Treatment of Cancer in the Older Aged Person.
Lodovico Balducci
Department of Tropical Medicine, Gastroenterology and Liver Disease, Ain Shams University, Cairo, Egypt
Correspondence to: Prof. Lodovico Balducci , Moffitt Cancer Center and Research Institute, Tampa, Florida, USA Lodovico.Balducci@moffitt.org
Published: September 29, 2010
Received: September 18, 2010
Accepted: September 28, 2010
Medit J Hemat Infect Dis 2010, 2(2): e2010029, DOI 10.4084/MJHID.2010.029
This article is available from: http://www.mjhid.org/article/view/6510
This is an Open Access article
distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Abstract
Cancer is a disease of
aging.[1] Currently 50% of all malignancies occur in
individuals 65 and over[1]
and by the year 2030 older individuals will account for 70% of all
neoplasms.
With the aging of the population the management of cancer in the older person with chemotherapy is beoming increasingly common. This treatment may be safe and effective if some appropriate measures are taken, including, an assessment of the physiologic age of each patient, modification of doses according to the renal function, use of meyelopoietic growth factors prophylactically in presence of moderately toxic chemotherapy, and provision of an adequate caregiver. Cure, prolongation of survival, and symptom palliation are universal goals of medical treatment. Prolongation of active life expectancy should be added to the treatment goal of the older aged person.
With the aging of the population the management of cancer in the older person with chemotherapy is beoming increasingly common. This treatment may be safe and effective if some appropriate measures are taken, including, an assessment of the physiologic age of each patient, modification of doses according to the renal function, use of meyelopoietic growth factors prophylactically in presence of moderately toxic chemotherapy, and provision of an adequate caregiver. Cure, prolongation of survival, and symptom palliation are universal goals of medical treatment. Prolongation of active life expectancy should be added to the treatment goal of the older aged person.
Introduction
The management of cancer in the older aged person is an increasingly common problem. Cancer is a disease of aging.[1] Currently 50% of all malignancies occur in individuals 65 and over[1] and by the year 2030 older individuals will account for 70% of all neoplasms.
The management of cancer in the older-aged person include some questions that are specfic of aging:
• Is the person life expectancy going to be shortened by cancer?
• Is the patient’s life expectancy long enough that he or she will experience the complications of cancer?
• Is the patient able to tolerate antineoplastic treatment?
• What are the long term side effects of cancer treatment in the older person?
• Does the patient have adequate social support to undergo cancer treatment?
We will address these questions using cytotoxic chemotherapy as a model. After an overview of aging and its assessment we will explore the pharmacologic changes of aging and the provisions to ameliorate the complications of chemotherapy.
Age and its assessment
Aging implies a decline in life expectancy and stress-coping ability, increased prevalence of comorbidity, increased risk of functional dependence and of the need of social support.[2] Though it is universal, aging is highly individualized and is poorly reflected in chronologic age. The management of the older aged person should be based on an assessment of physiologic rather than chronologic age.
Aging has been defined as loss of entropy and fractality[3] and as loss of homeostasis.[4] Entropy reflects the ability of a system to produce and to waste energy. A fractal is a unit subdividing into subunits of the same type, but whose number and size are unpredictable, similar to the branch of a tree. The maintenance of life is trusted to structural and dinamic fractals. In the mammalian body structural fractals include the respiratory, circulatory and nervous systems; the dinamic fractals include the process of cell generation. For example, in hemopoiesis, few pluripotent stem cells give origin to a number of committed progenitors from which the differentiated precursors of the circulating blood cells are derived. Commitment and differentiation involve the branching of the pluripotent stem cell into progressively larger cell populations. Entropy and fractality cannot be directly assessed by clinical means. The loss of cell replicating ability, expressed by a reduced length of leukocyte telomeres,5 may be seen as an expression of the loss of fractality. A number of studies have explored the possibility that the length of leukocyte telomeres provide a reliable assessment of physiologic age, but correlating the length of telomeres to the risk of functional dependence, geriatric syndromes, and other manifestations of age. The results so far have been inconclusive.[5-10]
Homeostasis is the ability of a system to restore basic conditions after stress imposed by environmental interactions. One may observe the dysregulation of a number of physiologic parameters, including blood pressure, insulin sensitivity, circulating levels of corticosteroid and cathecolamines. The so called “allostatic load” assesses the dysregulation of 12 different parameters and may estimate the physiologic age. Its clinical value so faris unestablished.[4] Chronic and progressive inflammation is arguably the best recognized manifestation of allostasis.. Aging is associated with increased concentration of circulating inflammatory markers, including inflammatory cytokines and fibrinolytic products. The concentration of Interleuking 6, D-Dimer, and C-reactive protein is associated with increased risk of death, functional dependence and geriatric syndromes[11-14] and may mirror the physiologic age of the person.
Inflammatory markers and the length of leukocyte telomeres are promising laboratory tests, but at present have limited clinical use.
The best validated instrument for the assessment of chronologic age is the Comprehensive Geriatric Assessment” (CGA), whose elements are illustrated in Table 1.[15-17]
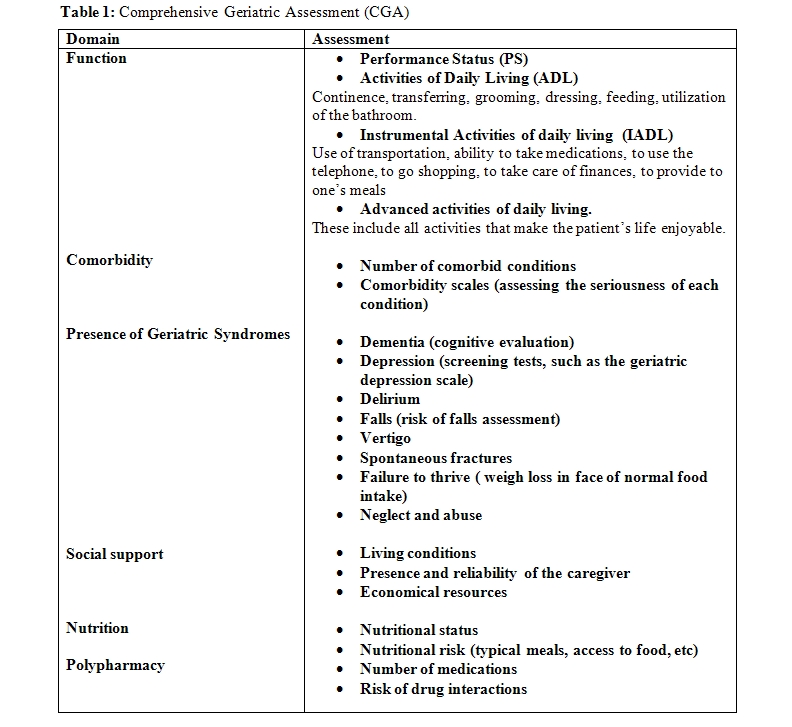
Dependence in one or more ADLs and the presence of one or more geriatric syndromes imply a marginal functional reserve associated with very limited tolerance of stress. These individuals can only survive thanks to a full time home caregiver, or to admission to an adult living facility. Dependence in one or more IADLs is associated increased risk of mortality,[18-21] of dementia, and of chemotherapy-induced toxicity.[20-21] These individuals need a caregiver to negotiate the outside world.
