Cumali Yalçın1, Fahir Özkalemkaş1, Vildan Özkocaman1, Tuba Ersal1, İbrahim Ethem Pınar1, Bedrettin Orhan1, Ömer Candar1, Sinem Çubukçu1, Tuba Güllü Koca1, Merve Nur Akyol2, Nevriye Gül Ada2, Cüneyt Özakın3, Esra Kazak4, Halis Akalın4 and Rıdvan Ali1.
1 Division of Hematology, Department of Internal Medicine, Uludag University Faculty of Medicine, Bursa, Turkey.
2 Department of Internal Medicine, Uludag University Faculty of Medicine, Bursa, Turkey.
3 Department of Microbiology, Uludag University Faculty of Medicine, Bursa, Turkey.
4 Department of Microbiology and Infectious Diseases, Uludag University Faculty of Medicine, Bursa, Turkey.
Correspondence to: Dr.
Cumali Yalçın. Address: Bursa Uludağ Üniversitesi Tıp Fakültesi Görükle
Kampüsü, 16059, Bursa/Turkey. Tel.: +905437279580, Fax: +902242940041.
E-mail:
doktorcumali@hotmail.com
Published: May 1, 2022
Received: February 10, 2022
Accepted: April 14, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022039 DOI
10.4084/MJHID.2022.039
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
This study aimed to evaluate the effects of the appropriate use of
empiric glycopeptide therapy in hematologic malignancy patients with
febrile neutropenia (FN).
Materials and Methods:
Patients with FN who were hospitalized in our clinic and started
empiric glycopeptide therapy were retrospectively analyzed. Empiric
glycopeptide treatment initial indications were determined according to
7 specific criteria in the IDSA guidelines. In addition, the duration
of glycopeptide use according to initial indications, causative
pathogens in culture positivity, frequency of VRE infection, and the
mortality rate was identified.
Results:
87 patients were included. Of these, 102 episodes of FN were analyzed.
Appropriate use of glycopeptides was observed in 98% of patients. The
most common initial indication for glycopeptide was skin or soft-tissue
infection, with 52% (n = 53). The mean duration of glycopeptide use was
11 (2–22) days. The time of glycopeptide use was longer in patients
with catheter-related infections than in those with severe mucositis
and hemodynamic instability (p = 0,041/p = 0,016). The duration of
glycopeptide use was shorter in patients with consolidation therapy
than in those without consolidation therapy. The mortality rate in
culture-positive patients was significantly higher than in
culture-negative patients (p = 0.041). At 72 h, glycopeptide therapy
was discontinued in 8 of 79 FN episodes within culture-negative
patients.
Conclusion: This
study showed that the mortality rate was higher in culture-positive
patients. Additionally, glycopeptides should be discontinued early with
no evidence of gram-positive infection.
|
Introduction
Febrile
neutropenia (FN) is a severe complication that usually occurs after
chemotherapy treatment in about 80% of patients with hematologic
malignancies.[1,2] FN is one of the most important
causes of comorbidity and mortality in cancer patients. The FN-related
mortality rate varies between 2% and 20% in studies.[3] Therefore, preventing infections and selecting appropriate treatments are vital in these patients.[4]
In the 1980s, Gram-negative bacteria were frequently detected in
pathogens isolated in cultures, while gram-positive bacteria have been
isolated more recently.[5]In culture, the most commonly isolated gram-positive pathogens are viridans group streptococci, coagulase-negative Staphylococcus, Staphylococcus aureus, and Enterococcus spp.
Therefore, it has become important according to which criteria the use
of empirical antibiotics for gram-positive bacteria should be started.
Clinical Practice Guideline for the Use of Antimicrobial Agents in
Neutropenic Patients with Cancer, 2010 Update by the Infectious
Diseases Society of America, identified seven criteria for the empiric
start of effective antibiotics against gram-positive bacteria.
According to the IDSA guidelines, discontinuation of glycopeptide
antibiotics is recommended within 72 h if there is no evidence of
gram-positive infection.[6] The disadvantage of
widespread vancomycin use is that it causes the development of
vancomycin-resistant organisms and multi-drug resistant organisms.[7]In
the literature, indications for initial glycopeptide were less
compatible according to the IDSA guidelines. The most important reason
for this is the addition of glycopeptide antibiotics to treatment due
to persistent fever in patients.[8] Therefore, this
study aimed to evaluate the compliance of empiric glycopeptide use with
IDSA guidelines in hematologic malignancy patients with FN.
