Irene Urbino1, Chiara Frairia1, Alessandro Busca2, Silvia Corcione3,4, Stefano D’Ardia1, Chiara Maria Dellacasa2, Valentina Giai1, Carolina Secreto1, Roberto Freilone1, Francesco Giuseppe De Rosa3, Semra Aydin1,5, Giovannino Ciccone6, Rosalba Rosato6, Marco Cerrano1 and Ernesta Audisio1.
1 Department of Oncology, Division of Hematology, Città della Salute e della Scienza, Turin, Italy.
2 Department of Oncology, SSCVD Trapianto di Cellule Staminali, Città della Salute e della Scienza, Turin, Italy.
3 Department of Medical Sciences, Unit of Infectious Diseases, University of Turin, Turin, Italy.
4 Tufts University School of Medicine, Boston, MA, USA.
5 Department of Oncology, Hematology, Immuno-oncology and Rheumatology, University Hospital of Bonn, Bonn, Germany.
6 Unit of Clinical Epidemiology, CPO, Città della Salute e della Scienza, Turin, Italy.
Correspondence to:
Urbino Irene, Department of Oncology, Division of Hematology, Città
della Salute e della Scienza di Torino, Corso Bramante 88, Torino –
Italy. Tel: (+39) 0116335551/5550. E-mail:
urbinoirene@gmail.com
Published: March 1, 2023
Received: January 21, 2023
Accepted: February 20, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023022 DOI
10.4084/MJHID.2023.022
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Acute myeloid leukemia (AML) patients are at high risk of infections
during post-induction neutropenia. Recently, the role of antibacterial
prophylaxis has been reconsidered due to concerns about the emergence
of multi-resistant pathogens. The aim of the present study was to
evaluate the impact of avoiding prophylaxis on the rate of induction
death (primary endpoint), neutropenic fevers, bloodstream infections
(BSIs), resistant pathogens BSIs and septic shocks (secondary
endpoints).
Methods: We
performed a retrospective single-center study including 373 AML
patients treated with intensive induction chemotherapy, divided into
two groups according to levofloxacin prophylaxis given (group A, gA) or
not (group B, gB).
Results: Neutropenic
fever was observed in 91% of patients in gA and 97% in gB (OR 0.35,
IC95% 0.08 – 1.52, p=0162). The rate of BSIs was 27% in gA compared to
34% in gB (OR 0.69, 0.38 – 1.25, p=0.222). The induction death rate was
5% in gA and 3% in gB (OR 1.50, 0.34 – 6.70, p=0.284). Fluoroquinolones
(FQ) resistant pathogens were responsible for 59% of total BSIs in gA
and 22% in gB (OR 5.07, 1.87 – 13.73, p=0.001); gram-negative BSIs due
to multi-drug resistant organisms were 31% in gA and 36% in gB (OR
0.75, 0.15 – 3.70, p=0.727).
Conclusions: Despite
its limitations (retrospective nature, single-center, different cohort
size), the present study showed that avoiding levofloxacin prophylaxis
was not associated with an increased risk of induction death. The
cumulative incidence of neutropenic fever was higher in non-prophylaxis
group, while no difference was observed for BSIs. In the prophylaxis
group we observed a higher incidence of FQ-resistant organisms.
|
Introduction
Bacterial
infections are a major cause of morbidity and mortality in patients
with acute myeloid leukemia (AML) during neutropenia following
induction chemotherapy.[1] According to the Infectious Diseases Society
of America (IDSA) guidelines,[2] the risk of infections in neutropenic
patients is classically divided into high (prolonged profound
neutropenia >7 days) and low risk (neutropenia expected to resolve
within 7 days). AML patients treated with intensive induction
chemotherapy are expected to have long aplasia periods (neutrophils
count < 500/mm3) lasting between 15 and 25 days, placing them at high risk for infections.
