Poor hematopoietic stem cell mobilizers in multiple myeloma: A single institution experience.
Guillermo J. Ruiz-Delgado,1,2,3 Avril López-Otero,1,3 Ana Hernandez-Arizpe,1,3 Aura Ramirez-Medina1 and Guillermo J. Ruiz-Argüelles.1,2,3
1 Centro de Hematología y Medicina Interna de Puebla. Puebla, MEXICO.
2 Laboratorios Clínicos de Puebla. Puebla, MEXICO.
3 Universidad Popular Autónoma del Estado de Puebla
Correspondence to: Guillermo J.
Ruiz-Delgado MD, Centro de Hematología y Medicina, Interna de Puebla,
8B Sur 3710, 72530 Puebla, Pue. Mexico. Teléfono: + 52 (222) 243 8100,
Telefax: + 52 (222) 243 8428. E-mail: gruiz2@clinicaruiz.com
Published: Jume 21, 2010
Received: May 31, 2010
Accepted: June 19, 2010
Medit J Hemat Infect Dis 2010, 2(2): e2010016, DOI 10.4084/MJHID.2010.016
This article is available from: http://www.mjhid.org/article/view/6020
This is an Open Access article
distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
In
a single institution, in a group of 28 myeloma patients deemed eligible
for autologous transplant, stem cell mobilization was attempted using
filgrastim: 26 individuals were given 31 autografts employing 1-4
(median three) apheresis sessions, to obtain a target stem cell dose of
1 x 106 CD34 +ve viable cells / Kg of the recipient. The median number
of grafted CD34 cells was 7.56 x 106 / Kg of the recipient; the
range being 0.92 to 14.8. By defining as poor mobilizers
individuals in which a cell collection of < 1 x 106 CD34 viable
cells / Kg was obtained, a subset of eight poor mobilizers was
identified; in two patients the autograft was aborted because of an
extremely poor CD34 +ve cell yield (< 0.2 x 106 CD34 +ve viable
cells / Kg of the recipient) after four apheresis sessions. The
long-term overall survival of the patients grafted with > 1 x 106
CD34 +ve viable cells / Kg was better (80% at 80 months) than those
grafted with < 1 x 106 CD34 +ve viable cells / Kg (67% at 76
months). Methods to improve stem cell mobilization are needed and may
result in obtaining better results when autografting multiple myeloma
patients.
Introduction
High-dose chemotherapy followed by autologous stem cell transplantation (SCT) is considered standard of care for patients with multiple myeloma (MM).[1,2] In this setting, autologous peripheral blood stem cells (PBSC) have largely replaced autologous bone marrow as the source of stem cells. Advantages of autologous PBSC grafts over bone marrow grafts include faster engrafment after high-dose chemotherapy, reduced contamination with tumor cells3 and lower morbidity and mortality.[3-5]
Hematopoietic stem cell mobilization is generally accomplished by administration of hematopoietic growth factors like granulocyte colony stimulating factor (G-CSF)[1-5] or a combination of myelosuppressive chemotherapy and hematopoietic growth factors. A number of factors have been reported to impact progenitor cell mobilization but the predictive factors vary from one study to the other.
The purpose of this study is to analyze the prevalence of poor mobilization in individuals with MM, prospectively studied and grafted in a single institution using the same autografting procedure.
Patients and Methods:
Results:
Discussion:
A number of factors have been reported to impact stem cell mobilization. These include patient age, weight, diagnosis, type and number of prior chemotherapeutic regimens administered, bone marrow involvement with the malignancy at baseline, extent of cell recovery from previous chemotherapy at the time of starting mobilization treatment, prior radiation therapy, time from diagnosis to harvest, and disease status.[13-15] Other factors include type of cytokine used and type and dose of chemotherapy regimen, if any, for mobilization.[13-15]
The number of CD34 +ve hematopoietic stem cells required to successfully conduct an autograft has not been clearly defined. Some authors have described that a minimum of 2 x 106 CD34 +ve viable cells / Kg of the recipient is required to rescue the hematopoiesis of the patient [13]. We have shown that a minimum of 1 x 106 CD34 +ve viable cells / Kg of the recipient may be useful to successfully autograft patients with multiple myeloma1 or other diseases.