Thrombotic Microangiopathy in Haematopoietic Cell Transplantation: an Update
Evi Stavrou and Hillard M. Lazarus.
Department of Medicine,
University Hospitals Case Medical Center, Case Western Reserve
University, Cleveland, OH 44106
Correspondence
to: Hillard
M. Lazarus, MD, FACP. Department of Medicine, University Hospitals Case
Medical
Center, 11100 Euclid Avenue, Cleveland, OH 44106. Telephone
216-844-3629, FAX
216-844-5979. E-mail: hillard.lazarus@case.edu
Published: November 3, 2010
Received: October 9, 2010
Accepted: October 29, 2010
Medit J Hemat Infect Dis 2010, 2(3): e2010033, DOI 10.4084/MJHID.2010.033
This article is available from: http://www.mjhid.org/article/view/6425
This is an Open Access article
distributed under the terms of
the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Allogeneic
hematopoietic cell transplantation (HCT) represents a vital procedure
for patients with various hematologic conditions. Despite advances in
the field, HCT carries significant morbidity and mortality. A rare but
potentially devastating complication is transplantation-associated
thrombotic microangiopathy (TA-TMA). In contrast to idiopathic TTP,
whose etiology is attributed to deficient activity of ADAMTS13, (a
member of the A Disintegrin And Metalloprotease with Thrombospondin 1
repeats family of metalloproteases), patients with TA-TMA have > 5%
ADAMTS13 activity. Pathophysiologic mechanisms associated with TA-TMA,
include loss of endothelial cell integrity induced by intensive
conditioning regimens, immunosuppressive therapy, irradiation,
infections and graft-versus-host (GVHD) disease. The reported incidence
of TA-TMA ranges from 0.5% to 75%, reflecting the difficulty of
accurate diagnosis in these patients. Two different groups have
proposed consensus definitions for TA-TMA, yet they fail to distinguish
the primary syndrome from secondary causes such as infections or
medication exposure. Despite treatment, mortality rate in TA-TMA ranges
between 60% to 90%. The treatment strategies for TA-TMA remain
challenging. Calcineurin inhibitors should be discontinued and replaced
with alternative immunosuppressive agents. Daclizumab, a
humanized monoclonal anti-CD25 antibody, has shown promising results in
the treatment of TA-TMA. Rituximab or the addition of defibrotide, have
been reported to induce remission in this patient population. In
general, plasma exchange is not recommended.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a useful therapeutic modality for a wide range of hematologic and non-hematologic conditions.[1-3] Peripheral blood progenitor cell collection, the new ‘gold standard’ in hematopoietic cell harvesting, and non-myeloablative peripheral blood progenitor cell transplantation, have reduced treatment-related mortality and enabled an increasing number of patients with comorbid conditions as well as older patients to receive therapy for conditions such as acute leukemia, myelodysplastic syndrome, multiple myeloma and lymphoma. The obstacles to successful HCT include the development of acute and chronic graft-versus-host disease (GVHD), opportunistic infections, and other complications, one of which is transplantation-associated thrombotic microangiopathy (TA-TMA).[4-6] The etiologies of this syndrome are diverse, and diagnosis of TA-TMA in this patient population requires a high degree of clinical suspicion. Moreover, management of TA-TMA remains a challenging task, mainly due to the poor response to therapeutic modalities that are beneficial in non-transplant-associated TMA.
Pathologic and clinical features
TMAs are defined by the association of microangiopathic hemolytic anemia, thrombocytopenia (platelet count < 100x109/L) and ischemic manifestations related to the formation of platelet-rich thrombi in the microcirculation.[7] TMAs include thrombotic thrombocytopenic purpura (TTP), and the hemolytic-uremic syndrome (HUS), and variants of these, which are characterized by ischemic manifestations involving the brain or gastrointestinal tract and/or kidneys, respectively.[8] TMA may be primary, or occur secondary to other disorders such as pregnancy, infections, autoimmune diseases and the post-HCT state.[9]
The clinical presentation of TMA invariably includes the presence of schistocytes on the peripheral blood film and consumptive thrombocytopenia. Surrogate markers include DAT (direct antiglobulin test)-negative hemolytic anemia, an elevated serum lactate dehydrogenase (LDH), decreased serum haptoglobin and indirect hyperbilirubinemia. Coagulation studies are usually normal. A “pentad” of signs and symptoms was traditionally associated with classic TTP: thrombocytopenia, microangiopathic hemolytic anemia (MAHA), neurologic abnormalities, renal abnormalities and fever. This complete set of symptoms occurs in only 40% of patients, and more than 70% have only the triad of MAHA, thrombocytopenia, and neurologic changes at the time of diagnosis.10 In current clinical practice, thrombocytopenia, schistocytosis, and an elevated serum LDH in the appropriate clinical setting provide sufficient criteria for the diagnosis.[7] The clinical manifestations of HUS are similar to TTP, although renal abnormalities, as opposed to neurologic dysfunction, often predominate.