The
use of antibacterial prophylaxis has been the standard of care since
2005 when Bucaneve et al. published a prospective randomized trial
showing that prophylactic treatment with levofloxacin was effective in
preventing febrile episodes and bacteremia in neutropenic patients with
cancer.[3] In 2007 antibacterial prophylaxis with fluoroquinolones (FQ)
was recommended by the European Conference on Infections in Leukemia
(ECIL) group for high-risk neutropenic patients.[4] However, in recent
years concerns have been raised about the worldwide emergence of
multi-drug resistant (MDR) pathogens.[5] As previously reported, the
incidence of MDR gram-negative bacteria has increased among AML
patients during subsequent consolidation chemotherapy.[6] In the era of
increasing antibiotic resistance, understanding antibacterial
prophylaxis's real efficacy is of utmost importance. Randomized
controlled and observational trials after 2006 report possible benefits
of FQ prophylaxis on febrile neutropenia and bloodstream infections
(BSI) rate but not on overall mortality.[7,8] More recently, some
international guidelines still recommended FQ prophylaxis in patients
who are at high risk for febrile neutropenia (National Institute for
Health and Care Excellence – NICE,[9] German Society of Hematology and
Medical Oncology – DGHO,[10] American Society of Clinical Oncology -
ASCO and IDSA,[11] National Comprehensive Cancer Network – NCCN).[12]
By contrast, Australian guidelines gave a low level (grade C) of
recommendation for antibacterial prophylaxis in high-risk patients;[13]
similarly, the European Society for Medical Oncology (ESMO) guidelines
on the management of febrile neutropenia discourage the use of
antimicrobial, including FQ, for prophylaxis.[14] In 2017 the ECIL
group analyzed the emergence of antimicrobial resistance in
gram-negative rods and questioned the recommendations for FQ
prophylaxis, underscoring the need for up-to-date, evidence-based data
on local epidemiology.[7]
Following these considerations, the aim
of the present study was to evaluate the impact of avoiding
antibacterial prophylaxis on infections and early mortality rates in
AML patients during post-induction aplasia.
Material and Methods
This
retrospective analysis was conducted at the Department of Oncology and
Hematology AOU. Città della Salute e della Scienza of Turin, Italy.
All consecutive adult patients with AML (excluding acute promyelocytic
leukemia) diagnosed between June 2001 and March 2019 and treated with
intensive induction chemotherapy were enrolled in the study. Patients
treated until December 2016 received antibacterial prophylaxis with
levofloxacin 500 mg QD during post-induction aplasia, as common past
practice in our center. Considering the locally increased incidence of
FQ-resistant and extended-spectrum beta-lactamase (ESBL) producing
gram-negative bacteria during consolidation chemotherapy and based on
the worldwide emergence of multi-resistant pathogens, from 2017, we
decided to discontinue the administration of FQ prophylaxis.
Consequently, for the study analysis, patients have been divided into
two groups on the basis of antibacterial prophylaxis administration:
group A included patients who received levofloxacin from June 2001 to
December 2016, and group B those who did not, from January 2017 to
March 2019.
All patients were treated with intensive induction
regimens containing cytarabine arabinoside and anthracyclines.[15]
Different doses of cytarabine were administered depending on the
chemotherapy schedule: high doses in fludarabine-based regimens[16,17]
and standard doses in a 7 + 3-like scheme.[18] Chemotherapy was
administered through a central venous catheter (CVC, Hohn, or Picc).
Patients presenting at diagnosis with infectious fever were excluded
from the analysis. Neutropenic fever was defined as a temperature ≥
38.0°C during post-induction aplasia. In all febrile patients,
empirical antibiotics were promptly started; the approach remained
similar for both analyzed periods and involved a broad-spectrum
antibiotic therapy with a beta-lactam mostly associated with an
aminoglycoside. CVC-related BSIs (CR-BSI) were defined by
differential time to positivity (DTP) criteria: growth of microbes from
a catheter blood sample should precede by at least 2 hours microbial
growth detected in a blood sample obtained from a peripheral vein.[19]
The definition of septic shock was established according to the 2016
Sepsis and Septic Shock Consensus Definitions.[20] Early induction
death was defined as all-cause mortality during the induction cycle,
referred to as the period from the first day of chemotherapy until the
post-induction bone marrow revaluation within a maximum of 35 days.
When
there were two bacteremia episodes in patients, to assess the incidence
of resistant organisms, both were counted. All bacteria specimens
isolated from blood cultures were considered, including the multiple
specimens detected in polymicrobial BSI. Data about colonized rectal
swabs and their impact on BSI in hospitalized patients were available
from 2017 when we started weekly testing for colonization with ESBLs
and carbapenem-resistant Enterobacteriaceae (CRE). For all the
isolates, MALDI-TOF MS analysis was used for bacterial identification,
and antimicrobial susceptibility testing was carried out using
Microscan WalkAway plus System (Beckman Coulter, Brea, CA, USA),
according to the manufacturer's instructions. Mastdiscs® combi Carba
plus disc system (Mast Group Ltd, Bootle, UK) was used to characterize
carbapenemase producers when meropenem MIC was >0.125 μg/ml.
Carbapenem resistance genes were detected using the Xpert Carba-R assay
(Cepheid, Sunnyvale, CA, USA). ESBL-E production was confirmed by
standard test (NBC 46, Beckman Coulter, Brea, California, USA).
Antimicrobial susceptibilities were interpreted according to EUCAST.
Statistical analysis.
