Mervat M. Khorshied1, Iman A. Shaheen1, Yasmeen M.M. Selim2, Asmaa O. Elshahawy1 and Ilham Youssry2.
1 Department of Clinical and Chemical Pathology, Faculty of Medicine, Cairo University, Egypt.
2 Department of Pediatrics, Pediatric Hematology and BMT Unit, Faculty of Medicine, Cairo University, Egypt.
Correspondence to:
Mervat Mamdooh Khorshied. Professor of Clinical and Chemical Pathology,
Consultant of Hematology, Faculty of Medicine, Cairo University, Egypt.
Postal address: 23 Gezeret El Arab street, Al Mohandeseen, Giza, Egypt,
Postal code: 12411. Tel.: +202 33037080 Cellular phone: 01001593441.
E-mail:
mervatkhorshied@kasralainy.edu.eg,
mervatkhorshied@hotmail.com ORCID ID: 0000-0003-2052-3768
Published: May 1, 2022
Received: January 29, 2022
Accepted: April 13, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022037 DOI
10.4084/MJHID.2022.037
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Oxidative stress plays a pivotal role in the pathophysiology of sickle
cell disease (SCD) and its associated disease complications. Superoxide
Dismutases (SODs) are protective enzymes against oxidative stress. SOD2
deficiency results in accumulation of oxidized red cell proteins,
increased rate of hemoglobin oxidation, decreased red cell membrane
deformability and subsequently decreased red cells survival.
Objective:
The current study was designed to determine the effect of SOD2 Val16Ala
gene polymorphism (rs4880) on SOD2 level and their possible impact on
SCD disease severity in a cohort of Egyptian SCD patients.
Methods:
Genotyping of SOD2 Val16Ala polymorphism by TaqMan allelic
discrimination assay for hundred SCD patients and a hundred age-sex
matched healthy controls revealed the genotypic and allelic frequencies
of the studied polymorphism in the SCD patients were close to that of
the controls.
Results:
Serum SOD2 level was significantly lower in those having the
polymorphic genotypes (p=0.005). SOD2 level inversely correlates with
the annual rate of hospitalization (r=-0.023, p= 0.038).
Conclusion: SOD2 Val16Ala polymorphism was associated with low serum SOD2 level that may predict disease severity.
|
Introduction
Sickle
cell disease (SCD) is coupled with a state of chronic inflammation,
ischemia-reperfusion injury and increased production of Reactive Oxygen
species (ROS) such as superoxide and hydrogen peroxide up to twice the
normal.[1] This state is often aggravated when patients are exposed to pathologic conditions like acidosis, infections and dehydration.[2,3] Oxidative stress plays a pivotal role in the pathophysiology of SCD and its associated complications.[4,5]
This high oxidative stress and an unbalanced oxidant/antioxidant status
could contribute to the pathogenesis of SCD related complications.[5,6]
Superoxide
Dismutase (SOD) is a key enzyme in the dismutation of superoxide
radicals which result from cellular oxidative metabolism into hydrogen
peroxide. It is an indispensable antioxidant defense system, especially
in the cells involved in aerobic cellular metabolism.[7]
There are three distinct types of superoxide dismutase (SOD) in human
cells: a homodimeric cytosolic Cu/Zn-SOD, an extracellular
homotetrameric glycosylated SOD, and a mitochondrial matrix
homotetrameric Mn-SOD.[8] Mitochondrial manganese
superoxide dismutase (Mn-SOD), encoded by the SOD2 gene, represents a
major cellular antioxidant. Its deficiency results in the accumulation
of oxidized red cell proteins, increased rate of hemoglobin oxidation,
decreased red cell membrane deformability and subsequently decreased
red cells survival.[9,10]
Previous reports showed
that sickled red blood cells produce twice as much superoxide, hydrogen
peroxide, and hydroxyl radicals as normal healthy controls.[11]
The antioxidant defense systems in sickle patients are suboptimal, thus
ineffectively neutralizing the excess pro-oxidant produced.[12,13]
Furthermore, individuals presenting a genetic predisposition to have
lower SOD levels would have difficulties compensating for the ROS
triggered by sickling cells.[14] The current study
was designed to determine the effect of SOD2 Val16Ala gene polymorphism
(rs4880) on SOD2 level and their possible impact on SCD disease
severity in a cohort of Egyptian SCD patients.