In addition to reduced life-expectancy and increased risk of treatment complications[22-24] comorbidity may be associated to changes in the natural history of cancer and polypharmacy. It is usefule to mention that diabetes has been associated with worsened prognosis of most common neoplasms including cancer of the large bowel, of the prostate and of the breast.[22,25] Anemia deserves a special mention as it is associated with increased risk of overall mortality in older individuals, of functional dependence and of chemotherapy-induced myelotoxicity.26 Polypharmacy may be responsible of iatrogenic morbidity and of unfavorable interactions with antineoplastic drugs.[27-28] In addition, drug interactions are a major cause of iatrogenic morbidity.
Some elements of the CGA may be utilized in models that predict the mortality and the risk of chemotherapy-related toxicity in older individuals.[20-21,29-31] In addition, the CGA identify reversible conditions that interfere with cancer treatment, including comorbidity, malnutrition, absence of a reliable caregiver.[32-34]
Any discussion of the assessment of physiologic age should include frailty. This is constructed as a condition of critically reduced physiologic reserve so that a minimal stress may cause loss of independence and start a chain of events that lead to the patient ‘s death.[3]
The clinical definition of frailty was first provided by the Cardiovascular Health Study (CHS).[35] Eighty-five hundred individuals 65 and over were followed for an average of 11 years. Based on five simple parameters they could be divided into three groups with different risk of mortality, disability, and admission to adult living facilities. The parameters of interest included:
• Involuntary weight loss of 10 lbs or more over a 6 months period;
• Decreased grip strength;
• Difficulty in starting movements;
• Reduced walk speed;
• Exhaustion.
The three groups of individuals were classified as: non-frail or fit (no abnormalities); pre-frail (up to two abnormalities), and frail (three or more abnormalities).
Another index of frailty validated both in older women and older men has been provided by the Study of Osteoporotic Fracture (SOF)36 and older men.[37] Based on the performance of simple exercises (rising five times from a chair) it appears as accurate a predictor of falls, death, fractures and disability as the CHS instrument. Canadian investigators developed a frailty index[38] involving the accumulation of 40 functional deficits. This appears too time-consuming for clinical practice. The CHS classification represents the golden standard for ongoing studies of frailty. Fatigue was the first harbinger of frailty in about 73% of the cases detected in the CHS [39]. This finding is germane to our discussion as fatigue is the most common chronic manifestation of cancer.
The interactions of aging and frailty should be clarified. In particular we should ask: is cancer a cause of frailty? Is chemotherapy a cause of frailty? Is frailty associated with increased risk of therapeutic complications?
Cytotoxic chemotherapy
Aging is associated with pharmacokinetic and pharmacodinamic changes that may enhance the toxicity of these agents (Table 2).[40]
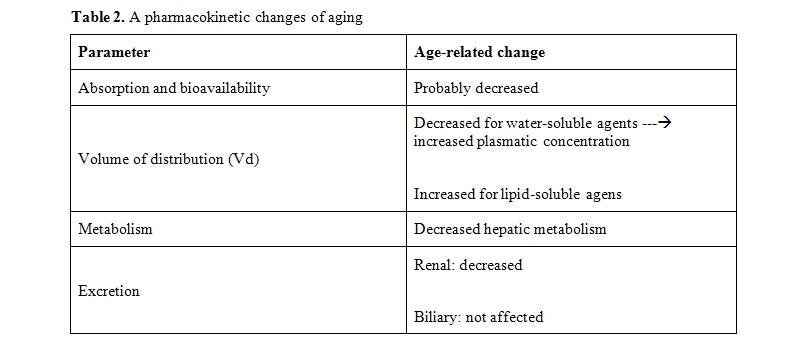
The most common pharmacokinetic changes of age include reduced glomerular filtration rate, which prevents renal excretion of drugs and their metabolites, and reduced Vd of hydrosoluble drugs, that is associated with increased AUC of these agents. The Vd is influences by body composition, serum albumin and hemoglobin concentration. Of these only the hemoglobin concentration can be modified at least in a short term. As discussed previously, anemia, is associated with increased risk of chemotherapy-related toxicity because several medications are bound to red blood cells. In presence of anemia the concentration of fre drugs is increased.
It is not clear at present whether an age-associated decline in food absorption affects the bio-availability of oral drugs. This issue need to be studies
Both the splanchnic circulation and the hepatic mass decrease with age. These changes conjure to reduce drug metabolism, especially when the cytochrome P450 reactions are involved. In addition, polypharmacy may modulate the cytochrome P450 system and inhibit or accelerate the metabolism of a number of drugs. The biliary excretion appears unaffected by age, but it is important to remember that some drugs, including idarubicin, daunorubicin, and morphine, give origine to active and toxic metaboites eliminated by the kidneys. The toxicity of these agents may then be enhanced in the presencce of declining GFR.
Some complications of cytotoxic chemotherapy become more common with aging.
The risk of neutropenia, neutropenic infections, and infectious deaths increase with the age of the patient.[41] Fortunately, the Granulocyte Colony Stimualting Factors (G-CSF) filgrastim, pegfilgrastim and lenograstim are effective in older individuals and reduce by more than 50% the risk of infections.[42-43] The use of these compounds has been associated with increased risk of myelodysplasia and acute myeloid leukemia in older individuals.[44] The risk of thrombocytopenia and anemia also increase with age, albeit to a lesser extent. These complications are rarely lethal but may cose a reduction of the dose intensity of chemotherapy and compromise the control of the tumor. Erythopoietic stimulating agents reverse the majority of chemotherapy-related anemias, but the use of these compounds is currently limited out of concern for hypertension, thrombosis and enhanced tumor growth.[45]
Mucositis, whose incidence and severity increase with age, causes dysphagia, diarrhea and rapid volume depletion in older individuals. Aggressive fluid resuscitation should be initiated in patients unable to ingest fluids. The prevention of mucositis has limited effectiveness and may include solutions of glutamine, supersaturated solutions of calcium phosphate, and keratynocyte growth factor.[46]
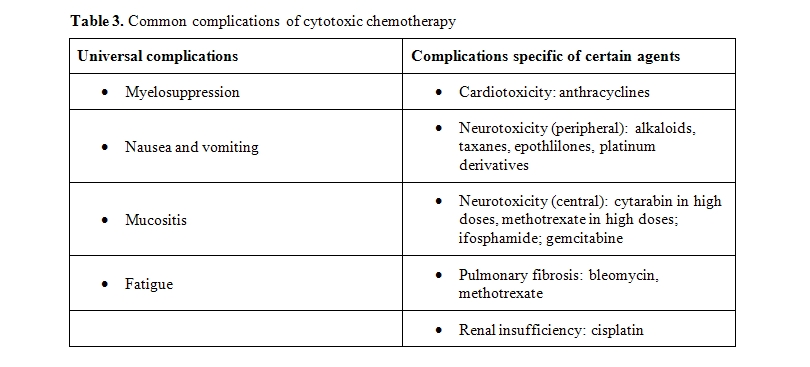
Fatigue is a feeling of exhaustion not releived by rest and is the most common long term complication of cancer chemotherapy.[47-48] The pathogenesis of chemotherapy induced fatigue is poorly understood, Correction of anemia may improve fatigue in some patients. In older individuals, fatigue is a a harbinger of frailty.[39]
Age is also a risk factor for anthracycline cardiomyopathy and for peripheral neuropathy.[40] The risk of cardiomyopathy may be reduced by the administration of doxorubicin as a continuous infusion, by the concomitant administration of doxorubicin and the antidote desrazoxane, or by the use of pegylated liposomal doxorubicin “in lieu” of doxorubicin. None of these approaches is routinely used in older individuals, because of cost, alternative toxicity and also because the dose of doxorubicin employed is rarely associated with cardiotoxic complications.