Presentation of TA-TMA is similar to other forms of TMA; multiple contributing pathogenic factors have been implicated.[4,11] These include endothelial cell injury due to toxic conditioning regimens (high-dose chemotherapy and total-body irradiation [TBI]), cytomegalovirus (CMV) infection, the use of calcineurin inhibitors such as cyclosporine, and a possible graft-versus-host effect on the endothelium.[4,12-14] Because anemia, thrombocytopenia, renal impairment, and changes in mental status are common and may have multiple causes in the transplant population, diagnosis may be difficult.[15] This observation currently is motivating experts in the field to reformulate a classification of TMAs more focused on pathophysiologic mechanisms rather than clinical symptoms.[16, 17]
Diagnostic criteria
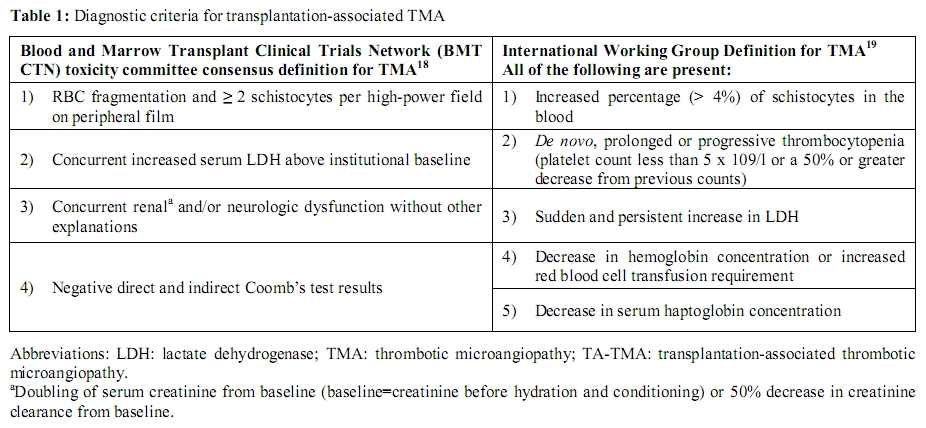
Until recently, there were no widely accepted criteria for the definition of hematopoietic progenitor cell TA-TMA. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) and the International Working Group separately formed toxicity committees to develop a consensus formulation of criteria for diagnosing clinically significant TA-TMA; these are listed in Table 1.[18,19]
Incidence and risk factors
The reported proportion of patients developing a clinically significant TA-TMA syndrome has varied greatly. George and coworkers15 presented a review of published reports on TMA after allogeneic HCT. Twenty-eight different definitions of this syndrome have been used in the 35 reviewed reports. Reflecting the different definitions, the incidence of TA-TMA varied in these reports from 0.5 to 63.6% of HCT recipients, the median frequency of diagnosis being 7.9%. The mortality in the different series ranged from 0% to 100%; the overall mortality rate was 61%. Of the deceased patients, 35 autopsy reports were identified. Three of the autopsies attributed death to HUS due to observation of isolated renal TMA. The remaining 32 deaths were attributed to other causes, the most common being systemic infection, including CMV, Aspergillus species, adenovirus and human herpesvirus-6 (HHV-6). Eleven autopsies stated that there was no evidence of TTP-HUS.[15] In another review, Pettitt and Clark4 estimated that TA-TMA occurs in 14% and 7% of allogeneic and autologous transplant recipients, respectively. A more recent report of more than 4000 HCT recipients estimated the frequency of severe TA-TMA to be 0.5% and 0.13% of allogeneic and autologous recipients, respectively.[6] This varying incidence of TA-TMA among reported series likely reflects the level of physician awareness, the different diagnostic criteria, and the heterogeneity of the transplant population.