Materials and Methods
In
this study, patients with FN who were hospitalized in our clinic and
started empiric glycopeptide therapy were retrospectively analyzed
between January 2020 and January 2021. The hospital where the research
was conducted is a tertiary health center with a capacity of 35 adult
hematology beds serving approximately 3 million people.
Hematologic
malignancy patients with FN who were 18 years of age or older were
included in the study. The patients' data were obtained from the
hospital information system and the patient file. Patients without
hematologic malignancy were not included in the study.
In
the FN episode, fever was defined as having a temperature above 38.3°C
once in oral or axillary measurement, or at least 1 hour continuously
above 38°C. Neutropenia was stated as a situation where the number of
neutrophils is expected to be less than 500/uL, or the neutrophil level
is between 500–1000/uL and will fall below 500/uL within 48 hours.
Empiric
glycopeptide treatment indications were determined according to 7
specific criteria specified by IDSA guidelines. These main criteria
were: hemodynamic instability or other evidence of severe sepsis, skin
or soft-tissue infection in any site, positive blood culture for
gram-positive bacteria before final identification and susceptibility
testing is available, clinically suspected serious catheter-related
infection, pneumonia documented radiographically, colonization with
methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus or penicillin-resistant Streptococcus pneumoniae, severe mucositis if fluoroquinolone prophylaxis has been given and ceftazidime is employed as empirical therapy.
The
cultures were taken (blood, urine, sputum, wound, and stool) during the
FN episode and then checked for gram-positive bacteria. In addition to
that, VRE surveillance was conducted by rectal swab.
The
primary endpoint was to determine the ratio of empiric glycopeptide use
in accordance with IDSA guidelines. In addition, FN-related mortality
and the frequency of VRE were determined. FN-related mortality was
defined as patient death in the presence of persistent or recurrent
fever or a documented infection at any time of the FN episode.
Statistical analysis. Statistical
analysis was conducted using SPSS version 23. Continuous variables were
described as mean and confidence interval (CI) or median and range,
while categorical variables were expressed as n (%). The difference
between independent variables was analyzed with a One-Way ANOVA with a post hoc Tukey Test. A p-value < 0.05 was considered statistically significant.
Ethical statement. This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our hospital.
Results
One
hundred two febrile neutropenic episodes were evaluated in 87 patients
included in this study. The median age of the patients was 51 (19–76),
and 57% were female. There were 59 patients with acute leukemia, 15
lymphomas, ten multiple myelomas, two myelodysplastic syndromes, and
one chronic myeloid leukemia. The mean duration of glycopeptide use was
11 (2–22) days. Seventy-eight (90%) patients were admitted for
chemotherapy, 6 (7%) for radiotherapy, and 3 (3%) for supportive
treatment (Table 1). Initial
glycopeptide therapy was vancomycin in 75 (73%) FN episodes and
teicoplanin in 27 (26%) FN episodes. Intravenous vancomycin dosage
regime was 1 gr every 12 hours, and intravenous teicoplanin dosage
regime was an initial dose of 6 mg/kg every 12 hours for two doses,
followed by 6 mg/kg every once daily. Unfortunately, we were not able
to measure vancomycin levels in our hospital. The reason for starting
teicoplanin as the initial treatment was a history of allergic
reactions to vancomycin, an allergic reaction had developed, or renal
failure.
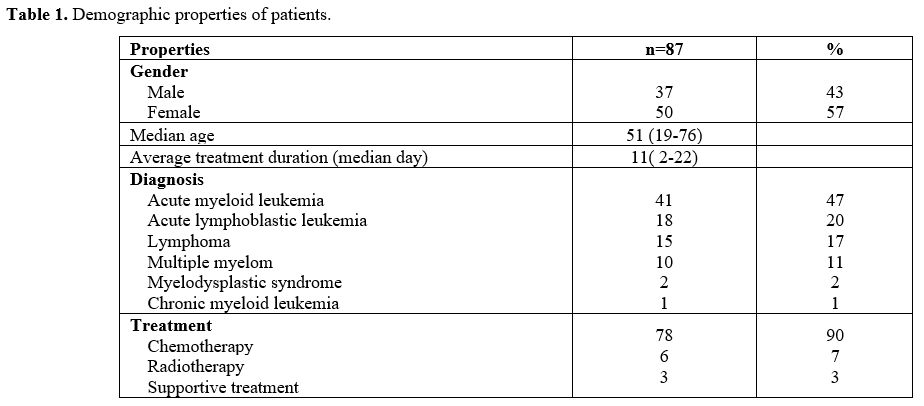 |
Table
1. Demographic properties of patients. |
When
all FN episodes in this study were examined, the most common indication
for starting empiric glycopeptide was skin or soft-tissue infection in
52% (n = 53). While severe mucositis, 17% (n = 17), was observed with
the second frequency, other treatment indications were given in Table 2.