[3-4] This apparent discrepancy may stem from several factors, one of them being the enumeration methods: we have also shown previously that a considerable variation might be due to the selection of antibodies, staining protocols, and acquisition strategies for the flow cytometric enumeration of these cells which may account even for two-fold differences;[16] both inter-instrument and inter-protocol variation provide explanation for the redundantly reported discrepancies concerning the numbers of CD34 cells that suffice to secure hematopoietic grafting and to define poor mobilizers.[16] Accordingly, the exact incidence of poor mobilizers in the setting of autologous grafting is unknown and has varied in the literature between 5 to 40% in different subsets of patients.[13] Using a lower threshold, we have found a prevalence of 24% (8 of 33 autografts) of poor mobilizers, which only in two cases led into aborting the autografting procedure. With the limitations of a small study, we found that certain features of the patients and/or disease were associated with a defective mobilization: Previous treatment with oral melphalan, previous autograft with intravenous melphalan and previous treatment with bortezomib. The low number of patients included in each subset of treatments makes hard to know of prior exposure to certain drugs per se interfered with mobilization, or whether patients exposed to some agents had other features that would compromise mobilization. On the other hand, it should be mentioned that some patients who had been treated previously with oral melphalan could be successfully autografted; accordingly, we believe that attempts of autografting should be tried even in patients previously treated with oral melphalan.
In order to improve the mobilization techniques for stem cell autografting, several approaches have been tried. The mobilization seems to be better if both chemotherapy and growth factors are employed.[17] Recently, Dugan et al. showed that plerixafor can safely be added to chemotherapy-based mobilization regimens and may accelerate the rate of increase in CD34 +ve cells on the second day of apheresis[18] whereas Di Persio et al. have shown similar results in patients with multiple myeloma.[19] Further studies are warranted to evaluate the effect of this drug in combination with chemomobilization on stem cell mobilization and collection on the first and subsequent days of apheresis, and its impact on resource utilization and results improvement.[19,20] Since there are data suggesting that failure of mobilization in myeloma patients results in increased expenses and increased use of resources,[21] methods to predict the failure to mobilize have been suggested.[22] Studies to analyze the balance of the costs of improved mobilization methods and the costs of failing to mobilize patients with myeloma who are to be autografted are mandatory to further explore this field, specially in developing countries, where autografting patients with multiple myeloma is still a cheaper therapeutic option than the use of the novel anty-myeloma drugs.[23]
Conclusions
In summary, we have found that in patients with multiple myeloma, using a threshold of 1 x 106 CD34 +ve viable cells / Kg of the recipient has allowed us to define a suboptimal mobilization which presents in one quarter of patients and seems to be associated with previous treatment with oral melphalan, previous autograft with intravenous melphalan and previous treatment with bortezomib, and that the long term overall survival of the poor mobilizers seems to be worse than that of individuals grafted with 1 x 106 CD34 +ve viable cells / Kg of the recipient. Efforts directed to improve stem cell mobilization seem to be warranted and could result in improving the results of autografting and the prognosis of patients with multiple myeloma.[24]
High-dose chemotherapy followed by autologous stem cell transplantation (SCT) is considered standard of care for patients with multiple myeloma (MM).[1,2] In this setting, autologous peripheral blood stem cells (PBSC) have largely replaced autologous bone marrow as the source of stem cells. Advantages of autologous PBSC grafts over bone marrow grafts include faster engrafment after high-dose chemotherapy, reduced contamination with tumor cells3 and lower morbidity and mortality.[3-5]
Hematopoietic stem cell mobilization is generally accomplished by administration of hematopoietic growth factors like granulocyte colony stimulating factor (G-CSF)[1-5] or a combination of myelosuppressive chemotherapy and hematopoietic growth factors. A number of factors have been reported to impact progenitor cell mobilization but the predictive factors vary from one study to the other.