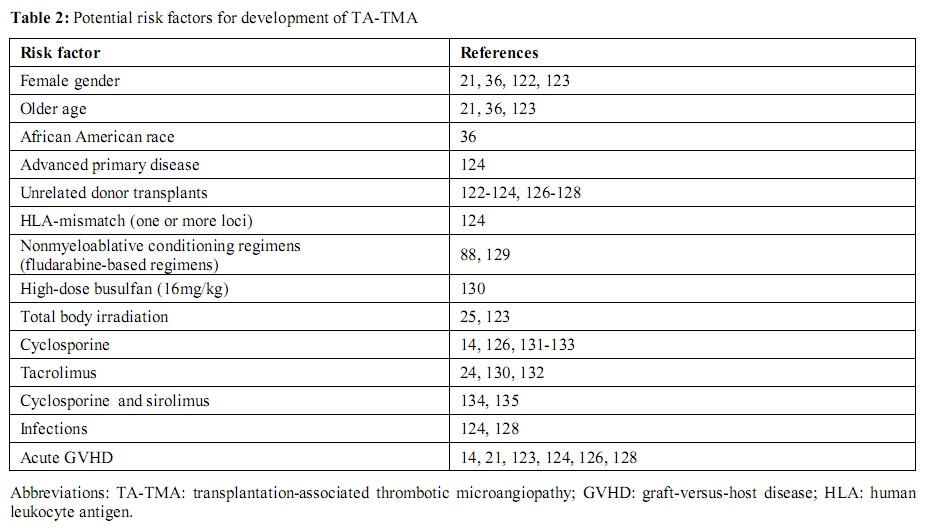
A variety of potential risk factors for the development of TA-TMA have been proposed (Table 2). Among the earliest reports, the use of cyclosporine (CsA) for the prevention of GVHD was recognized as a potential culprit.[20,21] Intensive immunosuppression with other inhibitors of the Ca2+-activated phosphatase, calcineurin (tacrolimus),[22-24] and (TBI)[25] have been associated with TA-TMA as well. In the following section we examine the pathophysiology and clinical characteristics of both primary and secondary TMAs. The heterogeneity in clinical background (idiopathic TTP vs. disease-associated TMA, including TA-TMA) and plasma concentration of markers (normal vs. decreased ADAMTS13 activity) is well emphasized and represents a barrier to the development of clear treatment guidelines.
Pathophysiology
Idiopathic TTP: Moake and colleagues [26] were the first to describe the presence of very high molecular weight [so-called ultralarge (UL)] multimers of von Willebrand factor (vWF) in the plasma of a patient with recurrent TTP. Once released from stimulated endothelial cells, UL-vWF, not present in plasma from healthy individuals, promote excessive aggregation of platelets, primarily in the microvasculature. Some ULvWF multimers remain on the endothelial cell surface as long strings that adhere to platelets. The molecules responsible for the binding of UL-vWF to endothelial cells and platelets are believed to involve integrin αvβ3 and glycoprotein Ib (GpIb), respectively.[27] Microvascular thrombosis and hemolytic anemia occur particularly in high shear stress locations, such as the microvasculature, that results in unfolding of ULvWF multimers and exposure of platelet binding sites.[7,28]
Moake hypothesized that a deficiency of a vWF cleaving protease might be responsible for the presence of ULvWF, [26] but it was Furlan and colleagues [29] et al., Tsai and Lian [30] who first isolated a plasma metalloprotease that cleaved the peptide bond between the tyrosine 1605 and methionine 1606 in the central A2 domain of vWF. These investigators subsequently found a deficiency of this vWF-cleaving protease in a retrospective cohort of patients clinically diagnosed as having TTP.[29,30] The protease was characterized in 2001 by Zheng and coworkers31 as a new (the thirteenth) member of the ADAMTS (A Disintegrin And Metalloprotease with Thrombospondin 1 repeats) family of metalloproteases and was thus called ADAMTS13.[16,31-34] The primary role of ADAMTS13 is to regulate the multimeric structure of vWF by cleaving the most hemostatically active ULvWF multimers.[35] Failure of this regulatory mechanism causes the highly adhesive, unusually large multimers of vWF to accumulate in plasma, which may lead to the microvascular thrombosis, tissue ischemia and infarction which are characteristic of TTP.
