Antonio Cristiano1*, Raffaele Palmieri1*, Emiliano Fabiani1,2, Tiziana Ottone1, Mariadomenica Divona1, Arianna Savi1, Francesco Buccisano1, Luca Maurillo1, Corrado Tarella3, William Arcese1 and Maria Teresa Voso1.
1 Department of Biomedicine and Prevention, University of Rome "Tor Vergata", Rome, Italy.
2 UniCamillus-Saint Camillus International University of Health Sciences, Rome, Italy.
3 European Institute of Oncology (IEO) and University of Milan, Milan, Italy.
* The first two authors equally contributed to this work.
Correspondence to:
Maria Teresa Voso, Department of Biomedicine and Prevention University
of Rome "Tor Vergata" Via Montpellier 1, 00133 Rome, Italy. E-mail:
voso@med.uniroma2.it
Published: May 1, 2022
Received: March 3, 2022
Accepted: April 15, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022041 DOI
10.4084/MJHID.2022.041
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
addition of Venetoclax (VEN) to Hypomethylating agents (HMAs)
significantly improves the probability of complete remission and
prolongs survival in patients with Acute Myeloid Leukemia (AML) when
compared to HMA alone. However, the mutated clone composition may
impact the probability of response and its duration.
Here, we
describe the molecular profile of a patient with AML rapidly evolved
from a previous therapy-related-Chronic MyeloMonocytic Leukemia, who
achieved safely complete remission after treatment with the
VEN/Azacitidine combination, even in the presence of SARS-COVID-2
infection.
The targeted NGS analysis showed that the VEN/AZA
combination led to the eradication of the FLT3-ITD and RUNX1 mutated
clone/s primarily associated with AML evolution, and subsequently, the SRSF2, NRAS, and ASXL1 mutated clone/s.
This
case also underlines the importance of the sequential use of targeted
NGS for disease monitoring: the deep molecular remission achieved by
this patient allowed to safely guide adjustments of drug dosage and
treatment intervals in the presence of neutropenia, helping to rule out
disease progression.
|
Introduction
Hypomethylating
agents (HMA) were considered, until recently, the standard of care for
older patients with acute myeloid leukemia (AML) ineligible for
conventional induction chemotherapy and for patients with high-risk
myelodysplastic syndromes (MDS).[1,2] The overall response rate to HMA in AML is about 50%, with complete remission (CR) achieved in about 15-30% of patients.[3]
Adding venetoclax (Venclyxto, VEN, ABBVie) to HMA significantly
improves the probability of CR and prolongs survival in elderly
patients with newly diagnosed AML compared to HMA alone.[4] However, the clone composition may impact on the probability of response and its duration.[5-6] Patients with AML and NPM1 and/or IDH2 mutations have the highest probability of response to VEN/HMA combinations, but also RUNX1 mutations appear to be associated with favorable responses. On the other hand, activating kinase mutations such as FLT3-ITD, N/KRAS, CBL, or KIT have been associated with treatment resistance.[7]
The impact of the combination treatment on the dynamics of the disease
clones during VEN combination treatments has been only partially
described. We
monitored the molecular profile of a patient with AML evolved from a
previous therapy-related chronic myelomonocytic leukemia (t-CMML)
during the treatment with a venetoclax/azacitidine (VEN/AZA)
combination. The improved quality of clinical response to VEN/HMA
versus HMA alone prompted us to reflect on the possible role of the
VEN/AZA combination on the molecular response at both clonal and
subclonal levels.
Case Report
A
74-year-old man with a previous history of prostate adenocarcinoma,
originally diagnosed in 2000, relapsed in 2016, and treated with
radiotherapy, was referred to our Hematology clinic in August 2020
because of anemia and persistent monocytosis.
At the first
hematology consultation, the patient was in good general condition,
complaining of fatigue and dizziness. Complete blood counts showed mild
anemia (hemoglobin: 9.5 g/dl) and monocytosis (2.1×109/L),
with normal platelet and white blood cell (WBC) counts. Bone marrow
(BM) aspirate revealed multilineage dysplasia in more than 50% of the
cells, a 20-30% infiltrate of monocytic/promonocytic cells, and 3%
myeloid blasts (CD34+, CD117+, CD13+, CD33+, CD38+, HLA-DR+),
consistent with the diagnosis of a t-CMML.
Conventional
cytogenetic analysis revealed normal karyotype, and fluorescent in-situ
hybridization was negative for chromosomal abnormalities involving
chromosomes 5, 7, 8, and 20. The CMML CPSS score was intermediate.[8]
The
patient was then started on human recombinant erythropoietin. However,
after only two months of therapy, he was admitted to our Hematology
ward because of fever and leukocytosis (WBC: 46.9×109/L),
with blasts in the peripheral blood and increased lactate dehydrogenase
levels (LDH: 628 UI/L). The BM aspirate displayed 60% monoblasts,
confirmed both by morphology and flow cytometry
(CD34-/CD117-/HLA-DR-/CD64+/CD11b+/CD13+/CD33+/CD45+), indicating AML
evolution. At the time of admission, a simultaneous SARS-CoV-2
infection was diagnosed.