Empiric glycopeptide was not started according to the appropriate
criteria in the two cases, and it was noted that the leading cause of
the start was prolonged fever.
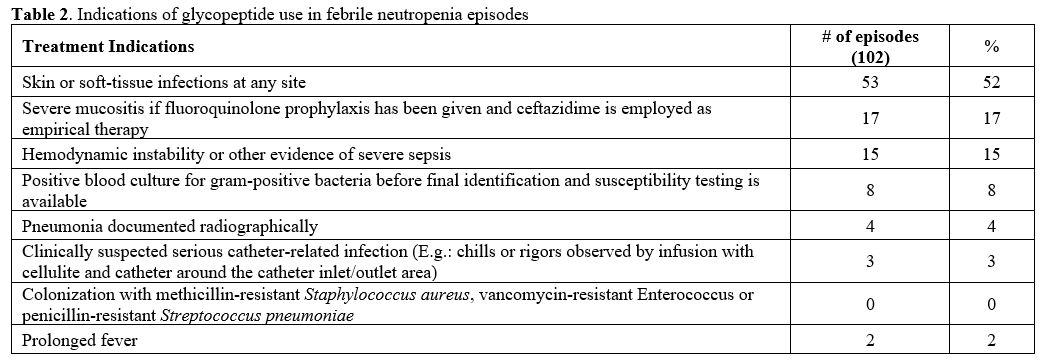 |
Table 2. Indications of glycopeptide use in febrile neutropenia episodes. |
There
was no significant difference in the duration of glycopeptide use
between the two groups when comparing culture-positive and
culture-negative patients. The duration of glycopeptide use was longer
in patients with catheter-related infections (median 16 days; range
16-22) than in those with severe mucositis (median 9 days; range 4-18)
and hemodynamic instability (median 6 days; range 2-22) (p = 0,041, p =
0,016 respectively). However, when patients receiving consolidation
therapy (median six days; range 5-14) for acute leukemia were compared
with patients receiving induction therapy (median 11 days; range 2-22),
the duration of antibiotic use was significantly shorter in the group
receiving consolidation therapy (p = 0.017) (Table 3).
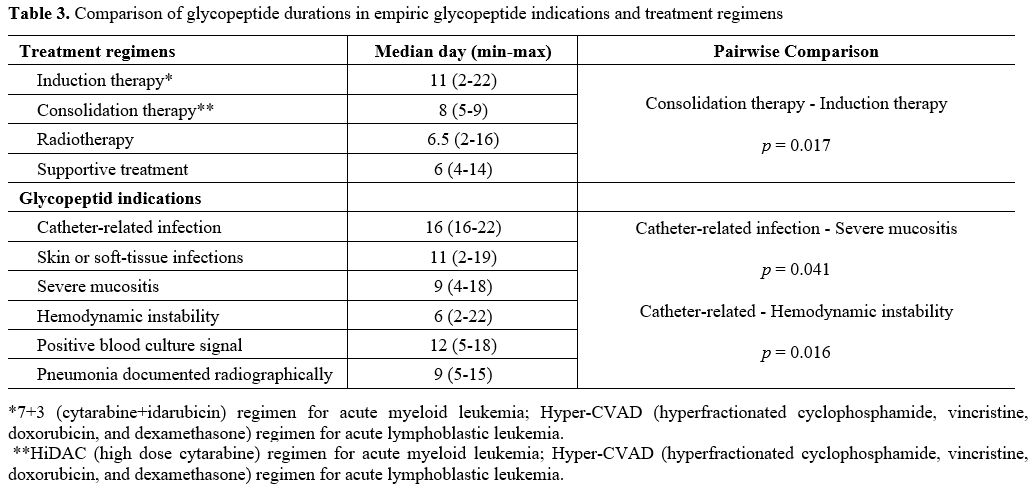 |
Table 3. Comparison of glycopeptide durations in empiric glycopeptide indications and treatment regimens. |
Gram-positive
pathogen isolated in cultures taken in 23 patients with FN episodes.