No antidote for peripheral neurotoxiciy exists. This complication may impair the independence of older individuals by impeding both walking and fine hand movements. The only prevention consists in trying to avoid combination of neurotoxic drugs (example cisplatin and paclitaxel) and in interrupting treatment when the neuropathy may interfere with an individual’s activity.
An important and unanswered question is whether age is a risk factor for more frequent and more severe manifestations of “chemobrain” that is a cognitive dysfunction caused by chemotherapy.
Age is a risk factor for delayed complications of chemotherapy. Myelodysplasia and acute myeloid leukemia may develop in 1-2% of patients 65 and over who had received anthracycline-containing treatment in the previous 10 years. The risk of these complications is enhanced by myelopoietic growth factors.[44] The incidence of a chronic cardiomyopathy, manifested by a progressive decline of the ejection fraction, is seen in approximately 19% of individuals 65 an older 5 years or longer after treatment with an anthracycline.[49]
Other potential long term complications of cancer chemotherapy include dementia, functional dependence and frailty.
Treatment goals
Cure, prolongation of survival, and symptom palliation are universal goals of medical treatment. Prolongation of active life expectancy should be added to the treatment goal of the older aged person.
The construct of active life expectancy[50] include preservation of functional independence (that is ability to carry on ADLs and IADLs) as well as preservation of the ability to perform activities that are pleasearuble and fulfilling(the so-called advanced activities of daily living). For this purpose it is important to obtain a so called” value history” at the beginning of treatment to detect what are the patients main objectives in the lasting years of his/her life and to make sure that the treatment is aimed to preserve these objectives.
Conclusions
With the aging of the population the management of cancer in the older person with chemotherapy is beoming increasingly common. This treatment may be safe and effective if some appropriate measures are taken, including, an assessment of the physiologic age of each patient, modification of doses according to the renal function, use of meyelopoietic growth factors prophylactically in presence of moderately toxic chemotherapy, and provision of an adequate caregiver. The goals of treatment should include prolongation of active life-expectancy.
The management of cancer in the older aged person is an increasingly common problem. Cancer is a disease of aging.[1] Currently 50% of all malignancies occur in individuals 65 and over[1] and by the year 2030 older individuals will account for 70% of all neoplasms.
The management of cancer in the older-aged person include some questions that are specfic of aging:
• Is the person life expectancy going to be shortened by cancer?
• Is the patient’s life expectancy long enough that he or she will experience the complications of cancer?
• Is the patient able to tolerate antineoplastic treatment?
• What are the long term side effects of cancer treatment in the older person?
• Does the patient have adequate social support to undergo cancer treatment?
We will address these questions using cytotoxic chemotherapy as a model. After an overview of aging and its assessment we will explore the pharmacologic changes of aging and the provisions to ameliorate the complications of chemotherapy.
Age and its assessment
Aging implies a decline in life expectancy and stress-coping ability, increased prevalence of comorbidity, increased risk of functional dependence and of the need of social support.[2] Though it is universal, aging is highly individualized and is poorly reflected in chronologic age. The management of the older aged person should be based on an assessment of physiologic rather than chronologic age.
Aging has been defined as loss of entropy and fractality[3] and as loss of homeostasis.[4] Entropy reflects the ability of a system to produce and to waste energy. A fractal is a unit subdividing into subunits of the same type, but whose number and size are unpredictable, similar to the branch of a tree. The maintenance of life is trusted to structural and dinamic fractals. In the mammalian body structural fractals include the respiratory, circulatory and nervous systems; the dinamic fractals include the process of cell generation. For example, in hemopoiesis, few pluripotent stem cells give origin to a number of committed progenitors from which the differentiated precursors of the circulating blood cells are derived. Commitment and differentiation involve the branching of the pluripotent stem cell into progressively larger cell populations. Entropy and fractality cannot be directly assessed by clinical means. The loss of cell replicating ability, expressed by a reduced length of leukocyte telomeres,5 may be seen as an expression of the loss of fractality. A number of studies have explored the possibility that the length of leukocyte telomeres provide a reliable assessment of physiologic age, but correlating the length of telomeres to the risk of functional dependence, geriatric syndromes, and other manifestations of age. The results so far have been inconclusive.[5-10]
Homeostasis is the ability of a system to restore basic conditions after stress imposed by environmental interactions. One may observe the dysregulation of a number of physiologic parameters, including blood pressure, insulin sensitivity, circulating levels of corticosteroid and cathecolamines. The so called “allostatic load” assesses the dysregulation of 12 different parameters and may estimate the physiologic age. Its clinical value so faris unestablished.[4] Chronic and progressive inflammation is arguably the best recognized manifestation of allostasis.. Aging is associated with increased concentration of circulating inflammatory markers, including inflammatory cytokines and fibrinolytic products. The concentration of Interleuking 6, D-Dimer, and C-reactive protein is associated with increased risk of death, functional dependence and geriatric syndromes[11-14] and may mirror the physiologic age of the person.
Inflammatory markers and the length of leukocyte telomeres are promising laboratory tests, but at present have limited clinical use.
The best validated instrument for the assessment of chronologic age is the Comprehensive Geriatric Assessment” (CGA), whose elements are illustrated in Table 1.[15-17]