Materials and Methods
Study population.
The current cross-sectional study included 100 Egyptian SCD patients in
a steady-state, which was defined as the period free of crisis
extending from at least 3 weeks since the last clinical event and 3
months or more since the last blood transfusion to at least one week
before the start of a new clinical event. Patients were selected from
the Hematology Outpatient Clinic of the New Cairo University Children's
Hospital. Consanguinity was found in 49% of the studied patients, and
their ages ranged between 1 and 37 years, with a mean age of 17.56±
6.77 years, 58 were males, and 42 were females, with a male to female
ratio of 1.3. In addition, demographic, clinical and laboratory data
were retrieved from patients' files. Diagnosis and phenotyping of SCD
patients was based on Alkaline Hemoglobin Electrophoresis and
High-Performance Liquid Chromatography (HPLC). Fifty-five (55%) patients
had sickle cell anemia (HbSS), forty-three (43%)
were double heterozygous for sickle-beta-thalassemia (HbSβ), one (1%)
patient was double heterozygous for hemoglobin C & S (HbSC) and one
(1%) patient was double heterozygous for hereditary persistence of
fetal hemoglobin & Hemoglobin S (HbS/HPFH).
Twenty-eight
(28%) patients were transfusion-dependent, and 14% were splenectomized.
More than 90% of our patients were on hydroxyurea therapy, and 43%
received iron chelation therapy in the form of deferoxamine (7/43),
deferiprone (35/43) and deferasirox (6/43); some were on combined
therapy with a median serum ferritin level of 540 ng/mL (IQR 171-992
ng/mL). Disease severity score was calculated, and SCD patients were
stratified into three groups; mild (<3), moderate (>3 ≤ 5) and
severe cases (>5) (Table 1).[15]
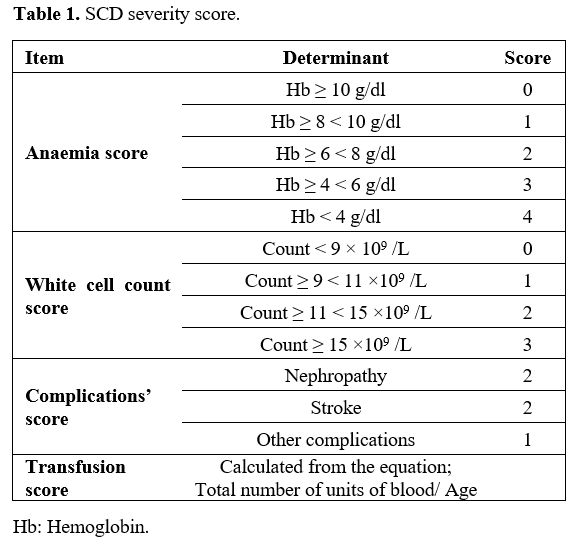 |
Table
1. SCD severity score. |
Hundred
healthy age and sex-matched children with normal hematological
parameters were included in the study as a control group. The study was
approved by the Research Ethics Committees of the Clinical Pathology
Department and Department of Paediatrics, Institutional Review Board
(IRB) - Faculty of Medicine, Cairo University. Informed consent was
obtained from the patients or their guardians before enrolment in the
study. All procedures performed were in accordance with the
recommendation of the Declaration of Helsinki, 1964 and its later
amendments or comparable ethical standards.
Genotyping of SOD2 Val16Ala polymorphism.
ACCORDING TO THE MANUFACTURER'S INSTRUCTIONS, genomic DNA was extracted
from whole blood samples using GeneJET Whole Blood Genomic DNA
Purification Mini Kit (cat no #K0781, Thermo Scientific, USA). The
concentration and purity of the DNA were assessed by spectrophotometer,
and samples were stored in the elution buffer at ‐20°C until being
used. Genotypic analysis was done by Applied Biosystem step one
Real-Time PCR System allelic discrimination assay. As previously
described, the assay was designed using Taq-Man SNP Genotyping Assays
(Assay ID: C_8709053_10, Applied Biosystems, Thermofisher, Foster City,
CA, USA).[14] 20% of the samples were randomly chosen
concerning case/control status and reanalyzed to validate our results.
The results were interpreted by different observers and were found to
be 100% concordant.
Measurement of SOD2 level in SCD.