The purpose of this study is to analyze the prevalence of poor mobilization in individuals with MM, prospectively studied and grafted in a single institution using the same autografting procedure.
Patients and Methods:
- Patients: Patients with MM diagnosed and autografted at the Centro de Hematología y Medicina Interna de Puebla, a part of the Clínica RUIZ, in Puebla, México between August 1993 to September 2008 were prospectively included in the study. The diagnosis of MM was based on the following findings:[1-2,4] a) Increased numbers (more than 30%) of abnormal, atypical or immature plasma cells in the bone marrow or histologic proof of plasmacytoma; b) presence of an M-protein in the serum or urine; or c) Bone lesions consistent with those of multiple myeloma. Individuals with plasma cell reactions to connective tissue disorders, liver disease, metastatic carcinoma or chronic infections were not included, nor patients with monoclonal gammopathy of undetermined significance,[3] smoldering multiple myeloma, solitary plasmacytoma or plasma cell leukemia. Individuals with primary amyloidosis (AL) were included only if features of MM predominated.[6,7] All patients were autografted in the Centro de Hematología y Medicina Interna de Puebla, México, and had received a median of 4 (range 1-7) prior chemotherapy regimens (Table 1). Normal renal and liver function tests were needed for inclusion as well as informed consent approved before any procedure.
- PBSC mobilization and apheresis: The PBSC mobilization schedule was started at least 30 days after the last dose of chemotherapy. Subcutaneous G-CSF (10 ug / Kg / day / 5 days) was given for mobilization of stem cells, starting day - 7. Using either a peripheral vein or a Majurkar-type subclavian catheter, the apheresis procedures were performed on days – 4 – 3, – 2 and – 1, using a Haemonetics V-50 PLUS machine (Haemonetics Corporation, Braintree MA) or a Baxter C-3000 PLUS machine (Baxter Healthcare, Deerfield IL), and the Spin-Nebraska protocol.8 The endpoint of collection was the processing of 5000 - 7000 ml of blood / m2 in each of the apheresis procedures, in order to obtain at least 3 x 108 mononuclear cells and / or 1 x 106 viable CD34 cells / Kg of the recipient’s weight.[1,3-5]
- Conditioning and autografting: Intravenous melphalan, 200 mg/m2 in a single I.V. dose was used on day – 1 in all patients. Ondansetron (8 mg i.v. every 12 h after chemotherapy), ciprofloxacin (250 mg. bid) and fluconazole (200 mg bid) were used in all patients. G-CSF (10 ug / Kg / day / 5 days) was re-started on day +5 and used until granulocytes were greater than 0.5 x 109/L. Antibiotics and antimycotics were used until granulocytes were above 0.5 x 109/L. All patients had daily laboratory workup and clinical studies.
- Apheresis product preservation, studies and infusion: The products of the apheresis and 1 ml aliquots were kept in ACD-A (Baxter Healthcare, Deerfield IL) at 4oC, in 300 ml transfer packs (Baxter Healthcare, Deerfield IL) composed of gas impermeable, polyvinyl chloride plastic film for up to 96 hours. Enumeration of the total white mononuclear cells (MNC) and CD34 +ve cells was done by flow-cytometry9 in an EPICS Elite ESP apparatus (Coulter Electronics, Hialeah, FL), using for the latter subpopulation the anti-CD34 monoclonal antibody HPCA-2 (Becton Dickinson, San José CA), gating in propidium iodide-excluding CD45(+) MNC population according to forward and 90° angle light scattering. Additional viability studies of the MNC prior to its re-infusion used propidium iodide exclusion and anti-cell antibodies on a flow cytometer. No purging procedures were performed. The apheresis products obtained on days – 4, – 3, – 2 and – 1 were reinfused to the patients on days 0, +1, +2 and +3 respectively after keeping them in the conventional blood bank refrigerator at 4 degress centigrade.[3,4]
- Criteria for assesment of the mobilization procedures: One to four apheresis sessions were performed to obtain a target stem cell dose of 1 x 106 CD34 +ve viable cells / Kg of the recipient. Cases in which less than this amount of CD34 +ve progenitor cells were obtained after four apheresis sessions were classified as “poor” mobilizers.
- Response assessment: Very good partial response (VGPR) was defined as a decrease of 90% in the serum paraprotein level. Partial remission (PR) as a decrease of 50% in the serum paraprotein level, and minimal response (MR) as a decrease of 25% in the serum paraprotein level.[1]