Dependence in one or more ADLs and the presence of one or more geriatric syndromes imply a marginal functional reserve associated with very limited tolerance of stress. These individuals can only survive thanks to a full time home caregiver, or to admission to an adult living facility. Dependence in one or more IADLs is associated increased risk of mortality,[18-21] of dementia, and of chemotherapy-induced toxicity.[20-21] These individuals need a caregiver to negotiate the outside world.
In addition to reduced life-expectancy and increased risk of treatment complications[22-24] comorbidity may be associated to changes in the natural history of cancer and polypharmacy. It is usefule to mention that diabetes has been associated with worsened prognosis of most common neoplasms including cancer of the large bowel, of the prostate and of the breast.[22,25] Anemia deserves a special mention as it is associated with increased risk of overall mortality in older individuals, of functional dependence and of chemotherapy-induced myelotoxicity.26 Polypharmacy may be responsible of iatrogenic morbidity and of unfavorable interactions with antineoplastic drugs.[27-28] In addition, drug interactions are a major cause of iatrogenic morbidity.
Some elements of the CGA may be utilized in models that predict the mortality and the risk of chemotherapy-related toxicity in older individuals.[20-21,29-31] In addition, the CGA identify reversible conditions that interfere with cancer treatment, including comorbidity, malnutrition, absence of a reliable caregiver.[32-34]
Any discussion of the assessment of physiologic age should include frailty. This is constructed as a condition of critically reduced physiologic reserve so that a minimal stress may cause loss of independence and start a chain of events that lead to the patient ‘s death.[3]
The clinical definition of frailty was first provided by the Cardiovascular Health Study (CHS).[35] Eighty-five hundred individuals 65 and over were followed for an average of 11 years. Based on five simple parameters they could be divided into three groups with different risk of mortality, disability, and admission to adult living facilities. The parameters of interest included:
• Involuntary weight loss of 10 lbs or more over a 6 months period;
• Decreased grip strength;
• Difficulty in starting movements;
• Reduced walk speed;
• Exhaustion.
The three groups of individuals were classified as: non-frail or fit (no abnormalities); pre-frail (up to two abnormalities), and frail (three or more abnormalities).
Another index of frailty validated both in older women and older men has been provided by the Study of Osteoporotic Fracture (SOF)36 and older men.[37] Based on the performance of simple exercises (rising five times from a chair) it appears as accurate a predictor of falls, death, fractures and disability as the CHS instrument. Canadian investigators developed a frailty index[38] involving the accumulation of 40 functional deficits. This appears too time-consuming for clinical practice. The CHS classification represents the golden standard for ongoing studies of frailty. Fatigue was the first harbinger of frailty in about 73% of the cases detected in the CHS [39]. This finding is germane to our discussion as fatigue is the most common chronic manifestation of cancer.
The interactions of aging and frailty should be clarified. In particular we should ask: is cancer a cause of frailty? Is chemotherapy a cause of frailty? Is frailty associated with increased risk of therapeutic complications?
Cytotoxic chemotherapy
Aging is associated with pharmacokinetic and pharmacodinamic changes that may enhance the toxicity of these agents (Table 2).[40]