The study's primary endpoint was the rate of induction death; secondary
endpoints were the rate of neutropenic fever, BSI, and septic shock. An
additional objective was to assess the potential role of FQ prophylaxis
on the emergence of antibiotic resistance, particularly the incidence
of FQ-resistant organisms and MDR gram-negative pathogens. The median
and standard deviation for continuous variables and percentage for
discrete variables were used to describe the sample. Mann-Whitney test
for continuous and Fisher exact test for categorical variables were
used to compare patients and disease characteristics between the study
groups. The effect of omitting antibacterial prophylaxis on the
induction death rate, neutropenic fever, BSI, and septic shocks was
estimated using four logistic regression models adjusted for age,
gender, cytarabine doses (> or < 1g/m2), duration of aplasia (≤ 15 days; 15 < days < 20; ≥
20 days) and genetic risk stratification (favorable, intermediate or
adverse).[15] Results are presented as Odds Ratio (OR). The cumulative
incidence of fever and BSI was calculated in patients receiving or not
levofloxacin prophylaxis, applying competing risk analysis with death
and progression disease as competing events. The Gray test compared
cumulative incidence curves between the two study groups. The
Kaplan-Meier curves were estimated to depict overall survival (OS) in
patients with or without prophylaxis and compared by log-rank test.
Results
Patient characteristics.
A total of 373 AML patients with a median age of 56 (range 18-76)
years, of whom 55% were males, were included in the present study.
Complete remission (CR) was achieved in 267 patients (72%), while 84
were resistant (22%), and 22 died (6%) during induction. The group
receiving levofloxacin prophylaxis (group A) included 315 patients,
while the group not receiving prophylaxis (group B) included 58
patients. The different periods of observation (16 years vs. 1.5 years)
were responsible for the group sizes. The median age at diagnosis was
different in the two groups (55 vs. 60 years in group A and B,
respectively, p=0.0025). Patients' characteristics are summarized in Table 1.
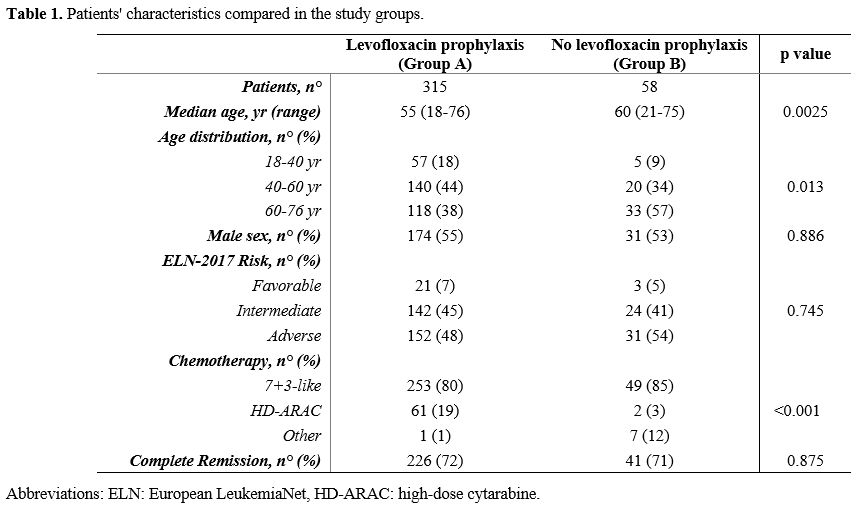 |
- Table 1. Patients' characteristics compared in the study groups.
|
Efficacy of FQ prophylaxis.
A total of 286 patients (91%) developed at least one episode of
neutropenic fever in group A and 56 patients (97%) in group B (OR 0.35,
IC95% 0.08 – 1.52, p=0.162). Among febrile patients, neither clinical
infections nor microbiological isolates (fever of unknown origin - FUO)
were found in 36% (n=104) of patients in group A and 23% (n=13) in
group B. A clinical infection was diagnosed in 43% (n=123) of patients
in group A (n=69 pneumonia, n=32 enterocolitis, n=22 other clinical
infections) and 75% (n=42) of patients in group B (n=15 pneumonia, n=22
enterocolitis, n=5 other clinical infections). Overall, 84 patients
(27%) in group A and 20 patients (34%) in group B had at least one
episode of bacteremia (OR 0.69, IC95% 0.38 – 1.25, p=0.222).
CVC-related BSIs were documented in 1% (n=3) of febrile patients in
group A and 5% (n=3) in group B. Of note, a higher cumulative incidence
(CI) of neutropenic fever during the 35 days after induction
chemotherapy was observed in patients who did not receive levofloxacin
prophylaxis (96.6 vs 90.6%, p=0.0003; Figure 1A) while the CI of BSI did not differ significantly between the two groups (26.7% in group A vs. 35.3% in group B, p=0.163; Figure 1B).
A septic shock was recorded in 15 (5%) febrile patients in group A vs.