Determination of serum SOD2 level in SCD patients was performed by
Enzyme-Linked Immunosorbent Assay (ELISA) (Catalog No: SG-10188,
SinoGeneclon, YuHang, China) according to manufacturers' instructions.
Statistical methods.
Data were statistically described in terms of mean ± Standard Deviation
(± SD), median and range (or IQR), or frequencies (number of cases) and
percentages when appropriate. Comparing numerical variables between the
study groups was done using the Mann Whitney U test for independent
samples for comparing two groups and the Kruskal Wallis test for
comparing more than two groups. For comparing categorical data,
Chi-square (χ2) test was performed. The exact test was used instead
when the expected frequency was less than 5. Correlation between
various variables was done using Pearson moment correlation equation
for linear relation of normally distributed variables and Spearman rank
correlation equation for non-normal variables/non-linear monotonic
relation. Two-sided p values less than 0.05 were considered
statistically significant. All statistical calculations were done using
the computer program IBM SPSS (Statistical Package for the Social
Science; IBM Corp, Armonk, NY, USA), release 22 for Microsoft Windows.
Results
Genotypic
analysis showed no statistically significant difference in the
genotypic and allelic distribution of SOD2 Val16Ala polymorphism
between SCD patients and controls (Table 2).
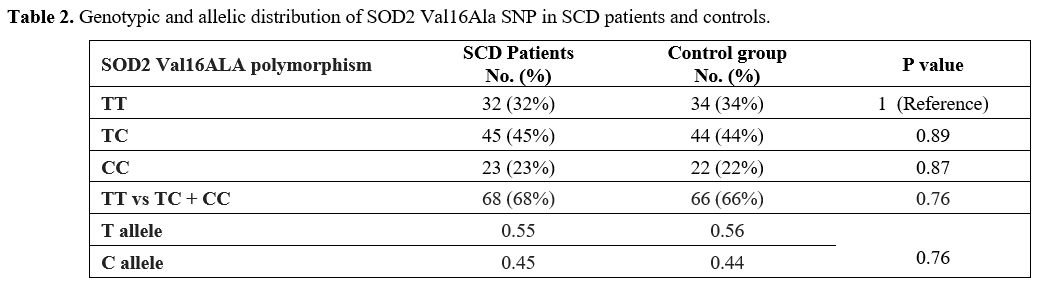 |
Table 2. Genotypic and allelic distribution of SOD2 Val16Ala SNP in SCD patients and controls. |
A
comparison between SCD patients having wild and the polymorphic
genotypes of SOD2 Val16Ala SNP revealed that hepatobiliary
complications (hepatomegaly and gall stones) were significantly lower
in polymorphic patients' genotypes (p=0.03). Neurological complications
(strokes and transient ischemic attacks) were reported in six patients,
and all of them had the homozygous polymorphic genotype (CC). SOD level
was significantly lower in patients having the polymorphic
(heteromutant and homomutant) genotypes (p=0.005) (Figure 1).
Otherwise, there was no statistically significant difference between
the two patients' groups regarding their demographic, clinical and
laboratory data (Table 3).
Based on the disease severity score, 27 patients (27%) were classified
as mild, 39 patients (39%) were classified as moderate, and 34 patients
(34%) were classified as severe. Comparing SOD2 levels between the SCD
patients stratified according to this score was statistically
insignificant (p=0.075) (Table 4).
No significant correlation was found between SOD2 level and any of the
different hemolytic indicators and disease-associated complications
apart from an inverse correlation between SOD level and the annual rate
of hospitalization (r=-0.023, p= 0.038) (Figure 2).