The most common pharmacokinetic changes of age include reduced glomerular filtration rate, which prevents renal excretion of drugs and their metabolites, and reduced Vd of hydrosoluble drugs, that is associated with increased AUC of these agents. The Vd is influences by body composition, serum albumin and hemoglobin concentration. Of these only the hemoglobin concentration can be modified at least in a short term. As discussed previously, anemia, is associated with increased risk of chemotherapy-related toxicity because several medications are bound to red blood cells. In presence of anemia the concentration of fre drugs is increased.
It is not clear at present whether an age-associated decline in food absorption affects the bio-availability of oral drugs. This issue need to be studies
Both the splanchnic circulation and the hepatic mass decrease with age. These changes conjure to reduce drug metabolism, especially when the cytochrome P450 reactions are involved. In addition, polypharmacy may modulate the cytochrome P450 system and inhibit or accelerate the metabolism of a number of drugs. The biliary excretion appears unaffected by age, but it is important to remember that some drugs, including idarubicin, daunorubicin, and morphine, give origine to active and toxic metaboites eliminated by the kidneys. The toxicity of these agents may then be enhanced in the presencce of declining GFR.
Some complications of cytotoxic chemotherapy become more common with aging.
The risk of neutropenia, neutropenic infections, and infectious deaths increase with the age of the patient.[41] Fortunately, the Granulocyte Colony Stimualting Factors (G-CSF) filgrastim, pegfilgrastim and lenograstim are effective in older individuals and reduce by more than 50% the risk of infections.[42-43] The use of these compounds has been associated with increased risk of myelodysplasia and acute myeloid leukemia in older individuals.[44] The risk of thrombocytopenia and anemia also increase with age, albeit to a lesser extent. These complications are rarely lethal but may cose a reduction of the dose intensity of chemotherapy and compromise the control of the tumor. Erythopoietic stimulating agents reverse the majority of chemotherapy-related anemias, but the use of these compounds is currently limited out of concern for hypertension, thrombosis and enhanced tumor growth.[45]
Mucositis, whose incidence and severity increase with age, causes dysphagia, diarrhea and rapid volume depletion in older individuals. Aggressive fluid resuscitation should be initiated in patients unable to ingest fluids. The prevention of mucositis has limited effectiveness and may include solutions of glutamine, supersaturated solutions of calcium phosphate, and keratynocyte growth factor.[46]