Results:
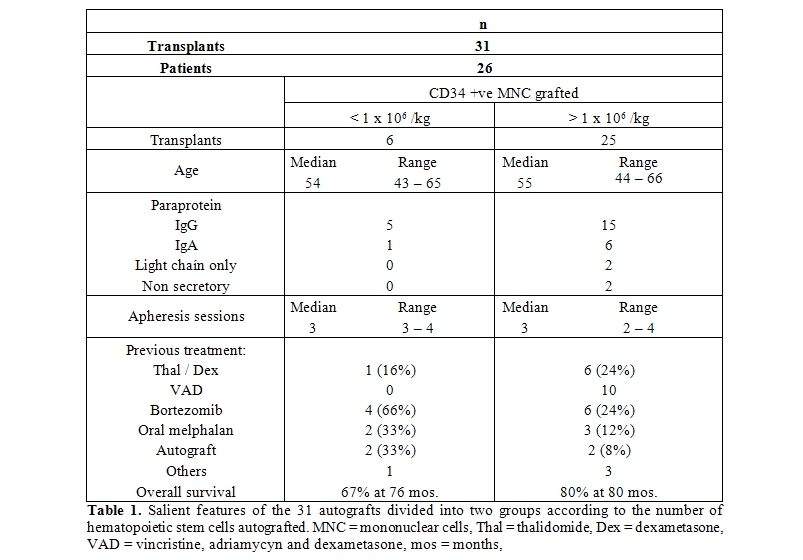
- Patients: All patients with multiple myeloma less than 70 years are offered an autograft in our institution; 28 consecutive patients were deemed eligible for autologous transplant and attempted stem cell mobilization; there were 13 females and 15 males. Since our autografting procedure does not involve hematopoietic stem cell freezing,[1,4-5] the procedure was aborted if less than 0.5 x 106 CD34 +ve viable cells / Kg of the recipient were collected; accordingly, the autograft was aborted in two patients because of a very poor CD34 +ve cell yield (less than 0.2 x 106 CD34 +ve viable cells / Kg of the recipient) after four apheresis sessions; one patient had developed an acute myelogenous leukemia after being treated with oral melphalan during 51 months and failed to mobilize after entering a complete remission of the leukemia; he was later on given an allogeneic graft with umbilical cord cells and remains disease-free 75 months after attempting the autograft.[10] The other patient had received an autologous graft using intravenous melphalan 51 months before attempting the second autograft; after failing mobilization with G-CSF he was later on mobilized with G-CSF plus plerixafor and subsequently re-autografted succesfully.[11] In the group of 26 patients who were autografted, the median age was 54 years (range 42 to 66). The type of paraprotein was IgG in 16 cases, IgA in 6 cases, light chain disease in 2 cases (both kappa). According to the International Staging System (ISS),[12] 21 patients were in stage I, 3 in stage II and 2 in stage III. All patients had been treated before the autograft: Six with thalidomide / dexametasone (thal/dex), 11 with bortezomib-containing regimens, 9 with vincristine / adriamycin / dexamethasone (VAD) and 5 with other schedules. No patient had received radiation therapy as part of the previous treatment and in all cases the autologous graft was performed as part of the initial therapy.
- PBSC mobilization and apheresis: A median of three apheresis sessions were needed to collect a minimum of 1 x 106 CD34 +ve viable cells / Kg of the recipient;[1] the range was 2 to 4 sessions to obtain enough CD34 +ve cells. Circulating CD34 +ve cells were not enumerated prior to the apheresis procedures.
- Conditioning and autografting: All patients were conditioned with a single dose of intravenous melphalan, 200 mg/ m2. In cases in which less than 1 x 106 CD34 +ve viable cells / Kg of the recipient, defined as poor mobilizers, the dose of melphalan was adjusted to 180 mg/m2 (90% of the planned dose).