The estimated annual incidence of idiopathic TTP is 3.7 to 11 cases per million.[36] In extremely rare cases, severe deficiency of ADAMTS13 (defined as <5% serum activity) is related to compound heterozygous or homozygous mutations of the ADAMTS13 gene (Upshaw-Shulman syndrome).[37-39] Defects in coding of the metalloprotease gene, located on chromosome 9q34, result in functionally deficient enzyme. In the vast majority of cases, severe ADAMTS13 deficiency is secondary to the development of anti-ADAMTS13 autoantibodies that can be detected in vitro.[29,30] Functional testing often is employed in which anti-ADAMTS13 autoantibodies are detected by their inhibitory effect on ADAMTS13 enzymatic activity.[40] More recently, physical methods of detection have been used and identify either immunoglobulin G (IgG) or IgM species via enzyme-linked immunosorbent assay (ELISA).[41,42] In more than 80% of acquired TTP, anti-ADAMTS13 antibodies are inhibitory IgG.[29,30] Less frequently, the mechanism for acquired TTP may be different, involving anti-ADAMTS13 noninhibitory IgG or IgM [41,42] autoantibodies that promote the clearance of ADAMTS13 from blood without inhibiting its activity.[41]
Adults with acquired idiopathic TTP require daily plasma exchange until neurologic symptoms have resolved and both a normal serum LDH and platelet count have been maintained for at least 2 to 3 days.[43-46] Plasma exchange is thought to remove antibodies against ADAMTS13, while replacing the deficient protease.[47,48] Plasma exchange results in the remission of TTP, which is usually fatal when untreated, in approximately 80%-90% of cases,[49-52] generally without persistent organ damage. Production of ADAMTS13 autoantibodies may also be suppressed by high-dose corticosteroid treatment, [52] although there is very little controlled data that demonstrates efficacy of steroids in the treatment of idiopathic TTP. Other therapies include use of the monoclonal antibody rituximab that is directed against the CD20 epitope on B lymphocytes; given weekly for 4-8 weeks, this agent will eliminate antibody-producing cells [53-57] and has been shown to induce remission in refractory TTP, and to reduce the otherwise high incidence of relapse in these patients.[56,58] Finally, splenectomy also has been shown to be effective in anecdotal cases of refractory TTP, and to reduce the incidence of relapse in small series.
Hemolytic-uremic syndrome (HUS): HUS refers to TMA that primarily affects the kidney, often causing oliguric or anuric renal failure.[59] HUS may present with a variety of manifestations, with one variant occurring after Escherichia coli O157:H7 gastroenteritis, primarily in children.[60] In adults, however, HUS occurs most commonly in association with pregnancy, with more than 90% of cases developing in the postpartum period.[61] The characteristic histologic lesion of HUS consists of vessel wall thickening, with swelling and detachment of the endothelial cells from the basement membrane. In HUS, microthrombi are rich in fibrin and contain relatively little vWF.[62] Their location is confined primarily to the kidneys and thus, renal failure is the dominant feature. HUS associated with renal failure in the absence of a diarrheal illness or other predisposing condition is commonly referred to as atypical HUS. It has been proposed that this group may, in part, consist of patients with complement system dysfunction owing to either a mutation of a complement-regulatory protein,[63] an antibody directed at one of these proteins,[64] or an activating mutation of a complement protein such as C3. Study of families with a history of HUS has implicated mutations in several proteins responsible for regulating the alternative complement pathway, namely complement factor H,[65] membrane cofactor protein (MCP), and factor I (IF),[66] as well as thrombomodulin.[67] Exposure to agents potentially toxic to the vascular endothelium (such as certain viruses, bacteria, toxins, immunocomplexes, and cytotoxic drugs) may initiate local intravascular thrombosis.[68] This action promotes C3bBb convertase formation and complement deposition within capillary vessels. Under normal conditions, however, factor H may effectively limit complement deposition and further extension of the process by modulating C3bBb activity.[69] In contrast, when the factor H bioavailability is reduced due to decreased activity or is congenitally defective, C3bBb convertase formation and complement deposition may become uncontrolled. As a result, the microangiopathic process is extended, leading to full-blown manifestations of the disease.