4 (7%) in group B (OR 0.68, IC95% 0.22 – 2.11, p=0.499). Among them, 4
patients in group A and no patients in group B required an intensive
care unit (ICU) admission. Mortality due to septic shock was 75% in the
prophylaxis group (n=11) and 25% in the non-prophylaxis group (n=1).
The induction death rate was comparable in both groups, being 5% (n=16)
in group A and 3% (n=2) in group B (OR 1.50, IC95% 0.34 – 6.70,
p=0.284). Kaplan-Meier OS curves are shown in Figure 2. Table 2 summarizes the overall results.
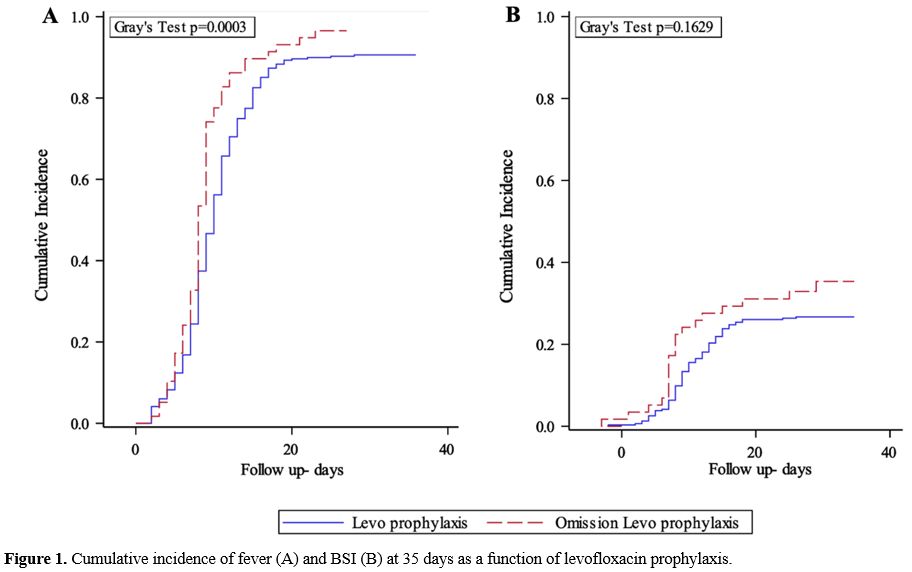 |
Figure 1. Cumulative incidence of fever (A) and BSI (B) at 35 days as a function of levofloxacin prophylaxis. |
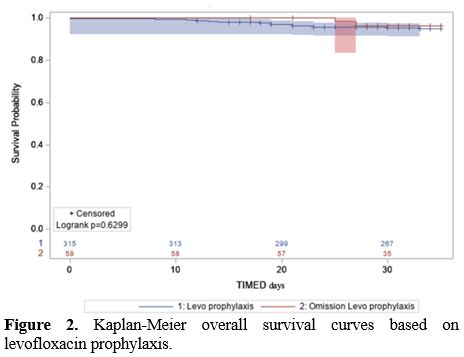 |
Figure 2. Kaplan-Meier overall survival curves based on levofloxacin prophylaxis. |
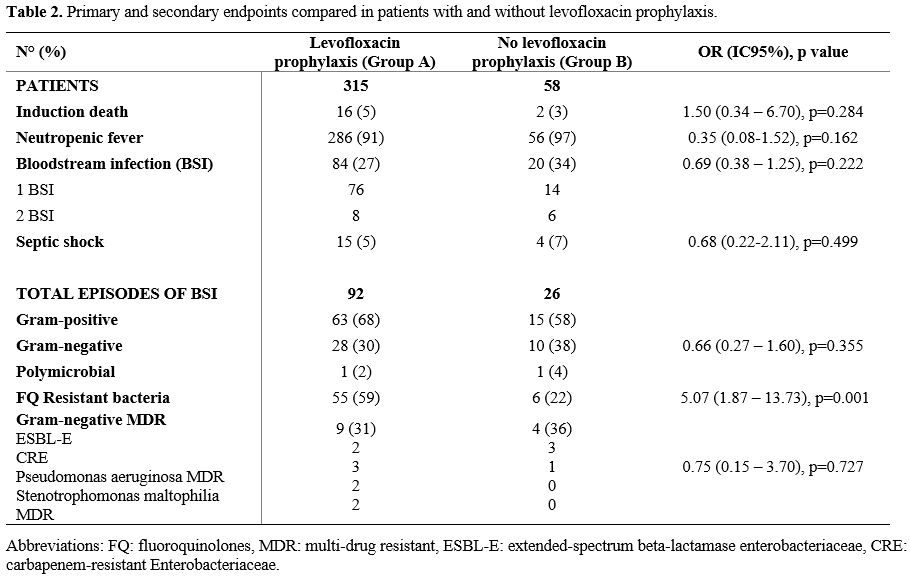 |
Table 2. Primary and secondary endpoints compared in patients with and without levofloxacin prophylaxis.
