Jassada Buaboonnam1, Chonthida Wangkittikal1, Nattee Narkbunnam1, Nassawee Vathana1, Chayamon Takpradit1, Kamon Phuakpet1, Phakatip Sinlapamongkolkul2, Kleebsabai Sanpakit1, Khemajira Karaketklang3 and Bunchoo Pongtanakul1.
1 Division of
Hematology and Oncology, Department of Pediatrics, Faculty of Medicine
Siriraj Hospital, Mahidol University, Bangkok, Thailand.
2 Department of Pediatrics, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand.
3 Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Correspondence to: Bunchoo
Pongtanakul, MD. Associate Professor of Pediatrics. Division of
Hematology and Oncology, Department of Pediatrics, Faculty of Medicine,
Siriraj Hospital, Mahidol University 2 Wanglang Road, Bangkok Noi,
Bangkok 10700, Thailand. Tel: +66 2 419 5960; Fax: +66 2 411 3010.
E-mail:
pongtanakul@yahoo.com
Published: January 1, 2023
Received: August 9, 2022
Accepted: December 15, 2022
Mediterr J Hematol Infect Dis 2023, 15(1): e2023004 DOI
10.4084/MJHID.2023.004
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Several disseminated intravascular coagulation (DIC) scoring systems
are used for prognosticating the clinical outcomes of patients with
DIC. However, research on children is scarce. Therefore, this study
compared the clinical outcomes of overt and non-overt DIC using the
International Society on Thrombosis and Hemostasis (ISTH) DIC scoring
system.
Methods: This retrospective study reviewed data on children aged one month to 15 years diagnosed with DIC between 2003 and 2014.
Results:
Of 244 patients, 179 (73.4%) had overt DIC, and 65 (26.6%) had
non-overt DIC. The most common causes were infection (84.8%), tissue
injury (7%), and malignancies (2.9%). The 28-day case fatality rate was
significantly higher for overt than non-overt DIC (76% vs. 15.6%; P < 0.001). DIC scores were significantly associated with mortality (R2
= 0.89). Each clinical parameter (platelet count, prothrombin time, and
fibrin degradation products) was associated with mortality (P = 0.01). On multivariable analysis, the factors associated with death were platelet counts ≤ 50 000 cells/mm3 (OR, 2.42; 95% CI, 1.08–5.42; P = 0.031); overt DIC score (OR, 7.62; 95% CI, 2.94–19.75; P < 0.001); renal dysfunction (OR, 2.92; 95% CI, 1.34–6.37; P = 0.007); shock (OR, 39.62; 95% CI, 4.99–314.84; P = 0.001); and acute respiratory distress syndrome (OR, 25.90; 95% CI, 3.12–214.80; P = 0.003).
Conclusions:
The 28-day case-fatality rate was significantly higher for patients
with overt than non-overt DIC and concordant with ISTH scores. ISTH DIC
scores can be used as a clinical predictor for DIC in children.
|
Introduction
Disseminated
intravascular coagulation (DIC) is caused by excessive hemostatic
system activation. The disease leads to consumptive coagulopathy,
microthrombi formation, and severe bleeding. Ultimately, it results in
multiorgan dysfunction, manifested in conditions such as trauma,
malignancy, and sepsis.[1] DIC is responsible for mortality in such conditions in both child and adult patients.[2,3]
Several
scoring systems have demonstrated value in diagnosing DIC. The
International Society on Thrombosis and Haemostasis (ISTH) DIC scoring
system draws upon prothrombin time, platelet count, fibrinogen, and
D-dimer levels, and it has been widely studied in mainly adult patients
with DIC.[4] The ISTH DIC system can prognosticate the outcomes of patients in critical condition due to sepsis and non-sepsis etiologies.[5,6]
Nevertheless, research on the efficacy of the scoring system for the
pediatric population is scarce. Therefore, evaluating ISTH DIC scores
in children with DIC may assist physicians in predicting clinical
outcomes. This study aimed to evaluate and compare the clinical
outcomes of children with overt DIC and non-overt DIC using the ISTH
scoring system.
Patients and Methods
This
retrospective study was performed on patients aged 28 days to 15 years
who had been diagnosed with DIC during admission at Siriraj Hospital,
Mahidol University, Thailand, between January 2005 and December 2014.