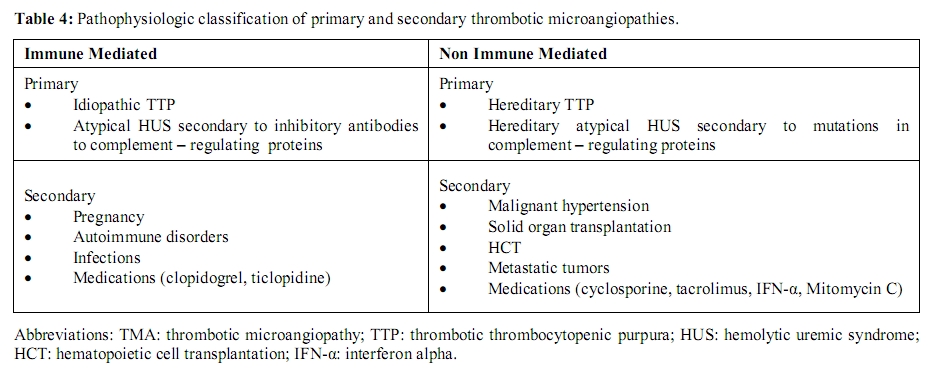
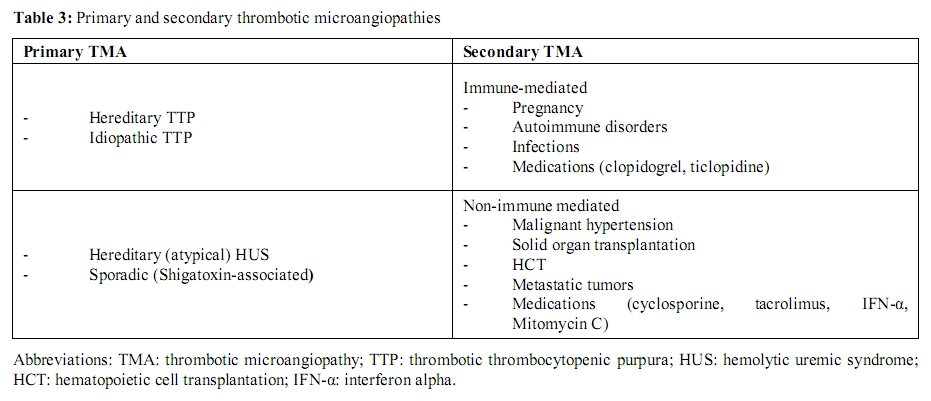
Secondary TMA: Secondary forms of TMA refer to a diverse group of disorders with frequently overlapping clinical features (Table 3). An alternative classification that takes into consideration the underlying pathophysiologic mechanisms (immune-mediated vs. nonimmune-mediated) is presented here (Table 4). Immune-mediated forms result from autoantibodies against ADAMTS13.[41,42,70] The second category (nonimmune-mediated) occurs from massive endothelial cell stimulation with consequent release of ULvWF multimers in amounts exceeding the system’s degradative ability, despite the presence of normal or only mildly reduced concentrations of ADAMTS13.[71] Distinction between these forms should limit diagnostic uncertainty and assist with management strategies, namely implementation of plasma exchange and use of immunosuppressive therapy.
The most common physiologic condition present in the immune-mediated forms, which is often associated with severe ADAMTS13 deficiency, is pregnancy.[29,36,72,73] Immune-mediated TMA in pregnancy should be distinguished from a number of pregnancy-associated TMAs such as preeclampsia, hemolysis with elevated liver enzymes and low platelets (HELLP) syndrome, acute fatty liver of pregnancy, and antiphospholipid syndrome.[74,75] Accurate diagnosis is essential since plasma exchange is indicated for pregnancy-associated TTP while fetal-placental delivery is therapeutic for HELLP syndrome.
The association between TTP and SLE has been well recognized in clinical and histologic reports.[76] Severe deficiency of ADAMTS13 activity is predominantly associated with the presence of inhibitory anti-ADAMTS13 IgG.