|
A
multivariate regression analysis did not show any impact of
levofloxacin prophylaxis on the incidence of neutropenic fever (OR
0.62, IC95% 0.13 - 2.97, p=0.552), bloodstream infection (OR 0.75,
IC95% 0.39 - 1.48, p=0.410), septic shock (OR 0.73, IC95% 0.20 - 2.66,
p=0.632) and induction death (OR 1.39, IC95% 0.27 - 7.21, p=0.696), see
Table 3. A prolonged duration
of aplasia (more than 20 days) was associated with an increased risk of
neutropenic fever (OR 6.07, IC95% 1.58 - 23.31, p= 0.009). Patients in
the adverse genetic risk category showed an increased risk of induction
death (OR 11.46, IC95% 2.45 – 53.62, p=0.002). Increasing age was
associated with early mortality (OR 1.64, IC95% 1.02 – 2.62, p=0.040)
and BSI occurrence (OR 1.25, IC95% 1.03 - 1.51, p=0.023).
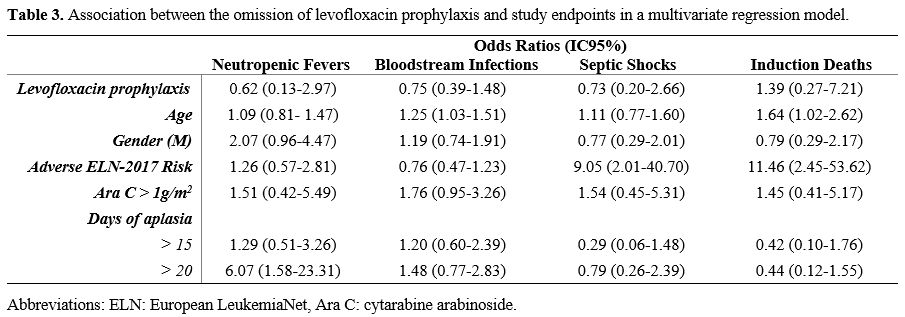 |
- Table 3. Association between the omission of levofloxacin prophylaxis and study endpoints in a multivariate regression model.
|
Role of FQ prophylaxis on antibiotic resistance.
Considering the 118 positive blood cultures, 120 bacterial isolates
have been detected, 93 in group A and 27 in group B. Two fungal
bloodstream infections (candidemia) were not included in the analysis.
Gram-positive
bacteria accounted for 67% (n=80) of the BSI, while gram-negative
organisms were identified in the remaining 33% (n=40) of BSI. Table 4 summarizes the frequency and the characteristics of bacterial bloodstream isolates.
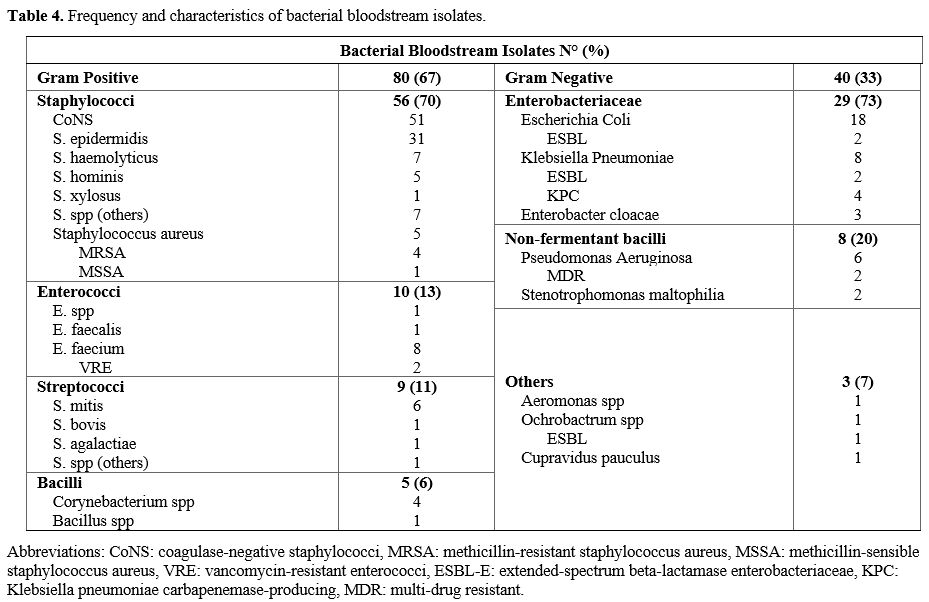 |
- Table 4. Frequency and characteristics of bacterial bloodstream isolates.
|
Overall,
FQ resistance was observed in 51% (n=61) of all bacteria; 55% (n=44) of
gram-positive pathogens, 43% (n=17) of gram-negative bacteria, and 55%
(n=16) of Enterobacteriaceae were FQ-resistant. MDR organisms (ESBLs,
CRE, and non-fermented MDR bacilli) represented 31% (n=13) of all
gram-negative bacteria.