VRE that was positive in culture was observed only in 3 (3%) episodes
of FN. The most common pathogens in culture were Enterococcus spp. and Staphylococcus spp. (Table 4).
FN-related mortality rate was 18.3% (n = 16) in all patients. Seven of
the patients with mortality were culture-positive patients. The
mortality rate in patients with culture-positive was significantly
higher than in patients with culture-negative (p = 0,041). The median
overall survival of all patients was 23.5 months (27.8–55.6, CI %95) (Figure 1).
There was no significant difference in overall survival between
patients with culture-positive and culture-negative (p > 0.05) (Figure 2).
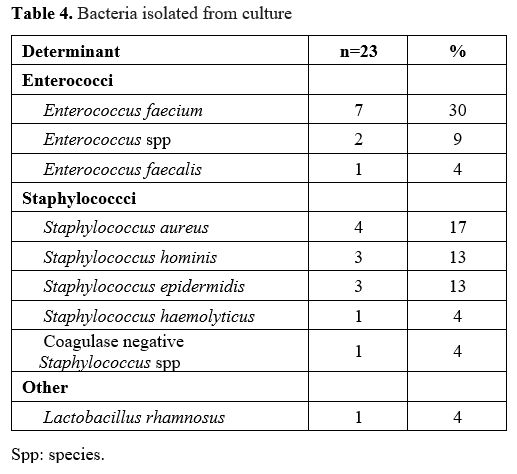 |
Table 4. Bacteria isolated from culture. |
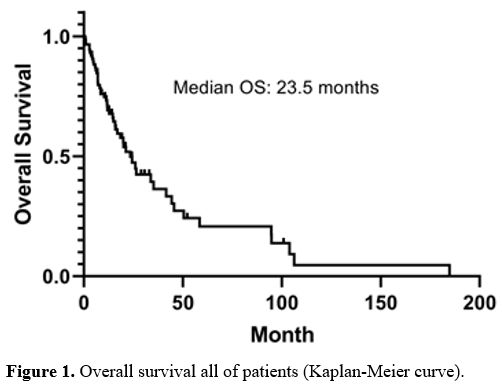 |
Figure
1. Overall survival all of patients (Kaplan-Meier curve). |
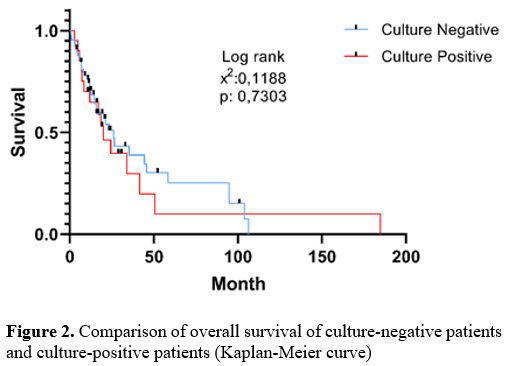 |
Figure
2. Comparison of overall survival of culture-negative patients and culture-positive patients (Kaplan-Meier curve). |
Discussion
In most studies, gram-positive bacteria have increased morbidity and mortality in febrile neutropenic patients.[9-11]
In our study, the mortality rate in patients with culture-positive was
significantly higher than in those with culture-negative, which
supports the studies conducted.
The use of empiric glycopeptide
treatment in FN has shown low starting rates with an appropriate
indication in many centers. Libuit et al. found compliance with the
IDSA guidelines for empirical vancomycin use in 66 adult cancer
patients with FN was 27.3%.[12] In a similar study by
Wright et al., it was shown that empirical vancomycin was used in
accordance with the guideline in 67% of patients with FN.[13]
In our study, empiric glycopeptide antibiotic initiation criteria were
98% compliant with IDSA guidelines. In addition, it was noted that
vancomycin was added to the treatment due to prolonged fever in only
two FN episodes. Therefore, our empiric glycopeptide treatment was
highly compliant with the IDSA guideline compared to other studies in
the literature.[11,12]
At 72 h, glycopeptide
therapy was discontinued in 8 of 79 FN episodes in which there was no
microbiological evidence of gram-positive infection. We thought that
the main reason for the prolonged use of glycopeptide in patients with
culture-negative were concerns about the deterioration in the clinical
condition of patients and prolonged fever.