- Apheresis product studies: The median number of transplanted CD34 +ve viable cell was 7.56 x 106 CD34 +ve viable cells / Kg of the recipient; the range was 0.92 to 14.8. In all cases the viability of the CD34 cells was above 85% prior to being reinfused to the patients.
- Assesment of the mobilization procedures: One to four (median three) apheresis sessions were performed to obtain a target stem cell dose of 1 x 106 CD34 +ve viable cells / Kg of the recipient. In six cases, less than 1 x 106 CD34 +ve viable cells / Kg of the recipient were obtained, in 16 cases 1 to 2 x 106 CD34 +ve viable cells / Kg of the recipient, whereas in 9 cases more than 2 x 106 CD34 +ve viable cells / Kg of the recipient.
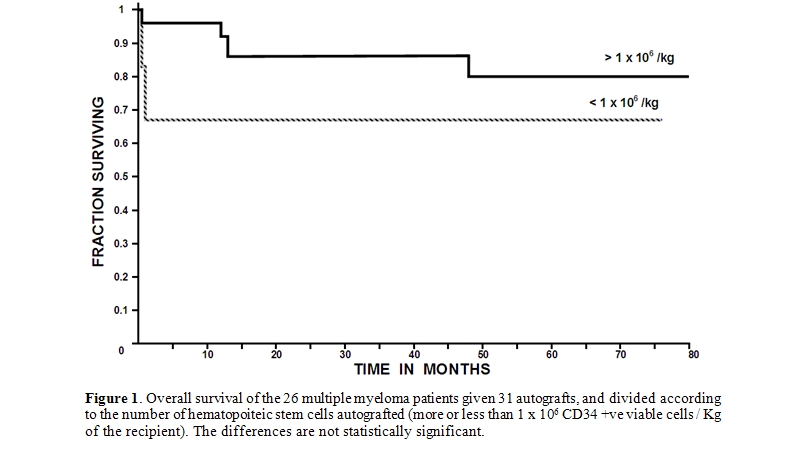
- Engraftment and response: In the whole group of patients, the time to achieve more than 0.5 x 109/L granulocytes had a median of 27 days (range 0 to 53), whereas the time to recover more than 20 x 109/L platelets had a median of 37 days (range 0 to 73). Of the 26 patients autografted, 19 achieved a complete remission, 6 a very good partial remission, 5 a partial remission and one a minor response. The 100-day mortality was 9.6% (3 of 31 transplants); Two patients died at day +9 and +11 as a result of sepsis during granulocytopenia and one died at day +100 due to a myocardial infarction; they had received 2.5, 1.5 and 3.4 x 106 CD34 +ve viable cells / Kg, respectively. The overall median post-transplant survival (OS) has not been reached, being above 80 months, whereas the 80-month OS is 77%. Relapses presented in 11/31 autografts, a median of 34 months after the procedure; five patients were rescued with a second autograft and two were given an allogeneic grafts. The autograft procedure was started in an outpatient basis in all instances, however four patients where admitted to the hospital as a result of: amebic colitis, Pseudomona aeruginosa sepsis, cerebrovascular episode and soft-tissue abscess, one case each. In the whole group of 31 autografts, a subset of six poor mobilizers was identified. The table 1 shows the salient data of these six poor mobilizers compared with those of the 25 autografts in which a better mobilization was obtained. It is interesting that the long-term overall survival of the multiple myeloma patients allografted with more than 1 x 106 CD34 +ve viable cells / Kg was better (80% at 80 months) than those autografted with less than 1 x 106 CD34 +ve viable cells / Kg (67% at 76 months); the differences however are not statistically significant according to the log-rank chi square method most likely as a result of the low number of patients included in the study, see table 1 and figure 1. There were not significant differences in time to recover granulocytes and platelets between patients receiving more or less than 1 x 106 CD34 +ve viable cells / Kg.