When comparing the characteristics of
bacterial bloodstream isolates in prophylaxis and non-prophylaxis
groups, gram-negative bacteria were found in 31% (n=29, of which 1 in a
polymicrobial BSI) of patients in group A vs. 41% (n=11, of which 1 in
a polymicrobial BSI) in group B (OR 0.66, IC95% 0.27 – 1.60, p=0.355).
Overall, FQ-resistant pathogens were responsible for 55 BSI (59%) in
group A and 6 (22%) in group B (OR 5.07, IC95% 1.87 – 13.73, p=0.001).
Gram-positive FQ-resistant bacteria were 63% (n=40) in group A and 25%
(n=4) in group B, while FQ resistance was observed in 52% (n=15) and
18% (n=2) of gram-negative pathogens in group A and B, respectively.
Bacteremia due to gram-negative resistant organisms (ESBLs, CRE, and
non-fermenting MDR bacilli) was 31% (n=9) in group A and 36% (n=4) in
group B (OR 0.75, IC95% 0.15 – 3.70, p=0.727).
Data on rectal
swabs colonization with resistant bacteria were only available from
patients in the non-prophylaxis group since routine testing was started
in 2017. Twenty-two percent of the patients (n=13/58) had a colonized
rectal swab, 12 of which with an ESBL-producing organism and 1 with a
KPC. Of them, 23% (n=3/13) developed a bloodstream infection due to the
same pathogen.
Discussion
In
the last few years, the emergence of MDR pathogens has become an
increasing worldwide problem, and the large-scale use of antibiotic
drugs has been questioned.[7] Consequently, defining the role of
antibacterial prophylaxis has become a major concern in the era of
antimicrobial resistance, particularly in endemic MDR environments.
The
present study explored the impact of avoiding FQ prophylaxis during
post-induction neutropenia in AML patients. Noteworthy, we found no
significant influence of prophylaxis omission on induction death. Also,
we did not observe a significant difference in febrile neutropenia rate
between patients receiving or not levofloxacin prophylaxis, even if a
trend towards a higher number of neutropenic fevers was observed in
patients who did not receive it (97% vs. 91%). The CI of neutropenic
fever was significantly higher in patients without prophylaxis,
partially due to the difference in the time to fever, which was shorter
in patients not receiving prophylaxis.
Interestingly, FUO episodes
were more common in the group receiving prophylaxis, while a clinical
infection was diagnosed more frequently in the other group. Although we
can debate if the absence of antibacterial prophylaxis could translate
into an increased incidence of clinically diagnosed infections, more
likely, these data reflect the diagnostic advances made in recent years
to reduce FUO incidence and increase infectious disease diagnoses. In
the present study, avoiding levofloxacin prophylaxis was not associated
with an increased incidence of BSI. Even though a numerically higher
frequency of BSI (34% vs. 27%) and gram-negative isolates (41% vs 31%)
was present in the non-prophylaxis group, the difference lacked
statistical significance. The frequency of septic shocks did not differ
significantly between the two groups. Interestingly, we observed that
septic shock mortality decreased from 75% in the prophylaxis group to
25% in the non-prophylaxis group. Although we cannot exclude a role of
prophylaxis, this difference is probably due to our improvements in the
management of septic patients.
Another objective was to assess
the potential impact of FQ prophylaxis on the emergence of antibiotic
resistance, an issue on which only a few and contrasting data are
available. As expected, the use of FQ prophylaxis was associated with a
significant increase in the incidence of FQ-resistant bacteria. Indeed,
among patients receiving levofloxacin prophylaxis, almost 60% of the
bacterial bloodstream isolates were FQ resistant, in contrast to only
about 20% of the bacteria in the non-prophylaxis group (63% vs. 25% in
gram-positive and 52% vs. 18% in gram-negative in group A and B,
respectively). No significant difference was observed in the
ESBL-producing organisms and CRE incidence among the two groups.
However, since modifying the epidemiological environment requires
months or even years, these data need to be considered preliminary. To
fully address this issue, it would be important to evaluate the
incidence of resistant pathogens also in the subsequent consolidation
cycles.
The increased rate of patients colonized with resistant
pathogens reported worldwide is considered a direct consequence of
wrong antibiotic prescriptions and the extensive use of antibacterial
prophylaxis.[21] Positive rectal swabs are an important risk factor for
BSI in neutropenic patients, and their increase should be considered
when balancing the potential benefits of FQ prophylaxis in this
setting.[22] In our study, 22% of patients from the available data had
a colonized rectal swab, mostly from ESBL organisms. Importantly,
almost a quarter of them experienced a related bacteremia due to the
same pathogen.