Fatigue is a feeling of exhaustion not releived by rest and is the most common long term complication of cancer chemotherapy.[47-48] The pathogenesis of chemotherapy induced fatigue is poorly understood, Correction of anemia may improve fatigue in some patients. In older individuals, fatigue is a a harbinger of frailty.[39]
Age is also a risk factor for anthracycline cardiomyopathy and for peripheral neuropathy.[40] The risk of cardiomyopathy may be reduced by the administration of doxorubicin as a continuous infusion, by the concomitant administration of doxorubicin and the antidote desrazoxane, or by the use of pegylated liposomal doxorubicin “in lieu” of doxorubicin. None of these approaches is routinely used in older individuals, because of cost, alternative toxicity and also because the dose of doxorubicin employed is rarely associated with cardiotoxic complications.
No antidote for peripheral neurotoxiciy exists. This complication may impair the independence of older individuals by impeding both walking and fine hand movements. The only prevention consists in trying to avoid combination of neurotoxic drugs (example cisplatin and paclitaxel) and in interrupting treatment when the neuropathy may interfere with an individual’s activity.
An important and unanswered question is whether age is a risk factor for more frequent and more severe manifestations of “chemobrain” that is a cognitive dysfunction caused by chemotherapy.
Age is a risk factor for delayed complications of chemotherapy. Myelodysplasia and acute myeloid leukemia may develop in 1-2% of patients 65 and over who had received anthracycline-containing treatment in the previous 10 years. The risk of these complications is enhanced by myelopoietic growth factors.[44] The incidence of a chronic cardiomyopathy, manifested by a progressive decline of the ejection fraction, is seen in approximately 19% of individuals 65 an older 5 years or longer after treatment with an anthracycline.[49]
Other potential long term complications of cancer chemotherapy include dementia, functional dependence and frailty.
Treatment goals
Cure, prolongation of survival, and symptom palliation are universal goals of medical treatment. Prolongation of active life expectancy should be added to the treatment goal of the older aged person.
The construct of active life expectancy[50] include preservation of functional independence (that is ability to carry on ADLs and IADLs) as well as preservation of the ability to perform activities that are pleasearuble and fulfilling(the so-called advanced activities of daily living). For this purpose it is important to obtain a so called” value history” at the beginning of treatment to detect what are the patients main objectives in the lasting years of his/her life and to make sure that the treatment is aimed to preserve these objectives.
Conclusions
With the aging of the population the management of cancer in the older person with chemotherapy is beoming increasingly common. This treatment may be safe and effective if some appropriate measures are taken, including, an assessment of the physiologic age of each patient, modification of doses according to the renal function, use of meyelopoietic growth factors prophylactically in presence of moderately toxic chemotherapy, and provision of an adequate caregiver. The goals of treatment should include prolongation of active life-expectancy.
References
- Yancik R; Ries LA: Cancer in the
Older Person: an International issue in an aging world. Sem Oncol,
2004, 31, 128-136