The clinical parameters of the patients at admission were rated using
the ISTH DIC scoring system as follows:[4]
• Platelet count: > 100 000 cells/mm3 = 0 points; between 50 000 and 100 000 cells/mm3 = 1 point; and < 50 000 cells/mm3 = 2 points
•
Prolonged prothrombin time: < 3 seconds = 0 points; between 3 and 6
seconds = 1 point; and > 6 seconds = 2 points
• Fibrinogen level: > 100 mg/dL = 0 points, and < 100 mg/dL = 1 point
• D-dimer level: no increase = 0 points; moderate increase = 2 points; and strong increase = 3 points
The
patients were classified into two groups: (1) “overt DIC”, for those
with ISTH DIC scores ≥ 5, and (2) “non-overt DIC”, for patients with
ISTH DIC scores < 5.
Patients who were previously diagnosed
with DIC were treated with blood components within 24 hours before the
diagnosis of DIC or had incomplete laboratory parameters of the ISTH
DIC scoring system were excluded. Specific organ dysfunctions were
classified according to the international pediatric sepsis consensus
conference[7] as follows,
Central nervous system dysfunction was defined as a Glasgow coma score ≤ 11 or decreased Glasgow coma score ≥ 3 from baseline.
Respiratory
system dysfunction was defined as the ratio of partial pressure of
arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2),
PaO2/FiO2 < 300 without evidence of cyanotic heart disease and
preexisting pulmonary disease or PaCO2 > 65 torr or > 20 mmHg of
baseline or requiring oxygen FiO2 > 0.5 to maintain oxygen
saturation ≥ 92% or requiring non-elective invasive or non-invasive mechanical ventilator
Renal dysfunction was defined as the serum creatinine ≥ 2 times for age or increased serum creatinine > 2 times from baseline.
Hepatic dysfunction was defined as total bilirubin ≥ 4 mg/dL or alanine transaminase > 2 times from baseline.
Descriptive
statistics were used to detail demographic and clinical characteristic
data. Continuous data are presented as medians and ranges, while
categorical data are reported as numbers and percentages. Pearson’s
chi-squared test was used to compare the proportions of groups with
categorical data, and Student’s t-test or the Mann–Whitney U test was
used to compare medians for continuous data. Univariable and
multivariable predictors of death were evaluated using binary logistic
regression analysis (backward method), with results presented as the
odds ratio (OR) and 95% confidence interval (CI). A probability (P)
value < 0.05 was considered statistically significant. All analyses
were performed using PASW Statistics for Windows, version 18.0 (SPSS
Inc, Chicago, IL, USA).
Results
Of
the 67 992 inpatient cases, 244 patients were diagnosed with DIC,
giving a frequency of DIC of 0.35. There were 118 male patients (48.4%)
and 126 female patients (51.6%); the age group breakdown of 1 month – 1
year, 1-5 years, 5-10 years, and more than 10 years were 71 (29.1%), 78
(32%), 50 (20.5%) and 45 (18.4%) patients, respectively. Infection was
the most common cause of DIC (84.8%), with tissue injury being the
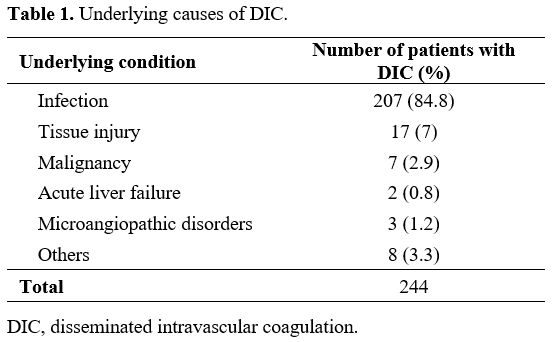
second most common cause (7%); other causes are detailed in Table 1.
Gram-negative bacterial infection was the most common cause of
infection-associated DIC. The other causative organisms are detailed in
Table 2. Of the 17 patients
with DIC secondary to tissue injury and surgery, open-heart surgery for
congenital heart disease (9 patients) was the most common cause.