Conclusions
In
the present study avoiding levofloxacin prophylaxis did not increase
induction mortality and did not have a significant impact on infectious
outcomes, even if a trend towards an earlier onset of neutropenic fever
was observed. Although limited by its retrospective nature, the
different periods, and the different sizes of the confronted groups,
the present analysis included a large and homogeneous cohort of
patients in terms of diagnosis (AML), treatment (intensive
chemotherapy), and site of observation (single center). The results are
concordant with the most recently published metanalyses addressing this
issue[7,8] and support the non-use of FQ prophylaxis, especially in
settings of endemic MDR spread.
References
- Vento
S, Cainelli F. Infections in patients with cancer undergoing
chemotherapy: aetiology, prevention, and treatment. Lancet Oncol.
2003;4(10):595-604. https://doi.org/10.1016/S1470-2045(03)01218-X PMid:14554236
- Freifeld
AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II,
Rolston KV, Young JA, Wingard JR. Infectious Diseases Society of
America. Clinical practice guideline for the use of antimicrobial
agents in neutropenic patients with cancer: 2010 update by the
infectious diseases society of america. Clin Infect Dis. 2011;52(4). https://doi.org/10.1093/cid/cir073 PMid:21258094
- Bucaneve
G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G,
Allione B, D'Antonio D, Buelli M, Nosari AM, Cilloni D, Zuffa E,
Cantaffa R, Specchia G, Amadori S, Fabbiano F, Deliliers GL, Lauria F,
Foà R, Del Favero A. Gruppo Italiano Malattie Ematologiche dell'Adulto
(GIMEMA) Infection Program. Levofloxacin to prevent bacterial infection
in patients with cancer and neutropenia. N Engl J Med.
2005;353(10):977-87. https://doi.org/10.1056/NEJMoa044097 PMid:16148283
- Bucaneve
G, Castagnola E, Viscoli C, Leibovici L, Menichetti F. Quinolone
prophylaxis for bacterial infections in afebrile high risk neutropenic
patients. EJC Suppl. 2007;5(2):5-12. https://doi.org/10.1016/j.ejcsup.2007.06.002
- European
Centre for Disease Prevention and Control. Surveillance of
antimicrobial resistance in Europe 2018. Stockholm: ECDC; 2019.
- De
Rosa FG, Motta I, Audisio E, Frairia C, Busca A, Di Perri G, Marmont F.
Epidemiology of bloodstream infections in patients with acute myeloid
leukemia undergoing levofloxacin prophylaxis. BMC Infect Dis.
2013;13:563. https://doi.org/10.1186/1471-2334-13-563 PMid:24289496 PMCid:PMC4219399
- Mikulska
M, Averbuch D, Tissot F, Cordonnier C, Akova M, Calandra T, Ceppi M,
Bruzzi P, Viscoli C. European Conference on Infections in Leukemia
(ECIL). Fluoroquinolone prophylaxis in haematological cancer patients
with neutropenia: ECIL critical appraisal of previous guidelines. J
Infect. 2018;76(1):20-37. https://doi.org/10.1016/j.jinf.2017.10.009 PMid:29079323
- Owattanapanich
W, Chayakulkeeree M. Efficacy of levofloxacin as an antibacterial
prophylaxis for acute leukemia patients receiving intensive
chemotherapy: a systematic review and meta-analysis. Hematology.
2019;24(1):362-368. https://doi.org/10.1080/16078454.2019.1589706 PMid:30880638
- Phillips
R, Hancock B, Graham J, Bromham N, Jin H, Berendse S. Prevention and
management of neutropenic sepsis in patients with cancer: summary of
NICE guidance. BMJ. 2012;345:e5368. https://doi.org/10.1136/bmj.e5368 PMid:22993392
- Christopeit
M, Schmidt-Hieber M, Sprute R, Buchheidt D, Hentrich M, Karthaus M,
Penack O, Ruhnke M, Weissinger F, Cornely OA, Maschmeyer G.
Prophylaxis, diagnosis and therapy of infections in patients undergoing
high-dose chemotherapy and autologous haematopoietic stem cell
transplantation. 2020 update of the recommendations of the Infectious
Diseases Working Party (AGIHO) of the German Society of Hematology and
Medical Oncology (DGHO). Ann Hematol. 2021;100(2):321-336. https://doi.org/10.1007/s00277-020-04297-8 PMid:33079221 PMCid:PMC7572248
- Taplitz
RA, Kennedy EB, Bow EJ, Crews J, Gleason C, Hawley DK, Langston AA,
Nastoupil LJ, Rajotte M, Rolston KV, Strasfeld L, Flowers CR.
