, TOCI-RAF Study Group.
Efficacy and safety of tocilizumab in severe COVID-19 pa-tients: a
single-centre retrospective cohort study. Eur J Intern Med
2020;76:43–49. https://doi.org/10.1016/j.ejim.2020.05.021
- Rojas-Marte
G, Khalid M, Mukhtar O, Hashmi AT, Waheed MA, Ehrlich S, Aslam A,
Sid-diqui S, Agarwal C, Malyshev Y, Henriquez-Felipe C, Sharma D,
Sharma S, Chukwuka N, Rodri-guez DC, Alliu S, Le J, Shani J. Outcomes
in patients with severe COVID-19 disease treated with tocilizumab: a
case-controlled study. QJM Mon J Assoc Physicians 2020;113:546–550.
https://doi.org/10.1093/qjmed/hcaa206
- Somers
EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, Zhou N,
Petty LA, Baang JH, Dillman NO, Frame D, Gregg KS, Kaul DR, Nagel J,
Patel TS, Zhou S, Lauring AS, Hanauer DA, Martin E, Sharma P, Fung CM,
Pogue JM. Tocilizumab for Treatment of Mechanically Ventilated Patients
With COVID-19. Clin Infect Dis Off Publ Infect Dis Soc Am
2021;73:e445–e454. https://doi.org/10.1093/cid/ciaa954
- Giesen
N, Sprute R, Rüthrich M, Khodamoradi Y, Mellinghoff SC, Beutel G, Lueck
C, Koldehoff M, Hentrich M, Sandherr M, von Bergwelt-Baildon M, Wolf
H-H, Hirsch HH, Wör-mann B, Cornely OA, Köhler P, Schalk E, von
Lilienfeld-Toal M. Evidence-based management of COVID-19 in cancer
patients: Guideline by the Infectious Diseases Working Party (AGIHO) of
the German Society for Haematology and Medical Oncology (DGHO). Eur J
Cancer Oxf Engl 1990 2020;140:86–104.
https://doi.org/10.1016/j.ejca.2020.09.009
- Goldenberg
NA, Sochet A, Albisetti M, Biss T, Bonduel M, Jaffray J, MacLaren G,
Mon-agle P, O'Brien S, Raffini L, Revel-Vilk S, Sirachainan N, Williams
S, Zia A, Male C, Pediat-ric/Neonatal Hemostasis and Thrombosis
Subcommittee of the ISTH SSC. Consensus-based clinical recommendations
and research priorities for anticoagulant thromboprophylaxis in
children hospital-ized for COVID-19-related illness. J Thromb Haemost
JTH 2020;18:3099–3105. https://doi.org/10.1111/jth.15073
- Loi
M, Branchford B, Kim J, Self C, Nuss R. COVID-19 anticoagulation
recommendations in children. Pediatr Blood Cancer 2020;67:e28485.
https://doi.org/10.1002/pbc.28485
- Kamidani
S, Rostad CA, Anderson EJ. COVID-19 vaccine development: a pediatric
per-spective. Curr Opin Pediatr 2021;33:144–151.
https://doi.org/10.1097/MOP.0000000000000978
- Frenck
RW, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL,
Walter EB, Senders S, Bailey R, Swanson KA, Ma H, Xu X, Koury K, Kalina
WV, Cooper D, Jennings T, Brandon DM, Thomas SJ, Türeci Ö, Tresnan DB,
Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001
Clinical Trial Group. Safety, Immunogenicity, and Efficacy of the
BNT162b2 Covid-19 Vaccine in Adolescents. N Engl J Med
2021;385:239–250. https://doi.org/10.1056/NEJMoa2107456
- Ali
K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, Ding B,
Dooley J, Girard B, Hillebrand W, Pajon R, Miller JM, Leav B, McPhee R.
Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N Engl J Med
2021. https://doi.org/10.1056/NEJMoa2109522
- Walter
EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, Barnett
ED, Muñoz FM, Maldonado Y, Pahud BA, Domachowske JB, Simões EAF, Sarwar
UN, Kitchin N, Cunliffe L, Rojo P, Kuchar E, Rämet M, Munjal I, Perez
JL, Frenck RW Jr, Lagkadinou E, Swanson KA, Ma H, Xu X, Koury K, Mather
S, Belanger TJ, Cooper D, Türeci Ö, Dormitzer PR, Şahin U, Jansen KU,
Gruber WC; C4591007 Clinical Trial Group. Evaluation of the BNT162b2
Covid-19 Vaccine in Children 5 to 11 Years of Age. N Engl J Med. 2021
Nov 9. https://doi.org/10.1056/NEJMoa2116298 Epub ahead of print.
PMID:34752019.
- Mevorach D, Anis E,
Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Has-in
T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D,
Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W,
Muhsen K, Levi Y, Green MS, Keinan-Boker L, Al-roy-Preis S. Myocarditis
after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med.