Postorgan transplantation ranked second with 5 patients (liver
transplantation, 4 patients; kidney transplantation, 1 patient),
followed by intra-abdominal surgery (2 patients) and thermal injury (1
patient). There were 7 patients with malignancies; of these,
hematologic malignancies (4 patients) were the most common cause (2
patients with acute lymphoblastic leukemia, 1 patient with acute
myeloid leukemia, and 1 patient with non-Hodgkin lymphoma). The other 3
patients had solid tumors: 1 with hepatoblastoma, another with
neuroblastoma, and the third with an endodermal sinus tumor. Hemorrhage
was the most common manifestation (153 patients; 62.7%), with
gastrointestinal hemorrhage being the most common site (41.8%),
followed by endotracheal hemorrhage (24.2%) and hematuria (7.8%).
Thrombosis was diagnosed in 20 patients (8.1%); venous thrombosis was
the most common site (45%), followed by peripheral gangrene (40%).
 |
Table
1. Underlying causes of DIC. |
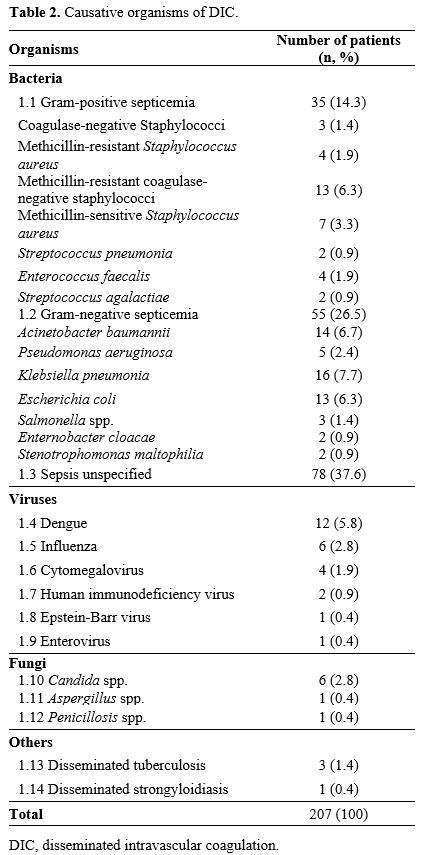 |
Table
2. Causative organisms of DIC.
|
The laboratory parameters of the patients with overt and non-overt DIC are compared in Table 3.
Of the 244 patients with DIC, 179 (73.3%) were diagnosed with overt
DIC, and the remaining 65 (26.7%) had non-overt DIC. The 28-day case
fatality rate for overt DIC (76%) was significantly higher than that
for non-overt DIC (15.4%; P
< 0.001). The median time from the diagnosis of DIC to death was 7.1
days (0–37 days), while the median time from diagnosis of DIC to
recovery was 14 days (2–61 days). The correlation between mortality and
the ISTH DIC scores is illustrated in Figure 1 (R2 = 0.89).
 |
Table 3. Comparison of laboratory parameters of patients with overt and non-overt DIC. |
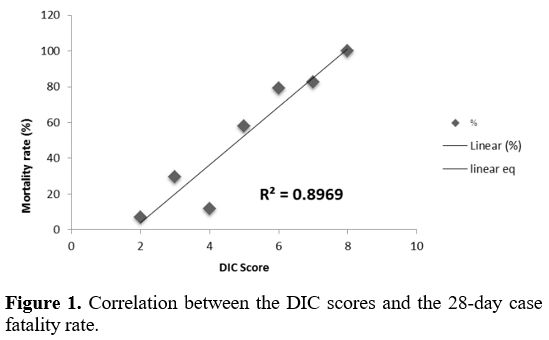 |
Figure 1. Correlation between the DIC scores and the 28-day case fatality rate.
|
In
terms of admission, 209 patients (85.7%) were admitted to the intensive
care unit (ICU), whereas the other 35 patients (14.3%) were not. The
rates of ICU admission of the patients with overt and non-overt DIC
were not significantly different (P
= 0.129). Anticoagulant was prescribed for 9 patients; the most
commonly used anticoagulant was unfractionated heparin (4 patients),
followed by low molecular weight heparin (3 patients) and warfarin (2
patients). Of the 9 patients requiring anticoagulant therapy, 4 had
venous thrombosis, 4 had arterial thrombosis, and one prophylactically
received an anticoagulant to prevent clotting after cardiac surgery.