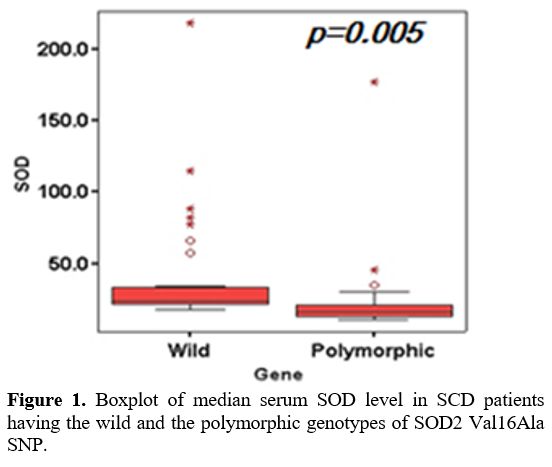 |
Figure 1. Boxplot of median serum SOD level in SCD patients having the wild and the polymorphic genotypes of SOD2 Val16Ala SNP. |
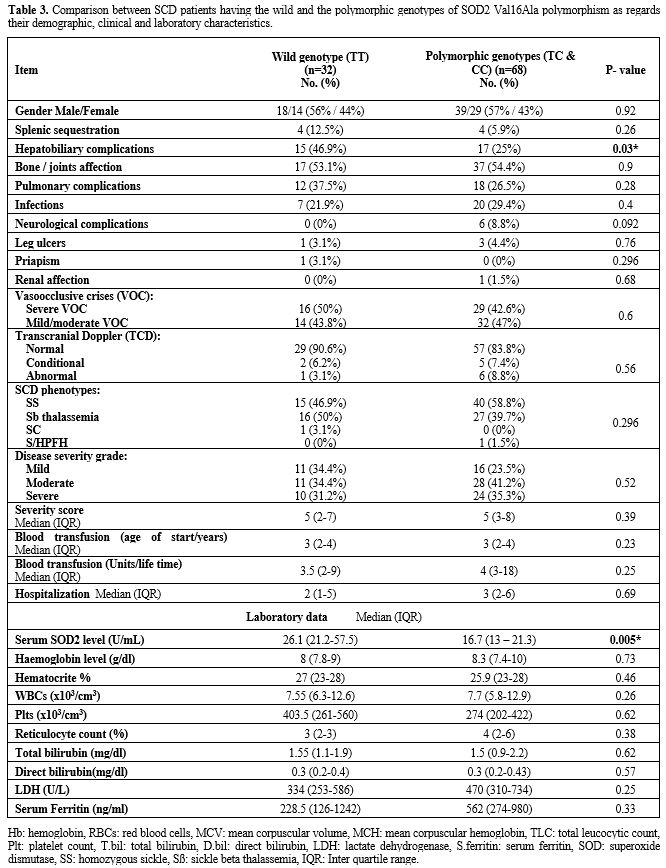 |
Table 3. Comparison
between SCD patients having the wild and the polymorphic genotypes of
SOD2 Val16Ala polymorphism as regards their demographic, clinical and
laboratory characteristics. |
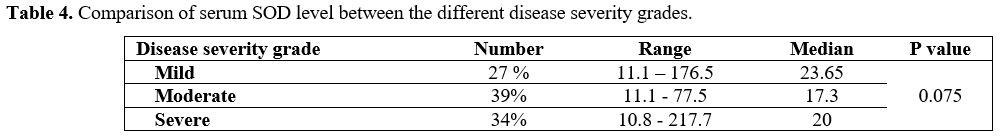 |
Table 4. Comparison of serum SOD level between the different disease severity grades. |
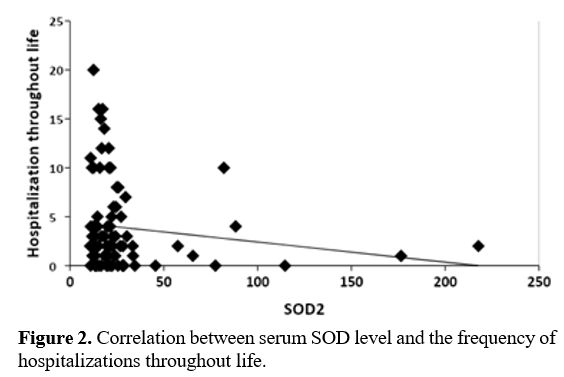 |
Figure
2. Correlation between serum SOD level and the frequency of hospitalizations throughout life.
|
Discussion
There
are contradictory reports on the level of various antioxidants in
patients with SCD, which warrant further investigation for
confirmation.[17] The antioxidant defense system is
significantly diminished through decreased expression and activity
levels of antioxidant enzymes, including superoxide dismutases (SOD),
catalases, and glutathione peroxidases.[1,12] Oppositely, Das and Nair[17]
stated that SOD levels were increased in sickle cell anemia patients
and postulated that this increase might be an adaptive defense
mechanism to counteract the increased oxidative stress. This imbalance
between oxidative stress and antioxidant defenses in SCD warranted
further investigations.
