The ATRA/ATO combination may cause hyperleukocytosis and is associated with side effects such as pseudotumor cerebri, hepatotoxicity, electrocardiogram abnormalities, and differentiation syndrome (DS).[5] The early diagnosis of DS can be challenging as signs and symptoms are frequently non-specific. Most commonly seen are unexplained fever, peripheral edema, dyspnea, weight gain, pulmonary infiltrates and pericardial or pleural effusion, hypotension, and renal failure.[5,6] The pathogenesis of this complication is not fully understood; ATRA/ATO combination determines the differentiation of immature promyelocytes into mature granulocytes, and DS symptoms are probably related to the burden of differentiating blasts and the subsequent release of cytokines and tissue infiltration. Dexamethasone (DXM) is considered the mainstay of treatment of DS and should be administered at the first signs or symptoms.[5,6] However, severe cases may require the interruption of ATRA/ATO treatment, administration of chemotherapy to control the WBC count, and intensive interventions such as respiratory support.[7] The information on ATRA/ATO side effects in childhood is still limited. We report the difficult management of DS in two pediatric cases during ATRA/ATO induction therapy.
In the period December 1, 2019 - December 31, 2020, 8 children with newly diagnosed APL underwent induction therapy with ATRA/ATO in our Center; in patients with WBC≥10x109/L, two doses of Gemtuzumab Ozogamicin (GO 3 mg/m2/day) were added, in agreement with the MD Anderson Cancer Center experience8. Prednisone (PDN, 0.5 mg/kg/day) was given from day 1 to day 15 of induction to prevent DS; if DS occurred, PDN was replaced by DXM (10 mg/m2/day i.v). Hydroxyurea (HU, 20-60 mg/kg/day) was given in case of sustained leukocytosis (WBC > 20x109/L for at least 3 days). Temporary discontinuation of ATRA, and if needed of ATO, was done in case of pseudotumor cerebri or other relevant DS symptoms.[5-8]