Recombinant factor VIIa was prescribed for 9 patients. Neither
treatment-associated hemorrhage nor thrombosis was observed. The most
typical indication was dengue hemorrhagic fever with severe hemorrhage
(6 patients). The other indications were cancer with severe hemorrhage
(2 patients) and chronic liver disease requiring a postsurgery bleeding
prophylactic (1 patient). The factors associated with the death of DIC
patients are presented in Table 4, and the laboratory parameters of the decedents and survivors are compared in Table 5. Univariable and multivariable factors associated with death in DIC are detailed in Table 6.
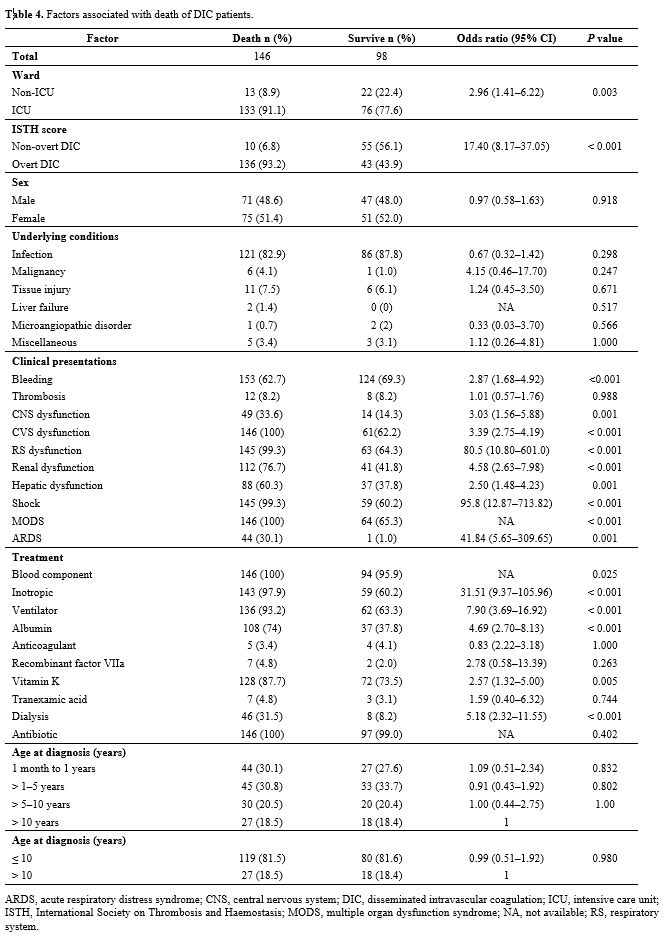 |
Table 4. Factors associated with death of DIC patients. |
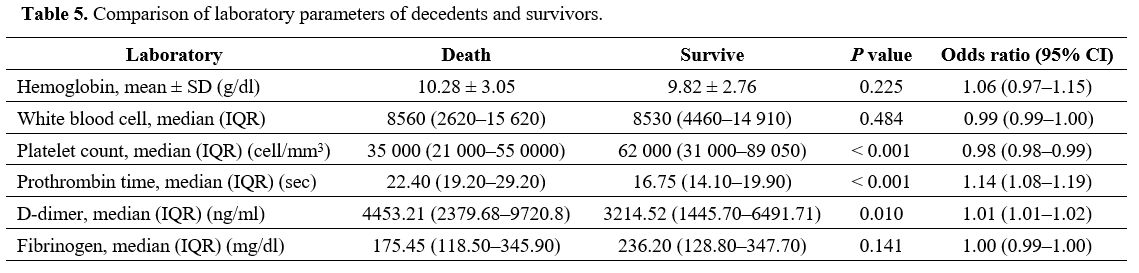 |
Table 5. Comparison of laboratory parameters of decedents and survivors. |
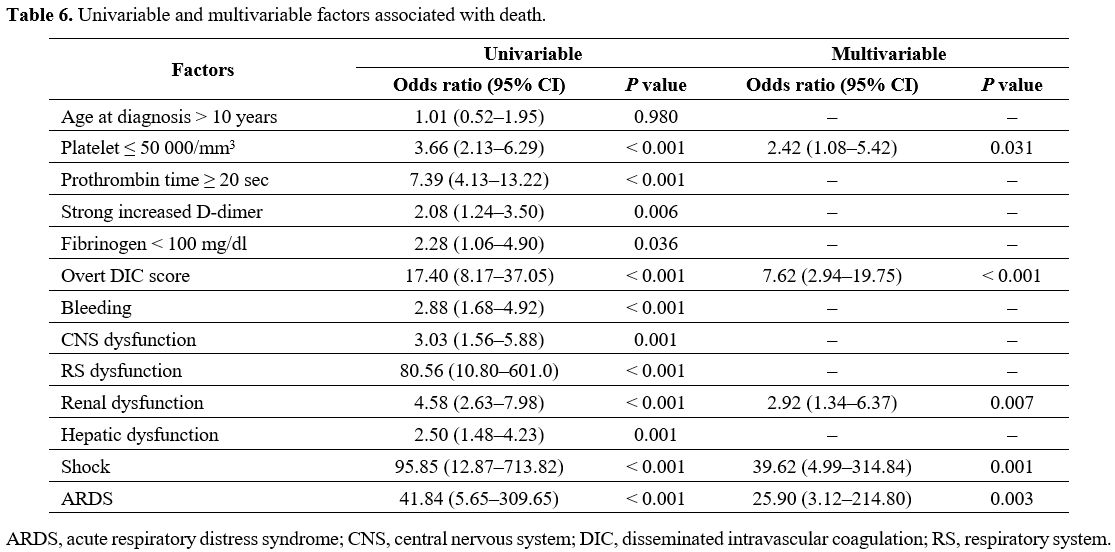 |
Table 6. Univariable and multivariable factors associated with death.
|
Discussion
Studies
regarding DIC in children are scarce. Oren et al. reported that the
frequency of DIC in the pediatric population was 1.12 hospitalized in a
Turkey University Hospital,[8] similar to the present study’s finding of 0.35. However, the frequency in children is lower than in adults (34.4).[9]
Additionally, the present investigation found that infections were the
most common etiology of DIC, which is consistent with previous studies
on adults and children.[8,9] On the other hand, the
proportion of children in the current study with infection
(approximately 80%) is markedly higher than the corresponding levels
previously reported for adults (30%–40%).[10]
Furthermore, the rate of malignancy-related DIC seems lower in children
than adults, which may indicate differences in the etiologies of the
disease in children and adults. Prevalent in tropical regions, dengue
hemorrhagic fever can cause thrombocytopenia, plasma leakage, and
decreased coagulation factors secondary to hepatic derangement, the