In our study, SOD2 Val16Ala polymorphic
(C) allele was 0.44 in healthy Egyptian controls. Similarly, it was
present in 45% of people with African ancestry[18,19] and close to that of Caucasians, being 0.5.[20]
SOD2 polymorphism has a wide range of allelic frequencies depending on
ethnicity being 0.65 in Latinos, 0.54 in South Asians, 0.52 in
Europeans (non-Finnish) and 0.16 in East Asians.[1] In
SCD patients, the heteromutant (TC) and homomutant (CC) genotypes were
detected in 45% and 23% of patients, respectively. The genotypic and
allelic frequencies of SOD2 Val16Ala polymorphism in our cohort of
Egyptian SCD patients were close to that reported in Brazilian
patients.[14] The polymorphic (C) allele frequency in our SCD patients was 0.44, while it was 0.35 in Turkish patients[20] and 0.52 in Brazilian patients.[14]
The Turkish and Brazilian studies and ours showed no statistical
difference in SOD2 allelic and genotypic frequencies between SCD
patients and healthy controls.
The high frequency of the SOD2
Val16Ala polymorphism and reported associations with sickle
complications emphasize the fundamental role that this polymorphism
could play in contributing to the phenotypic spectrum of the disease.[1]
In the current study, all the patients with neurological complications
had the homozygous polymorphic genotype (CC); nevertheless, there was
no statistically significant difference in the frequency of the
neurological complications between patients having the wild and those
having the polymorphic genotypes. However, Domingos et al.[22]
found that SOD2 Val16Ala polymorphism was independently associated with
risk of stroke (Odds ratio: 1.98; 95% confidence interval [CI]:
1.18-3.32; P = 0.009) and with the long term cumulative incidence of
stroke (hazard ratio: 2.24, 95% CI: 1.3-3.9; P = .004) in adults with
SCD.
Hepatobiliary complications (hepatomegaly and gall stones)
were significantly lower in patients with polymorphic genotypes (TC and
CC). It has been reported that human peripheral blood mononuclear cells
from the carriers of TT genotype of SOD2 produced more proinflammatory
cytokines such as IL-1, IL-6, TNF-α and IFN-γ than those having the CC
genotypes, which could affect the hepatocyte's function.[23] The pathology of chronic hemolytic status among SCD patients is a major contributing factor to gall stone formation.
The
frequency of patients with moderate and severe disease severity scores
was higher in patients with polymorphic genotypes than those with wild
ones, yet the difference did not reach a statistically significant
level. However, a recent study in Brazil on a pediatric SCD cohort
revealed that SOD2 polymorphism favors sickle vasoocclusive crises and
acute splenic sequestration.[13] The median serum
SOD2 level in our patients was 20.2 U/mL, almost two-fold that reported
in Nigerian sickle patients being 9.45±3.39 U/ml.[14]
In our study, the SOD2 level in patients with the polymorphic genotypes
of the studied SNP was higher than in the study of Farias et al..[14]
This difference could explain the association between decreased serum
SOD2 levels and both vasoocclusive crises (VOC) and acute splenic
sequestration crises found in their study and not in ours. The level of
enzymatic antioxidants such as SODs, catalases and glutathione
peroxidases were reduced in SCD.[24-26] In the
present study, serum SOD2 level was significantly lower in patients
with polymorphic genotypes (TC & CC). This datum is in line with
the study of Farias and coworkers.[14] A decreased SOD2 enzymatic level has also been observed in cryopreserved human hepatocytes[27] and isolated human erythrocytes with the Val16Ala polymorphism.[28] Studies about the effects of SOD2 as a marker of oxidative stress suggest a protective role of the T allele.[29,30]
Individuals carrying MnSOD TC/CC genotypes have increased DNA damage
induced by ROS compared with the TT genotype. Thus, although these
studies did not measure the functional enzyme activity, they support
the idea that the T allele could be a marker for ROS protection. Our
correlation studies revealed a significant inverse relationship between
SOD level and the annual rate of hospitalization, which may reflect the
protective role of SOD in SCD patients.
Although VOC is an important index of disease severity in sickle patients,[31]
there was no statistical correlation between SOD2 level and VOC or
other disease-associated complications in our study. Moreover, it did
not correlate with disease severity grades. On the contrary, Okocha et
al.[15] found an inverse correlation between SOD2
level and disease severity score in Nigerian patients. In this study,
the hemolytic indicators and serum ferritin did not correlate with SOD2
level or SOD2 Val16Ala polymorphism. Contrary to our results, Armenis
and coworkers[32] reported an inverse correlation
between serum SOD2 levels and serum ferritin and hemolytic indicators
in Greek SCD patients. Armenis et al. study[32] shows that SOD2 levels correlate with RBC count, reticulocyte count, platelet count, C-reactive protein and serum ferritin.