ADAMTS13 levels are not generally decreased with infections such as HIV, suggesting an alternative mechanism for TMA in these patients.[77,78] Cases of TMA associated with severe ADAMTS13 deficiency and inhibitory anti-ADAMTS13 IgG have been reported with influenza A,[79] legionella pneumonia [80] and brucellosis.[81]
Antibodies that inhibit plasma ADAMTS13 have also been demonstrated in patients with ticlopidine [82] or clopidogrel-associated TMA.[83] The immune dysregulation by these thienopyridine compounds might be analogous to the anti-RBC antibody ‘escape’, associated with the antihypertensive medication α-methyldopa.[84]
The most frequent concomitant conditions associated with TMA forms presenting with normal or mildly reduced levels of ADAMTS13 (greater than 10% serum activity) are malignant hypertension,17 metastatic tumors,85 solid organ transplantation, HCT (particularly allogeneic transplants), and the use of drugs such as cyclosporine, mitomycin, and α-interferon.[34]
Despite having some features in common, TA-TMA differs from de novo TTP in many aspects including the absence of severe ADAMTS13 deficiency, a different spectrum of clinical symptoms, poor response to plasma exchange, and the lack of evidence of systemic microthrombus formation.[15] Several small retrospective studies of TA-TMA encompassing a total of 33 HCT recipients also suggest that severe ADAMTS13 deficiency may be rare among this patient population.[86-88] Indeed, other prospective reports in HCT recipients suggest that the majority of these patients experience only a mild decrease in ADAMTS13 activity (usually after the cytotoxic conditioning) that can persist for weeks; however, severe ADAMTS13 deficiency was rare.[89,90] These data suggest that, unlike idiopathic TTP, ADAMTS13 deficiency is not the primary component of the pathophysiology of TA-TMA and other factors may play a more central role.
Role of endothelial cell injury: For a long time, many authorities have considered endothelial cell injury as the central and likely inciting factor that sustains the microangiopathic process in TMA, including the post-transplantation state. As early as 1942, Altschule [91] suggested that microvascular endothelial cell activation was the primary event causing platelet deposition in arterioles and capillaries with secondary “clearance of enormous numbers of platelets from the circulation”. Endothelial cells synthesize many substances involved in coagulation and fibrinolysis including vWF, thrombomodulin, tissue-type plasminogen activator (tPA), plasminogen activator inhibitor (PAI-1), protein S, prostacyclin (PGI2), and nitrous oxide (NO). Alterations in the concentration of these substances have been reported in idiopathic TTP and TA-TMA.[92,93] Whether these alterations represent an initiating effect or simply are reflective of endothelial cell injury, remains elusive. Levels of vWF antigen and soluble thrombomodulin were measured in patients with idiopathic TTP and TA-TMA.[94] vWF antigen and thrombomodulin levels were elevated in both patient groups compared to controls. Thrombomodulin concentrations were significantly higher in TA-TMA compared to idiopathic TTP, supporting a role for endothelial cell damage in the former. Gordon and colleagues [95] demonstrated that protein C deficiency correlated with thrombotic complications in patients undergoing HCT. Several groups have reported elevated plasma levels of fibrinogen, tPA, PAI-1, vWF antigen, thrombomodulin, and intercellular adhesion molecule 1 (ICAM-1).[96-100] Kanamori et al.[100] proposed that measurement of thrombomodulin levels on day 14 post-HCT may be useful in surveillance for TA-TMA. Cohen and colleagues [93] proposed that endothelial cell injury is pathognomonic of TA-TMA as well after they demonstrated absent endothelial cell PGI2 release and scanning electron microscopy (EM) evidence of endothelial cell damage with TMA after allogeneic HCT.