combined effects of which lead to DIC.[11] Dengue
hemorrhagic fever was the most common cause of viral-associated DIC in
the present study; however, this phenomenon might be uncommon in
countries where dengue is not endemic. Similarly, our investigation
determined that tropical diseases such as disseminated tuberculosis and
strongyloidiasis also caused DIC. Therefore, physicians in tropical
regions caring for patients with such diseases should be aware of DIC
as a peculiar clinical manifestation.
The hemostasis in infants,
especially neonates, differs from that in adults. The decreased
coagulation factors and natural anticoagulants gradually reach the
normal level at approximately six months of age, leading to the
counterbalance of hemostasis.[12] The prolonged
prothrombin time in such patients might not reflect the proper
hemostasis. Therefore, in this cohort, neonates with DIC were excluded
from the study.
The clinical severity of hemorrhage and organ
failure might be related to the etiologies of DIC in adults. Research
on adults showed that multiorgan failure was prevalent in
infection-associated DI; in contrast, hemorrhage was common in
noninfectious-associated DIC.[13,14] Furthermore, the
bleeding tendency in these adult studies appeared to be lower than that
of the present pediatric study. The different populations and DIC
etiologies of the adult and pediatric investigations may account for
the variations in the observed clinical manifestations. The present
work identified a thrombosis incidence of 8.1%, comparable with other
studies on adult and pediatric populations, and neither arterial nor
venous sites predominated.[8,15]
Consequently, clinical vigilance of thromboembolic complications is
needed in both pediatric and adult patients with DIC. In terms of
treatment-associated both hemorrhagic and thrombotic complications,
such complications were not observed in this cohort; this may result
from the scarcity of patients treated with recombinant factor VIIa,
tranexamic acid, and anticoagulant.