In
conclusion, our study revealed that SOD2 Val16Ala polymorphism was
associated with low serum SOD2 level. The combined analysis of SOD2
Val16Ala polymorphism and serum SOD2 level strongly suggests that the
studied SNP could significantly impact the serum levels and
subsequently influence the annual rate of hospitalization, which could
be considered a predictor for disease complications. For a greater
understanding of the pathogenesis of SCD, larger studies are needed to
evaluate whether genetic variations of the Mn-SOD gene contribute to
the disease-associated complications. In addition, further research is
warranted in larger sample sizes with other antioxidant enzymes to
clarify their impact on SCD associated morbidities.
Limitation of the study
It will be desirable to replicate this study with more SCD patients in both steady-state and crises.
Funding information
The authors did not receive support from any organization for the submitted work.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The
study was approved by the Research Ethics Committees of the Clinical
Pathology Department and Department of Paediatrics, Institutional
Review Board (IRB) - Faculty of Medicine, Cairo University.
Consent to participate
Informed
consents were obtained from the patients or their guardians before
enrolment in the study. All procedures performed were in accordance
with recommendation of the Declaration of Helsinki the 1964 and its
later amendments or comparable ethical standards.
References
- Dosunmu-Ogunbi AM, Wood KC, Novelli EM, Straub AC.
Decoding the role of SOD2 in sickle cell disease. Blood Adv. 2019;
3(17):2679-2687. https://doi.org/10.1182/bloodadvances.2019000527 PMid:31506286 PMCid:PMC6737422
- Silva
DG, Belini Junior E, Carrocini GC, et al. Genetic and biochemical
markers of hydroxyurea therapeutic response in sickle cell anemia. BMC
Med Genet. 2013;14:108. https://doi.org/10.1186/1471-2350-14-108 PMid:24106994 PMCid:PMC3851873
- Manwani
D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology
and novel targeted therapies. Blood 2013;122(24):3892-8. doi:
10.1182/blood-2013-05-498311.
https://doi.org/10.1182/blood-2013-05-498311 PMid:24052549 PMCid:PMC3854110
- Morris
CR, Suh JH, Hagar W, Larkin S, Bland DA, Steinberg MH, Vichinsky EP,
Shigenaga M, Ames B, Kuypers FA, Klings ES. Erythrocyte glutamine
depletion, altered redox environment, and pulmonary hypertension in
sickle cell disease. Blood 2008;8;111(1):402-10. https://doi.org/10.1182/blood-2007-04-081703 PMid:17848621 PMCid:PMC2200820
- Nur
E, Biemond BJ, Otten HM, Brandjes DP et al. Oxidative stress in sickle
cell disease; pathophysiology and potential implications for disease
management. Am J Hematol 2011;86(6):484-9. https://doi.org/10.1002/ajh.22012 PMid:21544855
- Voskou
S, Aslan M, Fanis P, Phylactides M, Kleanthous M. Oxidative stress in
β-thalassaemia and sickle cell disease. Redox Biol. 2015; :226-239. https://doi.org/10.1016/j.redox.2015.07.018 PMid:26285072 PMCid:PMC4543215
- Adegoke
S, Smith O, Akinlosotu M. Total oxidant status of children with sickle
cell anemia: Correlation with rate of pain episodes and haematological
indices. Pediatric Hematology Oncology Journal 2018; 3(3): 70-73. https://doi.org/10.1016/j.phoj.2018.10.002
- Martin
RC, Li Y, Liu Q, Jensen NS, Barker DF, Doll MA, Hein DW. Manganese
superoxide dismutase V16A single-nucleotide polymorphism in the
mitochondrial targeting sequence is associated with reduced enzymatic
activity in cryopreserved human hepatocytes. DNA Cell Biol. 2009;
28(1):3-7. https://doi.org/10.1089/dna.2008.0788 PMid:18821846 PMCid:PMC2851837
- Friedman
JS, Rebel VI, Derby R, et al. Absence of mitochondrial superoxide
dismutase results in a murine hemolytic anemia responsive to therapy
with a catalytic antioxidant. J Exp Med. 2001; 193(8):925-934. https://doi.org/10.1084/jem.193.8.925 PMid:11304553 PMCid:PMC2193409
- Mohanty
JG, Nagababu E, Friedman JS, Rifkind JM. SOD2 deficiency in
hematopoietic cells in mice results in reduced red blood cell
deformability and increased heme degradation. Exp Hematol. 2013;
41(3):316-321. https://doi.org/10.1016/j.exphem.2012.10.017 PMid:23142655 PMCid:PMC3741644
- Essien
EU. Increased susceptibility of erythrocyte membrane lipids to
peroxidation in sickle cell disease. Cent Afr J Med. 1994; 40(8):217-20.