Cytotoxic chemotherapy, the disease used to treat hematologic cancers, leads to myelosuppression and immunosuppression.[14]
Bradley et al. reported reducing the frequency of hospitalization for
FN in AML patients under consolidation chemotherapy use of G-CSF
prophylaxis.[15] This study demonstrated a shorter
duration of glycopeptide use in acute leukemia patients undergoing
consolidation therapy than induction therapy. It may be associated with
G-CSF prophylaxis in all patients undergoing consolidation therapy.
Enterococcal
infections are the leading cause (20%–30%) of hospital-acquired
infections in the United States in neutropenic patients. Prolonged use
of vancomycin and prolonged hospitalization increase the risk of
developing VRE.[9] In recent years, guidelines
limiting the use of empiric vancomycin in febrile neutropenic patients
have been associated with a reduced incidence of VRE.[15] Kirkizlar et al. found a VRE infection rate of 10.5% in febrile neutropenic patients with hematologic malignancy.[10]
Heisel et al. found a VRE infection rate of 14% VRE bloodstream
infections rate of 11.7% among all newly diagnosed VRE colonized acute
myeloid leukemia and myelodysplastic syndrome patients.[17]
In our findings, the frequency of VRE infection was 3%. However, the
frequency of VRE was found to be lower since it was evaluated only in
patients with FN who started empiric glycopeptide.
Neutropenic
patients are at high risk for catheter-related bloodstream infections
(CRBSI). Especially those with a neutrophil count of less than 100/uL
are at increased risk. CRBSI was higher in hematologic malignancies
than in solid tumors.[18,19] Ghanem et al. found the mean duration of antibiotic therapy in CRBSI was 20.2 days with hematologic and solid tumors.[20] The mean duration of antibiotic treatment in CRBSI in this study was 18 days and was longer as studies in the literature.[18,20]
FN-related mortality rates range from 2% to 20% in most studies.[21,22,23]
In a multicenter randomized study of 611 febrile neutropenic patients,
the mortality rate was 7.6% in the group where empiric vancomycin was
started. In contrast, the mortality rate was 5.6% in patients who
started linezolid.[24] In a study of 41,779 patients
with febrile neutropenia in the United States, the in-hospital
mortality rate was 9.5%. Mortality rates were 8% in solid tumors, 8.9%
in lymphoma patients, and 14.3% in leukemia patients.[23]
According to our results, the FN-related mortality rate was 18.3%
higher than in most studies because 68% (n = 59) of the patients were
diagnosed with acute leukemia. Mortality rates were 12.6% for acute
leukemia, 3.4% for lymphoma, and 2.2% for multiple myeloma patients.
Mert et al. found that the most frequently isolated gram-positive bacteria were coagulase-negative Staphylococcus (CNS) in patients with hematologic malignancies.[25] Özdemir et al. reported that the most commonly isolated gram-positive bacteria were CNS and Enterococcus faecium in febrile neutropenic patients.[26] In our study, the most frequently isolated gram-positive bacteria were CNS and Enterococcus faecium, similar to other studies.
Limitations of our study include its small sample size, its retrospective nature, and not being evaluated by other guidelines.
Conclusions
This
study is being conducted to evaluate the use of empiric glycopeptides
in hematologic malignancy patients with FN. Our empiric glycopeptide
treatment was highly compliant with the IDSA guideline. However, the
adherence to discontinuation at 72 h of treatment was not high. In
addition, our study showed mortality rate was higher in
culture-positive patients. Therefore, glycopeptides with no
microbiological evidence of gram-positive infection should be
discontinued early according to the IDSA guideline.
References
- Klastersky J. Management of fever in neutropenic
patients with different risks of complications. Clin Infect Dis.
2004;39 Suppl 1:32–37. https://doi.org/10.1086/383050 PMid:15250018
- Nucci M.How I treat febrile neutropenia. Mediterr J Hematol Infect Dis 2021, 13(1): e2021025 http://dx.doi.org/10.4084/MJHID.2021.025
- Kuderer
NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and
cost associated with febrile neutropenia in adult cancer patients.