Risk factors and prognosis: Non-modifiable risk factors for development of TA-TMA include female gender, African American race, and older age.[36,122,123] Prior medical history of severe hepatic dysfunction and advanced primary disease also increase the risk of developing TA-TMA.[124,125] Treatment-related risk factors include: unrelated donor transplants; [88,122-124,126-128] HLA-mismatched donors;124 fludarabine-based non-myeloablative conditioning regimens;88,129 busulfan and TBI myeloablative conditioning. [25,123,130] The incidence of TA-TMA did not differ according to graft source, e.g. bone marrow versus peripheral blood.122 As mentioned above, use of calcineurin inhibitors such as cyclosporine,[14,126,131-133] tacrolimus,[24,130,132] and sirolimus[132,134,135] are also associated with the development of TA-TMA. Infections and the development of GVHD also increase the risk of developing TA-TMA.[14,21,123,124,128] In the HCT patients, non-transplantation etiologies of TMA such as idiopathic TTP and HUS should always be considered in the differential diagnosis, as they may coexist with the primary hematologic disease. In TA-TMA, poor prognostic indicators include: patient age > 18 years, a graft source from an unrelated or haploidentical donor,[123] at least five schistocytes per high-power field on peripheral film,[136] TA-TMA in the absence of sirolimus,[134] and nephropathy.[122] Specifically, Uderzo and coworkers[123] reported three factors statistically significant in predicting outcome of TA-TMA: adult age, unrelated or haploidentical graft source, and high TMA index (elevated LDH-platelet ratio). A retrospective cohort analysis of myeloablative allogeneic HCT recipients showed that sirolimus exposure constitutes a risk factor for the development of TA-TMA (10.8% in the sirolimus-exposed subjects vs. 4.2% in the non-sirolimus group)[134] but is also a favorable prognostic indicator in terms of TA-TMA overall survival (58.3% for TA-TMA related to sirolimus exposure vs. 11.1% in the non-sirolimus group)134 and renal recovery (92% vs. 78% respectively).[134] Martinez and colleagues136 reported lower one-year survival in patients with TA-TMA than in patients without TA-TMA (27 ± 18.1% for TA-TMA with high schistocyte counts; 53 ± 15% for TA-TMA with low schistocyte counts; vs. 78 ± 7% in patients without TA-TMA, p< 0.0001). A survey of the European Group for Blood and Marrow Transplantation (EBMT)122 conducted among forty-five centers included 406 patients transplanted, and reported an incidence of TA-TMA of 6.7%. The only factor predictive of resolution of TA-TMA was the absence of nephropathy.[122]
Therapeutic modalities
At the present time there is no consensus on what constitutes appropriate therapy for patients with TA-TMA. Initial attempts should focus on the following: i) eliminating possible causative conditions such as treating underlying infections and controlling acute GVHD; and ii) pharmacologic therapy with medications such as daclizumab, defibrotide and rituximab. The rationale for use of these agents is based on empirical benefit.
Eliminating Risk Factors and Consideration of Plasma Exchange: Cyclosporine, tacrolimus and sirolimus should be discontinued immediately and replaced with alternative immunosuppressive medications. Corticosteroids, mycophenolate mofetil, azathioprine and methotrexate can be used as appropriate alternatives. Withdrawal of cyclosporine with initiation of plasma exchange/apheresis has shown response rates of up to 63%.[137] In all reported cases cyclosporine was discontinued at the time of diagnosis of TA-TMA, and the effect of this intervention in isolation cannot be determined as patients went on to have therapeutic plasma exchange.
Unlike the situation in idiopathic TTP, responses to plasma exchange alone are suboptimal in TA-TMA. Reported response rates vary between 0-49%,[138,139] compared with 78-91% in patients with idiopathic TTP.[45,140] Further, rise in platelet count, the usual marker for response to plasma exchange, cannot be relied upon in TA-TMA because platelet engraftment may not yet have occurred. In addition, plasma exchange procedures are associated with a significant number of complications, including systemic infections, catheter thrombosis, bleeding, pneumothorax, pericardial tamponade, and with plasma infusion, serum sickness and anaphylaxis.
Based on the incomplete responses and high complication rates, we do not advocate use of this procedure but rather alternative therapeutic approaches as discussed below.