2021 Oct 6:NEJMoa2109730. https://doi.org/10.1056/NEJMoa2109730. Epub
ahead of print. PMID: 34614328; PMCID: PMC8531987
- Monin
L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I,
Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, Abdul-Jawad
S, Kamdar S, Harvey-Jones E, Graham R, Cooper J, Khan M, Vidler J,
Kakkassery H, Sinha S, Davis R, Dupont L, Francos Qui-jorna I,
O'Brien-Gore C, Lee PL, Eum J, Conde Poole M, Joseph M, Davies D, Wu Y,
Swampillai A, North BV, Montes A, Harries M, Rigg A, Spicer J, Malim
MH, Fields P, Patten P, Di Rosa F, Papa S, Tree T, Doores KJ, Hayday
AC, Irshad S. Safety and immunogenicity of one versus two doses of the
COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of
a prospec-tive observational study. Lancet Oncol 2021;22:765–778.
https://doi.org/10.1016/S1470-2045(21)00213-8
- Benjamini
O, Rokach L, Itchaki G, Braester A, Shvidel L, Goldschmidt N, Shapira
S, Dally N, Avigdor A, Rahav G, Lustig Y, Ben David SS, Fineman R, Paz
A, Bairey O, Polliack A, Levy I, Tadmor T. Safety and efficacy of
BNT162b mRNA Covid19 Vaccine in patients with chronic lym-phocytic
leukemia. Haematologica 2021.
https://doi.org/10.3324/haematol.2021.279196
- Greenberger
LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL.
An-tibody response to SARS-CoV-2 vaccines in patients with hematologic
malignancies. Cancer Cell 2021;39:1031–1033.
https://doi.org/10.1016/j.ccell.2021.07.012
- Perry
C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, Tabib Y, Cohen
YC, Benyamini N, Beyar-Katz O, Neaman M, Vitkon R, Keren-Khadmy N,
Levin M, Herishanu Y, Avivi I. Efficacy of the BNT162b2 mRNA COVID-19
vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv
2021;5:3053–3061. https://doi.org/10.1182/bloodadvances.2021005094
- Herzog
Tzarfati K, Gutwein O, Apel A, Rahimi-Levene N, Sadovnik M, Harel L,
Benven-iste-Levkovitz P, Bar Chaim A, Koren-Michowitz M. BNT162b2
COVID-19 vaccine is significant-ly less effective in patients with
hematologic malignancies. Am J Hematol 2021;96:1195–1203.
https://doi.org/10.1002/ajh.26284
- Redjoul
R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response
after second BNT162b2 dose in allogeneic HSCT recipients. Lancet Lond
Engl 2021;398:298–299. https://doi.org/10.1016/S0140-6736(21)01594-4
- Maneikis
K, Šablauskas K, Ringelevičiūtė U, Vaitekėnaitė V, Čekauskienė R,
Kryžauskaitė L, Naumovas D, Banys V, Pečeliūnas V, Beinortas T,
Griškevičius L. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine
and early clinical outcomes in patients with haematological
malignancies in Lithuania: a national prospective cohort study. Lancet
Haematol 2021;8:e583–e592.
https://doi.org/10.1016/S2352-3026(21)00169-1
- Association
(EHA) TEH Expert opinions for COVID-19 vaccination in patients with
hematologic cancer. In: Eur. Hematol. Assoc. EHA.
https://ehaweb.org/covid-19/eha-statement-on-covid-19-vaccines/recommendations-for-covid-19-vaccination-in-patients-with-hematologic-cancer/
Accessed September 25 2021
- COVID-19 Vaccine. http://www.bccdc.ca/health-info/diseases-conditions/covid-19/covid-19-vaccine. Accessed September 25 2021
- Revon-Riviere
G, Ninove L, Min V, Rome A, Coze C, Verschuur A, de Lamballerie X,
An-dré N. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young
adults with cancer: A monocentric experience. Eur J Cancer Oxf Engl
1990 2021;154:30–34. https://doi.org/10.1016/j.ejca.2021.06.002
- Mark
C, Gupta S, Punnett A, Upton J, Orkin J, Atkinson A, Clarke L, Heisey
A, McGovern C, Alexander S. Safety of administration of BNT162b2 mRNA
(Pfizer-BioNTech) COVID-19 vac-cine in youths and young adults with a
history of acute lymphoblastic leukemia and allergy to
PEG-asparaginase. Pediatr Blood Cancer 2021;e29295.
https://doi.org/10.1002/pbc.29295
- Tande
AJ, Pollock BD, Shah ND, Farrugia G, Virk A, Swift M, Breeher L,
Binnicker M, Berbari EF. Impact of the COVID-19 Vaccine on Asymptomatic
Infection Among Patients Under-going Pre-Procedural COVID-19 Molecular
Screening. Clin Infect Dis Off Publ Infect Dis Soc Am 2021;ciab229.
https://doi.org/10.1093/cid/ciab229
[TOP]