- Balducci L, Colloca G, Cesari M, et al: Assessment and treatment of elderly patients with cancer. Surg Oncol, 2010, in press
- Walston J, Hadley EC, Ferrucci L,et al.:
Research Agenda for frailty in Older Adults. J Am Ger Soc, 2006 54,
991-2001

- Gruenewald TL, Seeman TE, Karlamangla
AS, et al: Allostatic Load and frailty in older individuals. J Am
Geriatr Soc. 2009 Sep;57(9):1525-31. Epub 2009 Jul 21

- Willeit P; Willeit J; Mayr A et al:
Telomere length and incident cancer and Cancer Mortality. JAMA, 2010,
304, 69-75

- Wong LS, van der Harst P, de Boer RA, et al: Aging, telomeres, and heart failure. Heart Fail Rev. 2010 in press
- Willeit P, Willeit J, Brandstätter A, et al
Cellular aging reflected by leukocyte telomere length predicts advanced
atherosclerosis and cardiovascular disease risk. Arterioscler Thromb
Vasc Biol. 2010 30(8):1649-56.

- Sahin E, Depinho RA. Linking functional
decline of telomeres, mitochondria and stem cells during ageing.
Nature. 2010 Mar 25;464(7288):520-8

- Babizhayev MA, Savelʼyeva EL, Moskvina SN, et al: Telomere Length is a Biomarker of Cumulative Oxidative Stress, Biologic Age, and an Independent Predictor of Survival and Therapeutic Treatment Requirement Associated With Smoking Behavior. Am J Ther. 2010 in press
- Risques RA; Arbeev KG; Yashin AI et al:
Leukocyte Telomere Length is associated with disability in the
older USA population. J Am Ger Soc, 2010, 58, 1289-1298

- Ferrucci L, Corsi A, Lauretani F, et al.:
The origin of Age-related pro-inflammatory state. Blood, 2005, 105,
2294-2299

- Maggio M, Guralnik JM, Longo DL, et al.:
Interleukin-6 in aging and chronic disease: a magnificent pathway. J
Gerontol A Biol Sci Med Sci. 2006 Jun;61(6):575-84

- Crimmins E, Vasunilashorn S, Kim JK, et
al.: biomarkers related to aging in the human population. Adv Clin
Chem. 2008; 46, 161-216

- Gurven M, Kaplan H, Winking J, Finch
C, Crimmins EM. aging and inflammation in two epidemiological
worlds. J Gerontol A Biol Sci Med Sci. 2008 Feb;63(2):196-201

- Extermann M; Hurria A: Comprehensive
Geriatric Assessment in older patients with cancer. J Clin Oncol, 2007,
25, 1824-1831

- Luciani A, Ascione G, Bertuzzi C, et
al Detecting disabilities in older patients with cancer:
comparison between comprehensive geriatric assessment and vulnerable
elders survey-13. J Clin Oncol. 2010 Apr 20;28(12):2046-50

- Brunello A, Sandri R, Extermann M.:
Multidimensional geriatric evaluation of the older cancer patient as a
clin Cancer treat Rev, 2009, 35, 487-492

- Störk S, Feelders RA, van den Beld AW, et
al.: Prediction of mortality risk in the elderly. Am J Med. 2006
Jun;119(6):519-25

- Carey EC, Covinsky KE, Lui LY, et
al: Prediction of mortality in community-living frail elderly
people with long-term care needs. J Am Geriatr Soc. 2008 Jan;56(1):68

- M. Extermann, I. Boler, R. Reich, et al: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score: Design and validation.. ASCO national conference 2010, Abstr 9000
- A. Hurria, K. Togawa, S. G. Mohile, et al.: Predicting chemotherapy toxicity in older adults with cancer: A prospective 500 patient multicenter study. ASCO Proc, 2010, Abstr 9001
- Extermann M: Interactions of Cancer and
Comorbidity. Cancer Control, 2007, 14, 13-22

- Kulminski AM, Ukraintseva SV, Kulminskaya IV,et al.: Cumulative deficits better characterizes susceptibility to death in elderly people than phenotypic frailty. Lessons from the Cardiovascular Health Study. J Am Ger Soc, 2008, 56, 898-903
- Pal SK, Hurria A.: Impact of age, sex, and
comorbidity on cancer treatment and disease progression J Clin
Oncol. 2010 Jul 19.

- Klepin H, Mohile S, Hurria A.: Geriatric
assessment in Older patients with Breast Cancer .J Natl Compr Canc
Netw. 2009 Feb;7(2):226-36

- Ferrucci L and Balducci L: Anemia of
aging: role of chronic inflammation and cancer. Semin Hematol, 2008,
45, 242-249

- Haider SI, Johnell K, Weitoft GR et al :
The influence of educational level on polypharmacyand inappropriate
drug use. A register based study of more than 600000 older people. J Am
Ger Soc, 2009, 57, 62-69

- Maggiore RJ, Gross CP, Hurria A.:
Polypharmacy in older Adults with Cancer. Oncologist. 2010;15(5):507-22

- Lee SJ, Lindquist K, Segal MRet al:
Development and validation of a prognostic index for 4-year mortality
in older adults. JAMA. 2006 Feb 15;295(7):801-8.