Discussion:
A number of factors have been reported to impact stem cell mobilization. These include patient age, weight, diagnosis, type and number of prior chemotherapeutic regimens administered, bone marrow involvement with the malignancy at baseline, extent of cell recovery from previous chemotherapy at the time of starting mobilization treatment, prior radiation therapy, time from diagnosis to harvest, and disease status.[13-15] Other factors include type of cytokine used and type and dose of chemotherapy regimen, if any, for mobilization.[13-15]
The number of CD34 +ve hematopoietic stem cells required to successfully conduct an autograft has not been clearly defined. Some authors have described that a minimum of 2 x 106 CD34 +ve viable cells / Kg of the recipient is required to rescue the hematopoiesis of the patient [13]. We have shown that a minimum of 1 x 106 CD34 +ve viable cells / Kg of the recipient may be useful to successfully autograft patients with multiple myeloma1 or other diseases.[3-4] This apparent discrepancy may stem from several factors, one of them being the enumeration methods: we have also shown previously that a considerable variation might be due to the selection of antibodies, staining protocols, and acquisition strategies for the flow cytometric enumeration of these cells which may account even for two-fold differences;[16] both inter-instrument and inter-protocol variation provide explanation for the redundantly reported discrepancies concerning the numbers of CD34 cells that suffice to secure hematopoietic grafting and to define poor mobilizers.[16] Accordingly, the exact incidence of poor mobilizers in the setting of autologous grafting is unknown and has varied in the literature between 5 to 40% in different subsets of patients.[13] Using a lower threshold, we have found a prevalence of 24% (8 of 33 autografts) of poor mobilizers, which only in two cases led into aborting the autografting procedure. With the limitations of a small study, we found that certain features of the patients and/or disease were associated with a defective mobilization: Previous treatment with oral melphalan, previous autograft with intravenous melphalan and previous treatment with bortezomib. The low number of patients included in each subset of treatments makes hard to know of prior exposure to certain drugs per se interfered with mobilization, or whether patients exposed to some agents had other features that would compromise mobilization. On the other hand, it should be mentioned that some patients who had been treated previously with oral melphalan could be successfully autografted; accordingly, we believe that attempts of autografting should be tried even in patients previously treated with oral melphalan.
In order to improve the mobilization techniques for stem cell autografting, several approaches have been tried. The mobilization seems to be better if both chemotherapy and growth factors are employed.[17] Recently, Dugan et al. showed that plerixafor can safely be added to chemotherapy-based mobilization regimens and may accelerate the rate of increase in CD34 +ve cells on the second day of apheresis[18] whereas Di Persio et al. have shown similar results in patients with multiple myeloma.[19] Further studies are warranted to evaluate the effect of this drug in combination with chemomobilization on stem cell mobilization and collection on the first and subsequent days of apheresis, and its impact on resource utilization and results improvement.[19,20] Since there are data suggesting that failure of mobilization in myeloma patients results in increased expenses and increased use of resources,[21] methods to predict the failure to mobilize have been suggested.[22] Studies to analyze the balance of the costs of improved mobilization methods and the costs of failing to mobilize patients with myeloma who are to be autografted are mandatory to further explore this field, specially in developing countries, where autografting patients with multiple myeloma is still a cheaper therapeutic option than the use of the novel anty-myeloma drugs.[23]
Conclusions
In summary, we have found that in patients with multiple myeloma, using a threshold of 1 x 106 CD34 +ve viable cells / Kg of the recipient has allowed us to define a suboptimal mobilization which presents in one quarter of patients and seems to be associated with previous treatment with oral melphalan, previous autograft with intravenous melphalan and previous treatment with bortezomib, and that the long term overall survival of the poor mobilizers seems to be worse than that of individuals grafted with 1 x 106 CD34 +ve viable cells / Kg of the recipient. Efforts directed to improve stem cell mobilization seem to be warranted and could result in improving the results of autografting and the prognosis of patients with multiple myeloma.[24]
References
- López-Otero A, Ruiz-Delgado GJ,
Ruiz-Argüelles GJ.: A simplified method for stem cell
autografting in multiple myeloma: A single institution experience. Bone
Marrow Transplant 2009; 44:715-9