- Hebbel
RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme
loss due to instability of sickle haemoglobin. Proc Natl Acad Sci U S
A. 1988 Jan; 85(1):237-41. doi: 10.1073/pnas.85.1.237. https://doi.org/10.1073/pnas.85.1.237 PMid:3422420 PMCid:PMC279519
- Vona
R, Sposi NM, Mattia L, Gambardella L, Straface E, Pietraforte D. Sickle
Cell Disease: Role of Oxidative Stress and Antioxidant Therapy.
Antioxidants (Basel) 2021; 10(2):296. https://doi.org/10.3390/antiox10020296 PMid:33669171 PMCid:PMC7919654
- Farias
ICC, Mendonça-Belmont TF, da Silva AS, do Ó KP, Ferreira F, Medeiros
FS, da Silva Vasconcelos LR, Bezerra MAC, da Silva Araújo A, de Moura
PMMF, Hatzlhofer BLD, Dos Anjos ACM, de Mendonça Cavalcanti MDS.
Association of the SOD2 Polymorphism (Val16Ala) and SOD Activity with
Vaso-occlusive Crisis and Acute Splenic Sequestration in Children with
Sickle Cell Anemia. Mediterr J Hematol Infect Dis.
2018;21;10(1):e2018012. https://doi.org/10.4084/mjhid.2018.012 PMid:29531649 PMCid:PMC5841937
- Okocha
E, Manafa O, Aneke C, Onwuzuruike E, Ibeh C, Chukwuama O. Serum
Superoxide Dismutase activity: A Predictor of Disease Severity in
Nigerian Sickle Cell Anemia Patients in Steady State. Med J DY Patil
Vidyapeeth 2017;10(5): 406-411. https://doi.org/10.4103/MJDRDYPU.MJDRDYPU_90_17
- Uche
E, Olowoselu F, Augustine B, Suleiman A, Ismail A, Oluwole E, Akinbami
A, Onwah L. Superoxide dismutase activity in sickle cell anemia
patients during crisis and in steady state. Journal of Applied
Hematology 2020;11(2):59. https://doi.org/10.4103/joah.joah_87_19
- Das
SK, Nair RC (1980) Superoxide dismutase, glutathione peroxidase,
catalase and lipid peroxidation of normal and sickled erythrocytes. Br
J Haematol. 1980;44(1):87-92. https://doi.org/10.1111/j.1365-2141.1980.tb01186.x PMid:7378296
- Shao
J, Chen L, Marrs B, Lee L, Huang H, Manton KG, Martin GM, Oshima J.
SOD2 polymorphisms: unmasking the effect of polymorphism on splicing.
BMC Med Genet. 2007; 8:7. https://doi.org/10.1186/1471-2350-8-7 PMid:17331249 PMCid:PMC1819367
- Iida
R, Tsubota E, Takeshita H, Yasuda T. Multiplex single base extension
method for simultaneous genotyping of non-synonymous SNP in the three
human SOD genes. Electrophoresis 2008; 29(23):4788-94. https://doi.org/10.1002/elps.200800332 PMid:19016244
- Ambrosone
CB, Ahn J, Singh KK, Rezaishiraz H, Furberg H, Sweeney C, Coles B,
Trovato A. Polymorphisms in genes related to oxidative stress (MPO,
MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res.
2005; 65(3):1105-11.
- Sogut S, Yonden Z,
Kaya H, Oktar S, Tutanc M, Yilmaz HR, Yigit A, Ozcelik N, Gali E.