- Carey EC, Covinsky KE, Lui LY, et
al: Prediction of mortality in community-living frail elderly
people with long-term care needs. J Am Geriatr Soc. 2008 Jan;56(1):68-75

- Schonberg MA, Davis RB, McCarthy EP,
et al: Index to predict 5-year mortality of community-dwelling
adults aged 65 and older using data from the National Health Interview
Survey. J Gen Intern Med. 2009 Oct;24(10):1115-22

- Extermann M; Overcash J; Lyman GH; et al
Comorbidity and performance status are independent in older cancer
patients. J Cin Oncol, 1998, 16, 1582-1587

- Ingram SS, Seo PH, Martell RE, et al l:
Comprehensive assessment of elderly Cancer patients: the feasiblility
of self-report methodology. J Clin Oncol, 2002, 20, 770-775

- Repetto L, Fratino L, Audisio RA, et al:
Comprehensive Geriatric assessment adds information to Eastern
Cooperative Group Performance Status in Elderly Cancer Patients:
an Italian Group for geriatric Oncology Study. J Clin Oncol, 2002, 15,
494-502

- Fried LP, Tangen CM, Walston J, et al
: Frailty in older adults: evidence for a
phenotype. J Gerontol A Biol Sci Med Sci. 2001
Mar;56(3):M146-56

- Ensrud KE, Ewing SK, Taylor BC, et
al. (2008); Comparison of two frailty indices for
prediction of falls, disability, fractures, and death in older women.
Arch Intern Med, 168, 382-389.

- Ensrud KE, Ewing SK, Cawthon PM, et al, Cummings SR; Osteoporotic Fractures in Men Research Group: A comparison of two frailty indices for prediciton of falls, disability, fractures and death in older men. J Am Ger Soc, 2009. 57, 492-49
- Searle SD, Mitnitski A, Gahbauer EA, Gill
TM, Rockwood K. A standard procedure for creating a frailty index. BMC
Geriatr. 2008, 30, 8-24.

- Xue QL, Bandeen-Roche K, Varadhan R et al.
Initial Manifestations of frailty criteria and the development of
frailty phenotype in the Women Health and Aging Study II. J Gerontol
Med Sci 2008, 63, 984-990

- Carreca I, Balducci L. Cancer chemotherapy
in the older cancer patient. Urol Oncol. 2009 Nov-Dec;27(6):633-42

- Lyman GH, Kuderer NM. A primer in
prognostic and predictive models: development and validation of
neutropenia risk models. Support Cancer Ther. 2005 Apr
1;2(3):168-75.

- Kuderer NM, Dale DC, Crawford J, et
al: Impact of primary prophylaxis with granulocyte
colony-stimulating factor on febrile neutropenia and mortality in adult
cancer patients receiving chemotherapy: a systematic review. J Clin
Oncol. 2007 Jul 20;25(21):3158-67.

- Balducci L, Al Halawani H, Charu V
et al: Elderly cancer patients receiving chemotherapy benefit from
first-cycle pegfilgrastim. Oncologist. 2007 Dec;12(12):1416-24.

- Lyman GH, Dale DC, Wolff DA, et al:
Acute myeloid leukemia or myelodysplastic syndrome in randomized
controlled clinical trials of cancer chemotherapy with granulocyte
colony-stimulating factor: a systematic review. J Clin Oncol. 2010 Jun
10;28(17):2914-24. Epub 2010 Apr 12

- Bohlius J, Schmidlin K, Brillant C, et al:
Erythropoietin or Darbepoetin for patients with cancer--meta-analysis
based on individual patient data. Cochrane Database Syst Rev. 2009 Jul
8;(3):CD007303. Review.

- Sonis ST. Regimen-related
gastrointestinal toxicities in cancer patients. Curr Opin Support
Palliat Care. 2010 Mar;4(1):26-30.

- Minton O, Richardson A, Sharpe M, et
al A systematic review and meta-analysis of the pharmacological
treatment of cancer-related fatigue. J Natl Cancer Inst. 2008 Aug
20;100(16):1155-66.

- Dy SM, Lorenz KA, Naeim A et al:
Evidence-based recommendations for cancer fatigue, anorexia,
depression, and dyspnea. J Clin Oncol. 2008 Aug 10;26(23):3886-95

- Broder H, Gottlieb RA, Lepor NE.:
chemotherapy and cardiotoxicity Rev Cardiovasc Med. 2008
Spring;9(2):75-8

- Manton KG, Gu X, Lowrimore GR.
Cohort changes in active life expectancy in the U.S. elderly
population: experience from the 1982-2004 National Long-Term Care
Survey. J Gerontol B Psychol Sci Soc Sci. 2008 Sep;63(5):S269-81.