Cancer. 2006;106:2258–2266. https://doi.org/10.1002/cncr.21847 PMid:16575919
- Virizuela
JA, Carratalà J, Aguado JM, Vicente D, Salavert M, Ruiz M, Ruiz I,
Marco F, Lizasoain M, Jiménez-Fonseca P, Gudiol C, Cassinello J,
Carmona-Bayonas A, Aguilar M, Cruz JJ. Management of infection and
febrile neutropenia in patients with solid cancer. Clin Transl Oncol.
2016;18:557-570. https://doi.org/10.1007/s12094-015-1442-4 PMid:26577106
- Wisplinghoff
H, Seifert H, Wenzel RP. Current trends in the epidemiology of
nosocomial bloodstream infections in patients with hematological
malignancies and solid neoplasms in hospitals in the United States.
Clin Infect Dis. 2003;36:1103–1110. https://doi.org/10.1086/374339 PMid:12715303
- Freifeld
AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II,
Rolston KV, Young JA, Wingard JR. Clinical practice guideline for
antimicrobial agents in neutropenic patients with cancer: 2010 update
by the infectious diseases society of America. Clin Infect Dis.
2011;52:56-93. https://doi.org/10.1093/cid/cir073 PMid:21258094
- Baden
LR, Swaminathan S, Angarone M Blouin G, Camins BC, Casper C, Cooper B,
Dubberke ER, Engemann AM, Freifeld AG, Greene JN, Ito JI, Kaul DR,
Lustberg ME, Montoya JG, Rolston K, Satyanarayana G, Segal B, Seo SK,
Shoham S, Taplitz R, Topal J, Wilson JW, Hoffmann KG, Smith C.
Prevention and Treatment of Cancer-Related Infections, Version 2.2016,
NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw.
2016;14:882-913. https://doi.org/10.6004/jnccn.2016.0093 PMid: 27407129
- Paul
M, Borok S, Fraser A, Vidal L, Leibovici L. Empirical antibiotics
against gram-positive infections for febrile neutropenia: systematic
review and meta-analysis of randomized controlled trials. J Antimicrob
Chemother. 2005;55:436–444. https://doi.org/10.1093/jac/dki028 PMid:15722392
- Khan HA, Ahmad A, Mehboob R. Nosocomial infections and their control strategies. Asian Pac J Trop Biomed. 2015;7:509-514. https://doi.org/10.1016/j.apjtb.2015.05.001
- Kirkizlar
TA, Akalin H, Kirkizlar O, Ozkalemkas F, Ozkocaman V, Kazak E, Ozakin
C, Bulbul EN, Ozboz ES, Ali R. Vancomycin-resistant enterococci
infection and predisposing factors for infection and mortality in
patients with acute leukaemia and febrile neutropenia. Leuk Res.
2020;99:106463. https://doi.org/10.1016/j.leukres.2020.106463 PMid:33130331
- Mermel
LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders
BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the
diagnosis and management of intravascular catheter-related infection:
2009 update by the infectious diseases society of America. Clin Infect
Dis. 2009;49: 1–45. https://doi.org/10.1086/599376 PMid:19489710
- Libuit
J, Whiteman A, Wolfe R, Washington CS. Empiric vancomycin use in
febrile neutropenic oncology patients. Open Forum Infect Dis.
2014;1:1–3. https://doi.org/10.1093/ofid/ofu006 PMid:25734080
- Wright
JD, Neugut AI, Ananth CV, Lewin SN, Wilde ET, Lu YS, Herzog TJ,
Hershman DL Deviations from guideline-based therapy for febrile
neutropenia in cancer patients and their effect on outcomes. JAMA
Intern Med. 2013;173:559–568. https://doi.org/10.1001/jamainternmed.2013.2921 PMid:23460379
- Cruciani
M, Rampazzo R, Malena M, Lazzarini L, Todeschini G, Messori A, Concia
E. Prophylaxis with fluoroquinolones for bacterial infections in
neutropenic patients: a meta-analysis. Clin Infect Dis.