Concordant with other studies,[16,17]
the 28-day case fatality rate of children with overt DIC or those with
organ dysfunction requiring advanced organ support was significantly
higher than that for children with non-overt DIC. Additionally,
mortality was significantly correlated with the ISTH DIC score and the
clinical parameters platelet number, prothrombin time, and D-dimer.
Similarly, our multivariable analysis revealed that overt DIC and
thrombocytopenia below 50 000/mm3
were associated with death. These results substantiate the role of the
ISTH DIC scoring system in predicting the clinical outcomes of DIC in
the pediatric population, with platelet number possibly being a pivotal
clinical factor in prognosticating the risk of death in DIC. Although
other factors (underlying diseases and age at diagnosis) were not
significantly associated with mortality, preexisting cancer tended to
be correlated with mortality. Therefore, patients with a high ISTH DIC
score, especially those with preexisting cancer, should be closely
monitored, and treatment interventions for underlying diseases should
be delivered promptly.
Compared to the score of 5, the ISTH score of 3 demonstrated a better mortality prediction in sepsis-associated DIC.[18] Furthermore, other DIC scoring systems, namely Texas Children’s Hospital criteria[19] and Japanese Association for Acute Medicine criteria[20]
were previously described and demonstrated a good prediction of
clinical outcome. However, the former system required sequential
evaluation by specialists while the latter required the anti-thrombin
level, which was somewhat not performed; these scoring systems might
not be applicable in our institute. Taken together, the heterogeneity
of results and scoring system substantiated the warrant of further
investigation of scoring systems in the pediatric population.
This
study had some limitations. First, since this was a retrospective
study, there is the possibility of missing or incomplete data. Second,
given that the population was drawn from a national tertiary referral
hospital, where some tropical diseases appeared to be prevalent, the
data may not be generalized to other populations or clinical settings;
therefore, the prevalence and causation of DIC may vary from other
studies.
Conclusions
Infection
was the most common cause of DIC. The children with overt DIC had a
higher mortality rate than those with non-overt DIC. The ISTH scoring
system can predict the clinical outcomes of children with DIC.
Acknowledgments
The authors are indebted to Mr David Park and Mrs. Sam Ormond for the English-language editing of this paper.
Author Contributions
BP
designed the study; CW, JB, NN, NV, CT, KP, KK, PS, and KS collected
and analyzed data; JB and BP wrote the manuscript; all authors read and
approved the final manuscript.
References
- Gando S, Levi M, Toh C-H. Disseminated
intravascular coagulation. Nat Rev Dis Primers 2016; 2: 16037. https://doi.org/10.1038/nrdp.2016.37 PMid:27250996
- Kongstad
C, Mikkelsen TS, Hvas AM. Disseminated intravascular coagulation in
children with cancer: A systematic review. Pediatr Hematol Oncol 2020;
37: 390-411. https://doi.org/10.1080/08880018.2020.1733717 PMid:32202958
- Niederwanger
C, Bachler M, Hell T, et al. Inflammatory and coagulatory parameters
linked to survival in critically ill children with sepsis. Ann
Intensive Care 2018; 8: 111. https://doi.org/10.1186/s13613-018-0457-8 PMid:30446841 PMCid:PMC6240023
- Taylor
FB, Jr., Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical
and laboratory criteria, and a scoring system for disseminated
intravascular coagulation. Thromb Haemost 2001; 86: 1327-1330. https://doi.org/10.1055/s-0037-1616068 PMid:11816725
- Helms
J, Severac F, Merdji H, et al. Performances of disseminated
intravascular coagulation scoring systems in septic shock patients. Ann
Intensive Care 2020; 10: 92. https://doi.org/10.1186/s13613-020-00704-5 PMid:32651674 PMCid:PMC7352012
- Grafeneder
J, Krychtiuk KA, Buchtele N, et al. The ISTH DIC score predicts outcome
in non-septic patients admitted to a cardiovascular intensive care
unit. Eur J Intern Med 2020; 79: 37-42. https://www.sciencedirect.com/science/article/pii/S0953620520302557 https://doi.org/10.1016/j.ejim.2020.06.017 PMid:32622514
- Goldstein
B, Giroir B, Randolph A. International pediatric sepsis consensus
conference: definitions for sepsis and organ dysfunction in pediatrics.