Ala-9Val polymorphism of Mn-SOD gene in sickle cell anemia. Genet Mol
Res. 2011; 10(2):828-33. https://doi.org/10.4238/vol10-2gmr1106 PMid:21574139
- Domingos
IF, Pereira-Martins DA, Borges-Medeiros RL, Falcao DA, Hatzlhofer BL,
Brewin JN, Gardner K, Mendonca TF, Cavalcanti MS, Cunha AF, Anjos AC,
Rodrigues ES, Kashima S, Cruz PR, Melo MB, Menzel S, Araujo AS, Costa
FF, Bezerra MA, Lucena-Araujo AR. Evaluation of oxidative
stress-related genetic variants for predicting stroke in patients with
sickle cell anemia. J Neurol Sci. 2020;414:116839. https://doi.org/10.1016/j.jns.2020.116839 PMid:32344219
- Radmanovic
B, Jovanovic J, Djordjevic N, Baskic D, Cukic J, Sazdanovic P,
Vojinovic RH, Sazdanovic M, Pantic K, Milovanovic DR. Superoxide
Dismutase 2 Val16Ala Polymorphism is Associated with
Amiodarone-Associated Liver Injury. Serbian Journal of Experimental and
Clinical Research. 2020; 4;1(ahead-of-print). https://doi.org/10.2478/sjecr-2019-0078
- Ray D, Deshmukh P, Goswami K, Garg N (2007) Antioxidant vitamin levels in sickle cell disorders. Natl Med J India. 20(1):11-13
- Biswal
S, Rizwan H, Pal S, Sabnam S, Parida P, Pal A. Oxidative stress,
antioxidant capacity, biomolecule damage, and inflammation symptoms of
sickle cell disease in children. Hematology 2019; 24(1):1-9. https://doi.org/10.1080/10245332.2018.1498441 PMid:30010491
- Ren
H, Ghebremeskel K, Okpala I, Lee A, Ibegbulam O, Crawford M. Patients
with sickle cell disease have reduced blood antioxidant protection. Int
J Vitam Nutr Res. 2008;78(3):139-147. https://doi.org/10.1024/0300-9831.78.3.139 PMid:19003736
- Martin
RC, Li Y, Liu Q, Jensen NS, Barker DF, Doll MA, Hein DW. Manganese
superoxide dismutase V16A single-nucleotide polymorphism in the
mitochondrial targeting sequence is associated with reduced enzymatic
activity in cryopreserved human hepatocytes. DNA Cell Biol.
2009;28(1):3-7. https://doi.org/10.1089/dna.2008.0788 PMid:18821846 PMCid:PMC2851837
- Bastaki
M, Huen K, Manzanillo P, Chande N, Chen C, Balmes JR, Tager IB, Holland
N. Genotype-activity relationship for Mn-superoxide dismutase,
glutathione peroxidase 1 and catalase in humans. Pharmacogenet Genomics
2006;16(4):279-86. https://doi.org/10.1097/01.fpc.0000199498.08725.9c PMid:16538174
- Hong
YC, Lee KH, Yi CH, Ha EH, Christiani DC. Genetic susceptibility of term
pregnant women to oxidative damage. Toxicol Lett. 129:255-262. https://doi.org/10.1016/S0378-4274(02)00014-0
- Park
SY, Lee KH, Kang D, Lee KH, Ha EH, Hong YC. Effect of genetic
polymorphisms of MnSOD and MPO on the relationship between PAH exposure
and oxidative DNA damage. Mutat Res. 2006;593:108-115. https://doi.org/10.1016/j.mrfmmm.2005.06.022 PMid:16084535
- Smith
OS, Ajose OA, Adegoke SA, Adegoke OA, Adedeji TA, Oderinu KA. Plasma
level of antioxidants is related to frequency of vaso-occlusive crises
in children with sickle cell anaemia in steady state in Nigeria.
Pediatric Hematology Oncology Journal. 2019; 4(1):17-22. https://doi.org/10.1016/j.phoj.2019.03.003
- Armenis
I, Kalotychou V, Tzanetea R, Moyssakis I, Anastasopoulou D, Pantos C,
Konstantopoulos K, Rombos I. Reduced peripheral blood superoxide
dismutase 2 expression in sickle cell disease. Annals of hematology
2019; 98(7):1561-72. https://doi.org/10.1007/s00277-019-03709-8 PMid:31098737
[TOP]