1996;23:795–805. https://doi.org/10.1093/clinids/23.4.795 PMid:8909847
- Bradley
AM, Deal AM, Buie LW, van Deventer H. Neutropenia-associated outcomes
in adults with acute myeloid leukemia receiving cytarabine
consolidation chemotherapy with or without granulocyte
colony-stimulating factor. Pharmacotherapy. 2012;32:1070-1077. https://doi.org/10.1002/phar.1150 PMid:23208834
- Ford
CD, Lopansri BK, Haydoura S, Snow G, Dascomb KK, Asch J, BoPetersen F,
Burke JP. Frequency, risk factors, and outcomes of vancomycin-resistant
Enterococcus colonization and infection in patients with newly
diagnosed acute leukemia: different patterns in patients with acute
myelogenous and acute lymphoblastic leukemia. Infect Control Hosp
Epidemiol. 2015;36:47-53. https://doi.org/10.1017/ice.2014.3 PMid:25627761
- Heisel
RW, Sutton RR, Mascara GP, Winger DG, Weber DR, Lim SH, Oleksiuk LM.
Vancomycin-resistant enterococci in acute myeloid leukemia and
myelodisplastic syndrome patients undergoing induction chemotherapy
with idarubicin and cytarabine, Leuk. Lymphoma. 2017;58:2565–2572.
- Ban
T, Fujiwara SI, Murahashi R, Nakajima H, Ikeda T, Matsuoka S, Toda Y,
Kawaguchi SI, Ito S, Nagayama T, Umino K, Minakata D, Nakano H, Morita
K, Ashizawa M, Yamamoto C, Hatano K, Sato K, Ohmine K, Kanda Y. Risk
Factors for Complications Associated with Peripherally Inserted Central
Catheters During Induction Chemotherapy for Acute Myeloid Leukemia.
Intern Med. 2021;8184-8121. https://doi.org/10.2169/internalmedicine.8184-21 PMid:34511570
- Mollee
P, Jones M, Stackelroth J, van Kuilenburg R, Joubert W, Faoagali J,
Looke D, Harper J, Clements A. Catheter-associated bloodstream
infection incidence and risk factors in adults with cancer: a
prospective cohort study. J Hosp Infect. 2021;78:26-30. https://doi.org/10.1016/j.jhin.2011.01.018 PMid:21459476
- Ghanem
GA, Boktour M, Warneke C, Pham-Williams T, Kassis C, Bahna P,
Aboufaycal H, Hachem R, Raad I. Catheter-related Staphylococcus aureus
bacteremia in cancer patients: high rate of complications with
therapeutic implications. Medicine (Baltimore) 2007;86:54-60. https://doi.org/10.1097/MD.0b013e318030d344 PMid:17220756
- Pathak
R, Giri S, Aryal MR, Karmacharya P, Bhatt VR, Martin MG. Mortality,
length of stay, and health care costs of febrile neutropenia-related
hospitalizations among patients with breast cancer in the United
States. Support Care Cancer. 2015;23:615–617. https://doi.org/10.1007/s00520-014-2553-0 PMid:25556610
- Caggiano
V, Weiss RV, Rickert TS, Linde-Zwirble WT. Incidence, cost, and
mortality of neutropenia hospitalization associated with chemotherapy.
Cancer. 2005;103: 1916–1924. https://doi.org/10.1002/cncr.20983 PMid:15751024
- Kuderer
NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and
cost associated with febrile neutropenia in adult cancer patients.
Cancer. 2006;106: 2258-2266. https://doi.org/10.1002/cncr.21847 PMid:16575919
- Jaksic
B, Martinelli G, Perez-Oteyza J, Hartman CS, Leonard LB, Tack KJ.
Efficacy and safety of linezolid compared with vancomycin in a
randomized, double-blind study of febrile neutropenic patients with
cancer. Clin Infect Dis. 2006;42(5): 597-607. https://doi.org/10.1086/500139 PMid: 16447103.
- Mert
D, Ceken S, Iskender G, Iskender D, Merdin A, Duygu F, Ertek M,
Altuntas F. Epidemiology and mortality in bacterial bloodstream
infections in patients with hematologic malignancies. J Infect Dev
Ctries. 2019;13:727-735.
- Özdemir SK,
Iltar U, Salim O, Yücel OK, Erdem R, Turhan Ö, Undar L. Investigation
of seasonal frequency and pathogens in febrile neutropenia. Memo. 2019;
12:119-122. https://doi.org/10.3855/jidc.11457 PMid:32069257
[TOP]