- Vela-Ojeda J, García-Ruiz-Esparza MA,
Padilla-González Y, Gómez-Almaguer D, Gutiérrez-Aguirre CH,
Gómez-Rangel D, Morales-Toquero A, Ruiz-Delgado GJ, Delgado-Lamas JL,
Ruiz-Argüelles GJ. Autologous peripheral blood stem cell
transplantation in multiple myeloma using oral versus I.V. melphalan.
Ann Hematol 2007, 86:277-282.

- Ruiz-Argüelles GJ, Ruiz-Argüelles A,
Pérez-Romano B, Marín-López A, Delgado-Lamas JL.: Non-cryopreserved
peripheral blood stem cells autotransplants for hematological
malignancies can be performed entirely on an outpatient basis. Am J
Hematol 1998, 58:161-164.

- Ruiz-Argüelles GJ, Ruiz-Argüelles A,
Pérez-Romano B, Marín-López A, Larregina-Díez A, Apreza-Molina MG.:
Filgrastim-mobilized peripheral-blood stem cells can be stored at 4
degrees and used in autografts to rescue high-dose chemotherapy. Am J
Hematol 1995; 48:100-103.

- Ruiz-Argüelles GJ, Gómez-Rangel D,
Ruiz-Delgado GJ, Ruiz-Argüelles A, Pérez-Romano B, Rivadeneyra L.
Results of an autologous non-cryopreserved, unmanipulated
peripheral blood hematopoietic stem cell transplant program: A single
institution, 10-year experience. Acta Haematol 2003; 110: 179-183.

- Caers Jo, Vandebroek I, De Raeve H, Michaux
L, Trullemans F, Schots R, van Camp B, Vanderkerken K.: Multiple
myeloma. An update on diagnosis and treatment. Eur J Haematol 2008;
81:329-343.

- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy
MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak
ME, Therneau TM, Greipp PR.: Review of 1027 patients with newly
diagnosed multiple myeloma. Mayo Clin Proc 2003; 78: 21-33.

- Kessinger A, Armitage JO, Landmark JD,
Smith DM, Weisenburger DD.: Autologous peripheral hematopoietic stem
cell transplantation restores hemopoietic function following marrow
ablative therapy. Blood 1988; 71: 723-727.

- Ruiz-Argüelles A.: Flow cytometry in the
clinical laboratory. Principles, applications and problems. Ann Biol
Clin 1992; 50:735-743.

- Ruiz-Argüelles GJ, Reyes-Núñez V, Garcés-Eisele J, Warwick RM, McKenna L, Ruiz-Reyes G, Granados J, Mercado-Díaz MA. Acquired hemoglobin S trait in an adult patient with secondary acute myelogenous leukemia allografted with matched unrelated umbilical cord blood cells using a non-ablative conditioning regimen. Haema 2005; 8: 492-496.
- Reyes-Torres V, Hernández-Arizpe A, López-Otero A, Ruiz-Delgado G, Kramis-Cerezo JL, Ruiz-Argüelles GJ.: El AMD3100 (plerixafor) puede mejorar la movilización de células hematopoyéticas para hacer trasplantes autólogos. Informe de un caso. Medicina Univ 2009; 11:202-206
- Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle
RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C,
Sonneveld P, Tosi P, Turesson I, Westin J.: International staging
system for multiple myeloma. J Clin Oncol 2005; 23:3412-20.