Pediatr Crit Care Med 2005; 6: 2-8. https://doi.org/10.1097/01.PCC.0000149131.72248.E6 PMid:15636651
- Ören
H, Cingöz I, Duman M, Yılmaz S, Irken G. Dsseminated intravascular
coagulation in pediatric patients: Clinical and laboratory features and
prognostic factors influencing the survival. Pediatr Hematol Oncol
2005; 22: 679-688. https://doi.org/10.1080/08880010500278749 PMid:16251173
- Okamoto
K, Wada H, Hatada T, et al. Frequency and hemostatic abnormalities in
pre-DIC patients. Thromb Res 2010; 126: 74-78. https://doi.org/10.1016/j.thromres.2010.03.017 PMid:20452653
- Wu
Y, Luo L, Niu T, et al. Evaluation of the new Chinese disseminated
intravascular coagulation scoring system in critically ill patients: A
multicenter prospective study. Sci Rep 2017; 7: 9057. https://doi.org/10.1038/s41598-017-09190-5 PMid:28831134 PMCid:PMC5567287
- Nurnaningsih,
Sunbanu SE, Rusmawatiningtyas D, Arguni E, Makrufardi F, Kumara IF.
Disseminated intravascular coagulation initial score as a predictor of
mortality in children with dengue shock syndrome: A retrospective
cohort study. Ann Med Surg 2022; 79: 103890. https://doi.org/10.1016/j.amsu.2022.103890 PMid:35860092 PMCid:PMC9289251
- Davenport P, Sola-Visner M. Hemostatic challenges in neonates. Front Pediatr 2021; 9: 627715. https://doi.org/10.3389/fped.2021.627715 PMid:33738269 PMCid:PMC7960661
- Okajima
K, Sakamoto Y, Uchiba M. Heterogeneity in the incidence and clinical
manifestations of disseminated intravascular coagulation: a study of
204 cases. Am J Hematol 2000; 65: 215-222. https://doi.org/10.1002/1096-8652(200011)65:3<215::AID-AJH7>3.0.CO;2-7 PMid:11074538
- Ohbe
H, Yamakawa K, Taniguchi K, et al. Underlying disorders, clinical
Phenotypes, and treatment diversity among patients with disseminated
intravascular coagulation. Jma J 2020; 3: 321-329. https://doi.org/10.31662/jmaj.2020-0023
- Mahanupap
P, Angchaisuksiri P, Rattanasiri S. Disseminated intravascular
coagulation in Ramathibodi hospital. J Hem Transfus Med 2010; 20:
27-38.
- Khemani RG, Bart RD, Alonzo TA,
Hatzakis G, Hallam D, Newth CJL. Disseminated intravascular coagulation
score is associated with mortality for children with shock. Intensive
Care Med 2009; 35: 327-333. https://doi.org/10.1007/s00134-008-1280-8 PMid:18802683
- Kunwar
S, Alam M, Ezekwueme F, et al. Diagnostic scores and treatment options
for acute disseminated intravascular coagulation in children. Cureus
2021; 13: e17682. https://doi.org/10.7759/cureus.17682
- Slatnick
LR, Thornhill D, Deakyne Davies SJ, et al. Disseminated intravascular
coagulation is an independent predictor of adverse outcomes in children
in the emergency department with suspected sepsis. J Pediatr 2020; 225:
198-206.e192. https://doi.org/10.1016/j.jpeds.2020.06.022 PMid:32553867 PMCid:PMC7529972
- Soundar
EP, Jariwala P, Nguyen TC, Eldin KW, Teruya J. Evaluation of the
international society on thrombosis and haemostasis and institutional
diagnostic criteria of disseminated intravascular coagulation in
pediatric patients. Am J Clin Pathol 2013; 139: 812-816. https://doi.org/10.1309/AJCPO64IWNLYCVVB PMid:23690126 PMCid:PMC5281061
- Jhang
WK, Ha EJ, Park SJ. Evaluation of disseminated intravascular
coagulation scores in critically ill pediatric patients. Pediatr Crit
Care Med 2016; 17. https://doi.org/10.1097/PCC.0000000000000705 PMid:27028791
[TOP]