Antimicrobial Prophylaxis for Adult Patients With Cancer-Related
Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J
Clin Oncol. 2018;36(30):3043-3054. https://doi.org/10.1200/JCO.18.00374 PMid:30179565
- Tallman
MS, Wang ES, Altman JK, Appelbaum FR, Bhatt VR, Bixby D, Coutre SE, De
Lima M, Fathi AT, Fiorella M, Foran JM, Hall AC, Jacoby M, Lancet J,
LeBlanc TW, Mannis G, Marcucci G, Martin MG, Mims A, O'Donnell MR, Olin
R, Peker D, Perl A, Pollyea DA, Pratz K, Prebet T, Ravandi F, Shami PJ,
Stone RM, Strickland SA, Wieduwilt M, Gregory KM; OCN; Hammond L, Ogba
N. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice https://doi.org/10.6004/jnccn.2019.0028 PMid:31200351
- Slavin
MA, Lingaratnam S, Mileshkin L, Booth DL, Cain MJ, Ritchie DS, Wei A,
Thursky KA; Australian Consensus Guidelines 2011 Steering Committee.
Use of antibacterial prophylaxis for patients with neutropenia.
Australian Consensus Guidelines 2011 Steering Committee. Intern Med J.
2011;41(1b):102-9. https://doi.org/10.1111/j.1445-5994.2010.02341.x PMid:21272174
- Klastersky
J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M,
Herrstedt J. ESMO Guidelines Committee. Management of febrile
neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol.
2016;27(suppl 5):v111-v118. https://doi.org/10.1093/annonc/mdw325 PMid:27664247
- Döhner
H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H,
Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T,
Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF,
Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in
adults: 2017 ELN recommendations from an international expert panel.
Blood. 2017;129(4):424-447. https://doi.org/10.1182/blood-2016-08-733196 PMid:27895058 PMCid:PMC5291965
- Parker
JE, Pagliuca A, Mijovic A, Cullis JO, Czepulkowski B, Rassam SM,
Samaratunga IR, Grace R, Gover PA, Mufti GJ. Fludarabine, cytarabine,
G-CSF and idarubicin (FLAG-IDA) for the treatment of poor-risk
myelodysplastic syndromes and acute myeloid leukaemia. Br J Haematol.
1997;99(4):939-44. https://doi.org/10.1046/j.1365-2141.1997.4763281.x PMid:9432047
- Cerrano
M, Candoni A, Crisà E, Dubbini MV, D'Ardia S, Zannier ME, Boccadoro M,
Audisio E, Bruno B, Ferrero D. FLAI induction regimen in elderly
patients with acute myeloid leukemia. Leuk Lymphoma.
2019;60(13):3339-3340. https://doi.org/10.1080/10428194.2019.1620943 PMid:31159609
- Lichtman
MA. A historical perspective on the development of the cytarabine
(7days) and daunorubicin (3days) treatment regimen for acute
myelogenous leukemia: 2013 the 40th anniversary of 7+3. Blood Cells Mol
Dis. 2013;50(2):119-30. https://doi.org/10.1016/j.bcmd.2012.10.005 PMid:23154039
- Mermel
LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders
BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the
diagnosis and management of intravascular catheter-related infection:
2009 Update by the Infectious Diseases Society of America. Clin Infect
Dis. 2009;49(1):1-45. https://doi.org/10.1086/599376 PMid:19489710 PMCid:PMC4039170
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss
RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der
Poll T, Vincent JL, Angus DC. The Third International Consensus
Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA.
2016;315(8):801-10. https://doi.org/10.1001/jama.2016.0287 PMid:26903338 PMCid:PMC4968574
- Cattaneo
C, Di Blasi R, Skert C, Candoni A, Martino B, Di Renzo N, Delia M,
Ballanti S, Marchesi F, Mancini V, Orciuolo E, Cesaro S, Prezioso L,
Fanci R, Nadali G, Chierichini A, Facchini L, Picardi M, Malagola M,
Orlando V, Trecarichi EM, Tumbarello M, Aversa F, Rossi G, Pagano L.
SEIFEM Group. Bloodstream infections in haematological cancer patients
colonized by multidrug-resistant bacteria. Ann Hematol.
2018;97(9):1717-1726. https://doi.org/10.1007/s00277-018-3341-6 PMid:29705860
- Vehreschild
MJ, Hamprecht A, Peterson L, Schubert S, Häntschel M, Peter S,
Schafhausen P, Rohde H, Lilienfeld-Toal MV, Bekeredjian-Ding I, Libam
J, Hellmich M, Vehreschild JJ, Cornely OA, Seifert H. A multicentre
cohort study on colonization and infection with ESBL-producing
Enterobacteriaceae in high-risk patients with haematological
malignancies. J Antimicrob Chemother. 2014;69(12):3387-92. https://doi.org/10.1093/jac/dku305 PMid:25103492
[TOP]