Molecular analysis for recurrent fusion genes (BCR/ABL1, RUNX1/RUNX1T1, DEK/NUP214, CBFbeta/MYH11) and common AML mutations (NPM1, FLT3, IDH1, IDH2) identified an FLT3-ITD mutation, with an allelic ratio (AR) of 0.22 by capillary electrophoresis (CE).
Given
the concomitant SARS-CoV-2 infection and the t-AML, the patient was
considered unfit for standard induction therapy and received a standard
dose of Azacitidine (75 mg/m2 for seven days, every day 28-day cycle) in combination with venetoclax (400 mg once daily, 28-day cycles).
Targeted
Next Generation Sequencing (t-NGS) was performed on DNA samples
extracted from BM mononuclear cells (MNC) at t-CMML diagnosis, at the
time of AML progression, and during treatment (at 6 and 12 months) (Figure 1).
We used the MYeloid Solution panel (MYS_1) by Sophia GENETICS (Saint
Sulpice, Switzerland) to screen for somatic mutations in 30 genes
frequently mutated in myeloid malignancies, using a variant allelic
frequency (VAF) cut-off of 1%.
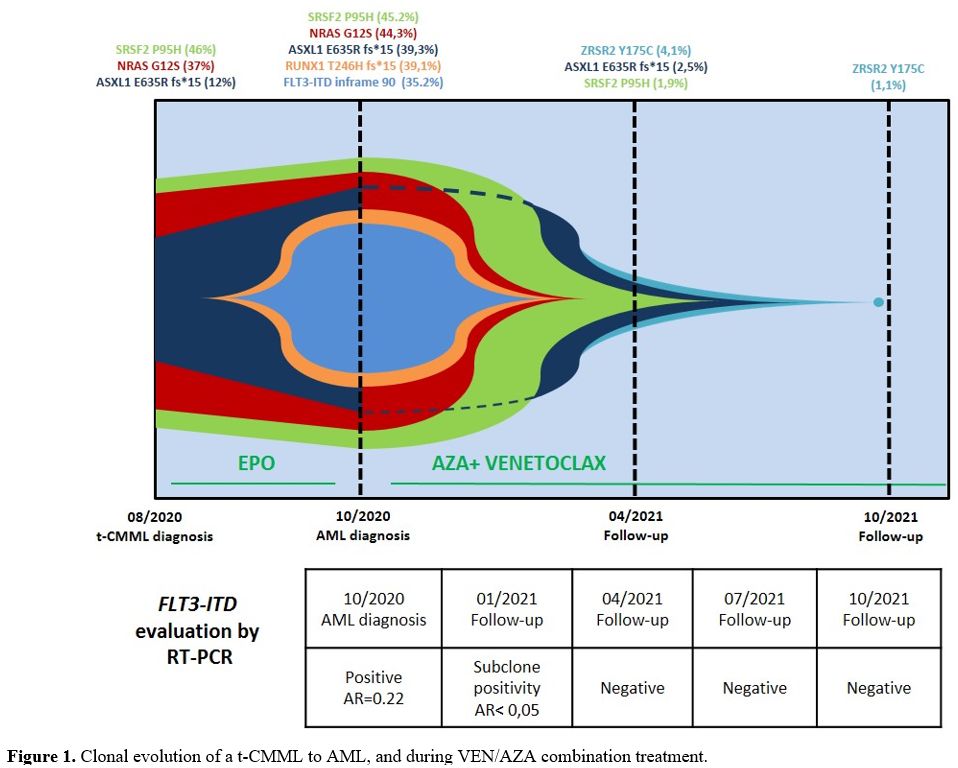 |
Figure
1. Clonal evolution of a t-CMML to AML, and during VEN/AZA combination treatment.
|
Somatic variants in 3 genes were detectable at t-CMML diagnosis, including SRSF2 p.Pro95His (P95H, VAF: 46%), NRAS p.Gly12Ser (G12S, VAF: 37%), and ASXL1 p.Glu635Arg_fs*15 (E635R _fs*15, VAF: 12%). These last variants are associated with a high probability of rapid CMML progression.[9]
Leukemic evolution was associated with the acquisition of additional mutations, including the FLT3-ITD mutation (inframe_90, VAF=35.2%, also detected by CE), and a RUNX1
p.Thr246Hisfs*15 variant (T246Hfs*15, VAF=39.1%), consistent with a
high-risk AML profile, according to ELN 2017 stratification.[10]
After
two cycles of VEN/AZA treatment, hematologic CR was achieved, residual
disease assessment showed a decrease in subclonal levels for FLT3-ITD
mutation on both DNA and RNA (AR <0.05), whereas it became negative
after six cycles. However, MRD monitoring by flow cytometry (FC) was
impaired due to the monocytic phenotype of AML blasts and the
impossibility of defining the leukemia-associated immunophenotype.