Table 1, summarizes patients clinical and biological characteristics at disease presentation; 5 patients were standard-risk (SR; WBC<10x109/L) and 3 high-risk (HR; WBC≥10x109/L). All 5 SR patients developed hyperleukocytosis during induction, requiring discontinuation of ATRA and/or ATO and administration of HU. Three of them presented with DS. Out of 3 HR patients, the first did well, the second developed pseudotumor cerebri treated with DXM and acetazolamide with complete resolution, and the third presented DS with respiratory failure. All eight patients achieved a complete molecular remission and are alive and disease-free; all are off therapy.
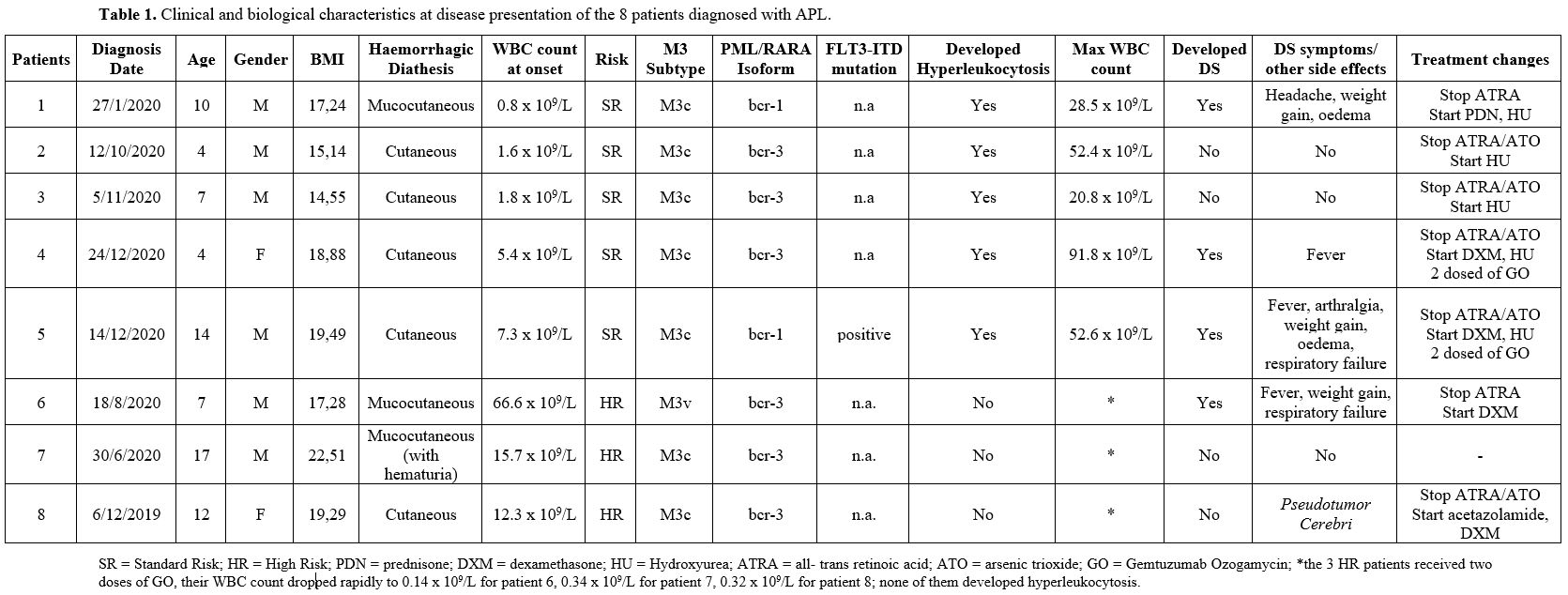 |
Table 1. Clinical and biological characteristics at disease presentation of the 8 patients diagnosed with APL. |
Of particular interest is the description of two cases of DS that occurred in 2 SR patients.
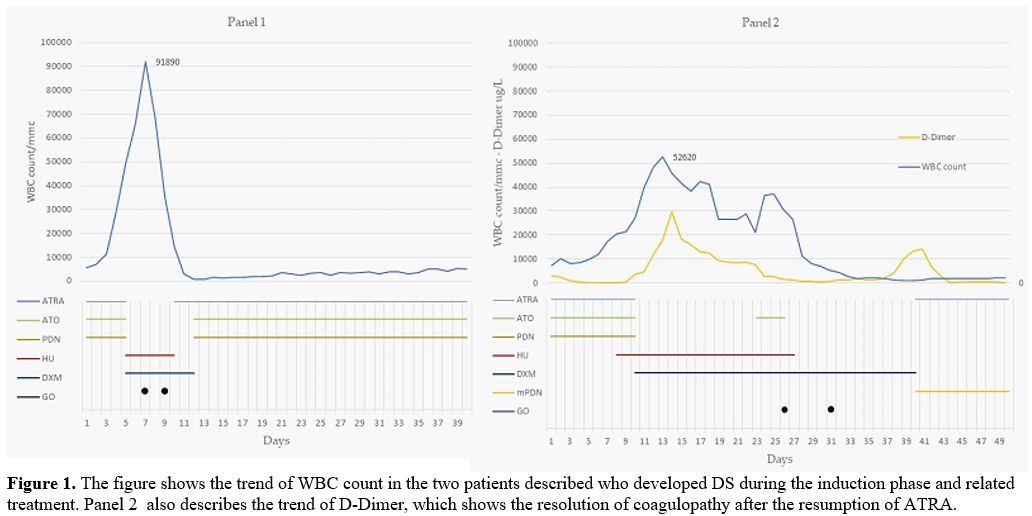
Patient 4 (Figure 1, Panel 1): a 4 years old girl presented with WBC count 5.4x109/L; on day 5, due to progressive hyperleukocytosis (WBC 49.1x109/L), worsening of unexplained fever and clinical status, treatment with ATRA and ATO was interrupted; DXM and HU were started. WBC count, however, continued to increase (WBC 91.8x109/L, day 7), with peripheral blood smear showing 83% of blasts. Two doses of GO were thus administered, inducing profound aplasia lasting ten days without infectious complications. On day 12, treatment with ATRA, ATO, and PDN was resumed without any problems until the end of the induction phase on day 40 (32 and 36 days of ATO and ATRA, respectively).