- Hosing C, Saliba RM, Ahlawat S, Korbling
M, Kebriaei P, Alousi A, de Lima M, Okoroji JG, McMannis J, Qazilbash
M, Anderlini P, Giralt S, Champlin RE, Khouri I, Popat U.: Poor
hematopoietic stem cell mobilizers: A single institution study of
incidence and risk factors in patients with recurrent or relapsed
lymphoma. Am J Hematol 2009, 84:335-337.

- Corso A, Caberlon L, Pagnucco G, Klersy C,
Zappasodi P, Alessandrino EP, Vanelli L, Mangiacavalli S, Lazzarino M,
Bernasconi C.: Blood stem cell collections in multiple myeloma:
definition of a scoring system. Bone Marrow Transplant 2000;
26:283-6

- Lee JL, Kim SB, Lee GW, Ruy MH, Kim EK,
Kim S, Kim WK, Lee JS, Suh C.: Collection of peripheral blood
progenitor cells: analysis of factors predicting the yields. Transfus
Apher Science 2003; 29:29-37.

- Rivadeneyra-Espinoza L, Pérez-Romano B,
González-Flores A, Guzmán-García MO, Carvajal-Armora F, Ruiz-Argüelles
A. Instrument- and protocol-dependent variation in the enumeration of
CD34+ cells by flow cytometry. Transfusion 2006;46:530-6

- Mark T, Stern J, Furst JR, Jayabalan D,
Zafar F, LaRow A, Pearse RN, Harpel J, Shore T, Schuster MW, Leonard
JP, Christos PJ, Coleman M, Niesvizky R. Stem cell mobilization with
cyclophosphamide overcomes the suppressive effect of lenalidomide
therapy on stem cell collection in multiple myeloma. Biol Blood Marrow
Transplant. 2008 Jul;14(7):795-8.

- Dugan MJ, Maziarz RT, Bensinger WI, et al.
Safety and preliminary efficacy of plerixafor (Mozobil) in combination
with chemotherapy and G-CSF: an open-label, multicenter, exploratory
trial in patients with multiple myeloma and non-Hodgkin's lymphoma
undergoing stem cell mobilization. Bone Marrow Transplant 2010; 45:39.47

- DiPersio JF, Stadtmauer EA, Nademanee A,
Micallet INM, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Fruehauf S,
Horwitz M, Cooper D, Bridger G, Calandra G.: Plerixafor and G-CSF
versus placebo and G-CSF to mobilize hematopoietic stem cells for
autologous stem cell transplantation in patients with multiple myeloma.
Blood 2009; 113:5720-6

- Giralt S, Stadmauer EA, Harousseau JL,
Palumbo A, Bensinger W et al.: International myeloma working group
(IMWG) consensus statement and guidelines regarding the current status
of stem cell collection and high dose therapy for multiple myeloma and
the role of plerixafor (AMD 3100). Leukemia 2009; 23:1904-12

- Gertz MA, Wolf RC, Micallef IN, Gastineau
DA. Clinical impact and resource utilization after stem cell
mobilization failure in patients with multiple myeloma and lymphoma.
Bone Marrow Transplant. 2010 Jan 11. [Epub ahead of print]

- Putkonen M, Rauhala A, Pelliniemi TT,
Remes K. Sepsis, low platelet nadir at mobilization and previous IFN
use predict stem cell mobilization failure in patients with multiple
myeloma. Cytotherapy. 2007;9(6):548-54.

- Ruiz-Arguelles GJ.: Whither the bone
marrow transplant. Hematology 2010; 15:1-3

- Ruiz-Argüelles GJ, Gómez-Rangel D, Ruiz-Delgado GJ, Aguilar-Romero L.: Multiple myeloma in México: A single institution, twenty-year experience. Arch Med Res 2004; 35; 163-167.