After six cycles, NRAS and RUNX1 mutations became undetectable, and the ASXL1 and SRSF2 mutation burden steeply decreased (VAF from 39.3% to 2.5% and from 45.2% to 1.9%, respectively), while a new small ZRSR2
p.Tyr175Cys (Y175C, VAF=4.1%) clone was acquired. Finally, after 12
treatment cycles, most gene mutations became undetectable, with the ZRSR2 mutation burden decreasing to 1.1% (Figure 1).
At
the time of reporting, the patient is receiving the fourteenth VEN/AZA
cycle, is in CR, and in excellent general conditions. Although he
underwent recurrent neutropenia, he did not show infectious
complications, and the treatment schedule was adjusted to 5 days
azacytidine 75 mg/sqm and 14 days venetoclax 100 mg, during cycles of
35 days.
Discussion
In
this case report, we describe the evolution of the mutational profile
of a patient with a t-CMML, rapidly progressing to AML, who was treated
with the VEN/AZA combination, achieved hematologic CR, and undetectable
somatic mutations by t-NGS.
The mutation profile of t-CMML is usually characterized by a high frequency of cytogenetic abnormalities.[11]
Despite the normal karyotype, our patient presented with mutations in
myeloid genes commonly associated with unfavorable CMML outcome (in
particular NRAS and ASXL1)
and presented a rapid progression to AML (9,11). Of note, the weakening
of the immune system, due to the co-occurrence of SARS-CoV-2 infection
at the time of AML onset, may have contributed to disease progression.
After AML onset, the patient was started on VEN/AZA combination
treatment and achieved CR after two cycles. The treatment was well
tolerated and confirmed the applicability of this reduced intensity
regimen as induction to patients with concomitant SARS-CoV-2 infection.[12]
Treatment
with Azacitidine or decitabine single agent has been shown to have a
limited impact on the mutation burden in AML and MDS. Indeed, although
HMA has been shown to reduce the size of mutated clones initially, they
are rarely eradicated, even in patients in morphologic remission.[13-15]
Furthermore, although considered disease-modifying, HMAs are not
curative in AML and MDS, and mutations may persist or be acquired at
disease recurrence and progression. This is also the case of TP53
mutations, which may become undetectable following 10-day decitabine
treatment, but tend to early recur and are associated with high rates
of disease relapse.[15] Although speculatively, our
data suggest that the addition of venetoclax to Azacitidine may have
played a crucial role in eradicating leukemic clones carrying specific
mutations that hardly would have been influenced by HMAs monotherapy,
in line with previously published data.
After four cycles of VEN/AZA combination treatment in our patient, FLT3-ITD, RUNX1 and NRAS mutations became undetectable, and there was a significant reduction of the ASXL1 and SRSF2 mutation burden.
We hypothesize that the VEN/AZA combination eradicated the FLT3-ITD and RUNX1 mutated clone/s primarily associated with AML evolution and subsequently affected the underlying SRSF2, NRAS, and ASXL1 mutated clone, which was present at the time of t-CMML diagnosis. The ZRSR2 mutated
subclone may have been initially masked by the overwhelming leukemic
population and reappeared at low VAF after restoring normal
hematopoiesis as part of clonal hematopoiesis of indeterminate
potential (CHIP).
This case shows that the addition of
venetoclax to HMA significantly improves the quality of response at the
molecular level: this is associated with prolonged disease remission,
as in our patient. These data also underline the importance of the
sequential use of t-NGS for disease monitoring: the deep molecular
remission achieved by this patient allowed to safely guide adjustments
of drug dosage and treatment intervals in the presence of neutropenia,
helping to rule out disease progression.
The achievement of
molecular negativity using the VEN/AZA combination will have to be
confirmed on larger patient series, best by using sensitive NGS
techniques. That will also help establish the role of MRD studies in
this intermediate-intensity treatment of conventional chemotherapy.
Acknowledgments
This
study was supported in part by AIRC 5x1000 call "Metastatic disease:
the key unmet need in oncology" to MYNERVA project, #21267 (MYeloid
NEoplasms Research Venture AIRC. A detailed description of the MYNERVA
project is available at http://www.progettoagimm.it), by Ricerca
finalizzata, code NET-2018-12365935 and PRIN grant N. 2017WXR7ZT to
MTV.