Patient 5 (Figure 1, Panel 2): a 14 years old boy presented with WBC count 7.3x109/L; on day 10, he developed fever, arthromyalgia, chest pain, weight gain, and sustained hyperleukocytosis (WBC 27.2x109/L). Due to the poor clinical status, treatment with ATRA/ATO was interrupted, and DXM and HU were started with slow resolution of symptoms. However, the patient continued to present hyperleukocytosis (WBC 30x109/L), thrombocytopenia, and worsening coagulopathy with atypical not differentiating promyelocytes in peripheral blood. On day 23, treatment with ATO was resumed. Three days later, the patient developed progressive weight gain and respiratory failure requiring support with CPAP. ATO was interrupted again, and two doses of GO (days 26 and 31) were administered with progressive improvement of respiratory function and decreased WBC count (nadir WBC 0.94x109/L, on day 40). Unfortunately, coagulopathy worsened after a transient improvement, in association with unexplained fever and 80% of bone marrow blasts. Treatment with ATRA was resumed and DXM was substituted for methylprednisolone with rapid resolution of signs and symptoms and continued without ATO until the end of the induction phase on day 72 (10 and 33 days of ATO and ATRA, respectively). In this patient, hyperleukocytosis was associated with striking alterations of blasts morphology; the majority of them looked like atypical monocytes, raising the suspicion of the presence of another leukemic clone. These cells were sorted and documented to be positive for the PML-RARA rearrangement.
In pediatric APL, the experience with ATRA/ATO in a frontline chemo-free treatment is still limited and is currently under investigation in clinical trials. Previous experiences in a limited number of children have been reported.[3,9] Treatment has resulted highly effective with the achievement of molecular CR in almost all treated patients. The major risks of the treatment with differentiating agents in APL are the increase of WBC count and DS. The incidence of hyperleukocytosis in children during ATRA/ATO induction seems to be higher than in adults, reaching more than 60% in some series.[10] Patients with hyperleukocytosis (WBC ≥ 20x109/L) are at higher risk to develop DS, which can occur at any time during the induction therapy, most commonly around day 7.[3,4,9] DS symptoms are frequently non-specific, and the alternative causes explaining the clinical features should be ruled out. The prompt treatment with steroids reduces the severity of DS and continues both ATRA and ATO in the majority of adult and pediatric patients.[4,5] However, the clinical manifestations of DS may be serious and life-threatening, requiring the interruption of treatment, administration of chemotherapy, and intensive supportive care. In the pediatric age, the diagnosis and management of this complication, and the modulation of therapy with differentiating agents, are not yet fully established. In a PETHEMA study, other pretreatment variables, such as FLT3-ITD mutation, microgranular FAB subtype, short PML-RARA isoform (bcr3), male gender, and serum creatinine, were predictive of severe DS; however, the multivariate analysis reduced the significant variables to WBC count and serum creatinine level.[11] An increased BMI at diagnosis is also associated with a higher risk of developing DS; in some pediatric and adult APL series, BMI was one of the most powerful predictors of the syndrome.[12]
In our experience, ATRA/ATO toxicity, in keeping with the literature, occurred mostly in the first two weeks of treatment. This experience confirms that the management of DS in childhood may be challenging, requiring either discontinuation of one or both differentiating agents and intensive supportive treatment, including the administration of HU, which, especially in a young child, may not be sufficient to control WBC count increase and DS symptoms. Of interest, 2 SR patients benefited from treatment with two GO doses. However, both developed profound and protracted aplasia, suggesting that a different dosage or schedule may be appropriate. Since APL is rare in the pediatric age, it remains difficult to establish solid evidence-based guidelines; therefore, it is paramount to gather all possible information on the clinical course of such patients.
Overall, and even with the limitations of the number of patients treated, our data suggest that differentiating agents ATRA/ATO-related complications may be more pronounced during the induction phase for APL in children than in adults. The relevance of these aspects requires thorough attention in managing chemo-free schedules and needs to be confirmed in larger cohorts. Controlled large studies are thus still needed to optimize the ATRA/ATO combination treatment of APL in childhood to reduce DS toxicity without jeopardizing the outcome.