Author Contributions
AC,
RP, EF, AS, FB, LM, WA, CT and MTV performed research, analyzed the
data, and wrote the paper; TO and MD analyzed the data. All Authors
critically reviewed and approved the final version of the manuscript.
Competing Interests
MTV received honoraria from Celgene/BMS and Abbvie.
References
- Dombret H, Seymour JF, Butrym A, Wierzbowska A,
Selleslag D, Jang JH, et al. International phase 3 study of Azacitidine
vs conventional care regimens in older patients with newly diagnosed
AML with >30% blasts. Blood. 2015;126(3):291-9. https://doi.org/10.1182/blood-2015-01-621664
- Fenaux
P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A,
et al. International Vidaza High-Risk MDS Survival Study Group.
Efficacy of Azacitidine compared with that of conventional care
regimens in the treatment of higher-risk myelodysplastic syndromes: a
randomised, open-label, phase III study. Lancet Oncol.
2009;10(3):223-32. https://doi.org/10.1016/S1470-2045(09)70003-8
- Voso
MT, Santini V, Fabiani E, Fianchi L, Criscuolo M, Falconi G, et al. Why
methylation is not a marker predictive of response to hypomethylating
agents. Haematologica. 2014;99(4):613-9. https://doi.org/10.3324/haematol.2013.099549
- DiNardo
CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al.
Azacitidine and Venetoclax in Previously Untreated Acute Myeloid
Leukemia. N Engl J Med. 2020;383(7):617-629. https://doi.org/10.1056/NEJMoa2012971
- Rahmani NE, Ramachandra N, Sahu S,
Gitego N, Lopez A, Pradhan K, et al. ASXL1 mutations are associated
with distinct epigenomic alterations that lead to sensitivity to
venetoclax and azacytidine. Blood Cancer J. 2021;11(9):157. https://doi.org/10.1038/s41408-021-00541-0
- Cherry
EM, Abbott D, Amaya M, McMahon C, Schwartz M, Rosser J, et al.
Venetoclax and Azacitidine compared with induction chemotherapy for
newly diagnosed patients with acute myeloid leukemia. Blood Adv.
2021;5(24):5565-5573. https://doi.org/10.1182/bloodadvances.2021005538
- DiNardo
CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al.
Molecular patterns of response and treatment failure after frontline
venetoclax combinations in older patients with AML. Blood.
2020;135(11):791-803. https://doi.org/10.1182/blood.2019003988
- Such
E, Germing U, Malcovati L, Cervera J, Kuendgen A, Della Porta MG, et
al. Development and validation of a prognostic scoring system for
patients with chronic myelomonocytic leukemia. Blood.
2013;121(15):3005-15. https://doi.org/10.1182/blood-2012-08-452938
- Elena
C, Gallì A, Such E, Meggendorfer M, Germing U, Rizzo E, et al.
Integrating clinical features and genetic lesions in the risk
assessment of patients with chronic myelomonocytic leukemia. Blood.
2016;128(10):1408-17. https://doi.org/10.1182/blood-2016-05-714030
- Döhner
H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al.
Diagnosis and management of AML in adults: 2017 ELN recommendations
from an international expert panel. Blood. 2017;129(4):424-447. https://doi.org/10.1182/blood-2016-08-733196
- Patnaik
MM, Vallapureddy R, Yalniz FF, Hanson CA, Ketterling RP, Lasho TL, et
al. Therapy related-chronic myelomonocytic leukemia (CMML): Molecular,
cytogenetic, and clinical distinctions from de novo CMML. Am J Hematol.
2018;93(1):65-73. https://doi.org/10.1002/ajh.24939
- Paul
S, Rausch CR, Jain N, Kadia T, Ravandi F, DiNardo CD, et al. Treating
leukemia in the Time of COVID-19. Acta Haematol. 2021;144(2):132-145. https://doi.org/10.1159/000508199
- Craddock
C, Quek L, Goardon N, Freeman S, Siddique S, Raghavan M, et al.
Azacitidine fails to eradicate leukemic stem/progenitor cell
populations in patients with acute myeloid leukemia and myelodysplasia.
Leukemia. 2013;27(5):1028-36. https://doi.org/10.1038/leu.2012.312
- Falconi
G, Fabiani E, Piciocchi A, Criscuolo M, Fianchi L, Lindfors Rossi EL,
et al. Somatic mutations as markers of outcome after Azacitidine and
allogeneic stem cell transplantation in higher-risk myelodysplastic
syndromes. Leukemia. 2019;33(3):785-790. https://doi.org/10.1038/s41375-018-0284-9
- Welch
JS, Petti AA, Miller CA, Fronick CC, O'Laughlin M, Fulton RS, et al.
TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic
Syndromes. N Engl J Med. 2016 Nov;375(21):2023-2036. https://doi.org/10.1056/NEJMoa1605949
[TOP]