Daclizumab: Daclizumab is a humanized monoclonal anti-CD25 antibody, which targets the chain of the IL-2 receptor.[141] This agent is 90% humanized, retaining only 10% of the original murine compartments in the critical hypervariable segments for binding specificity. Daclizumab has been used to decrease the incidence of acute rejection in solid organ transplants including renal,[142] hepatic, cardiac,[143,144] and lung transplantation.[143,145] Daclizumab also has been used successfully in T-cell mediated autoimmune diseases such as multiple sclerosis,[146-148] pure red cell aplasia,[149] and aplastic anemia.[150]
In the HCT setting, intravenous daclizumab (with a serum half-life of 20 days) has been used to prevent or treat acute GVHD;[151] more recently, this agent has been used for the treatment of TA-TMA. Adverse effects include an increased risk for bacterial, candida and aspergillosis infections, as well as CMV reactivation. Through its effect on depleting alloreactive T-cells, daclizumab can substitute for a calcineurin inhibitor. Wolff et al.[132]used daclizumab at an initial loading dose of 2mg/kg and then 1mg/kg weekly. Nine of 13 affected patients attained complete remission after therapy.[132] Four of the patients who had a complete remission from TMA also had complete resolution of active GVHD. A fifth, complete remitter patient from both TMA and GVHD died of primary disease relapse; the remaining eight patients died from infections, GVHD or multiorgan failure.[132] The long half-life and potent immunosuppressive effect make this agent a promising treatment modality that merits further investigation.
Defibrotide: Defibrotide is a large, single-stranded polydeoxyribonucleotide, derived from porcine mucosa by controlled depolymerization. It has been found to have potent anti-thrombotic, anti-ischemic, anti-inflammatory, and thrombolytic properties, without significant systemic anticoagulant effects.[127,152-158] This drug exerts its properties by inhibition of TNFα-mediated endothelial cell apoptosis in vitro,148 decreasing the activity of PAI-1 and increasing endogenous tissue plasminogen activator (tPA) function.[159]
Hepatic veno-occlusive disease (VOD) is a potentially lethal complication of both allogeneic and autologous HCT,[156,160,161] especially after prior exposure to the immunoconjugate gemtuzumab ozogamicin.[162] In some studies, the incidence of hepatic VOD after HCT approaches 20% with mortality ranging from 7% to 50%.[163] The pathogenesis of VOD involves injury to the sinusoidal endothelial cells, leading to occlusion of small vessels with fibrin deposition and disruption of hepatic function. Previous attempts at therapy using either heparin or tPA have been unsuccessful.[164,165] Defibrotide therapy has improved outcomes for hepatic VOD that develops after HCT (30% to 60% CR rate).[166-170] Given the similarities in pathophysiology with TA-TMA, including loss of small vessel endothelial cell integrity, Corti and coworkers[155] reported that 3 of 12 affected patients with TA-TMA given oral defibrotide achieved partial remission, while 5 of 12 patients achieved a complete response.[155] Because the effects of defibrotide are exerted locally within the vascular bed, it is usually well tolerated with no significant systemic effects on coagulation such as seen during treatment with tPA.
Rituximab: Rituximab, discussed above, has been used with increasing frequency for the treatment of various hematologic and rheumatologic disorders including idiopathic TTP,[171-173] acquired coagulation factor inhibitors,[174,175] antiphospholipid antibody syndrome,[173] systemic lupus erythematosus176 and rheumatoid arthritis.[177,178] Rituximab use in relapsed, refractory TTP is linked to its ability to eliminate antibodies to ADAMTS13.171 Au et al.[179] treated five TA-TMA patients refractory to a week of plasma exchange and prednisolone with rituximab 375mg/m2/week for four doses. Four attained complete remission; two patients recovered after receiving two weeks of rituximab.[179] At a median follow-up of 305 days, 3 of 4 responders remained in remission [179] but the fourth responder died of sepsis. The only non-responder died of multi-organ failure after three weeks.[179] ADAMTS13 antigen levels were marginal or low either post-HCT or at the onset of TMA and did not change significantly after rituximab-induced remission.[179] It remains unclear whether these patients actually had TA-TMA (or TTP), thus making the use of rituximab in this setting uncertain. In addition, there are no established guidelines for recommending duration of rituximab treatment. Given the lack of reliable markers for remission (serum ADAMTS13 activity and anti-ADAMTS13 antibody levels), maintenance rituximab therapy cannot be recommended.
Other modalities and future directions: Single agent response rates for the antiplatelet agents aspirin and dipyridamole approximate 10%,[49,180] a result indistinguishable from the natural history of TTP. Antiplatelet agents have not been convincingly shown to increase the response to plasma exchange [45,51,181] and may promote bleeding in the setting of acute thrombocytopenia and invasive procedures.[51,182] Hence, their use as first-line treatment of TMA, including TA-TMA, cannot be recommended. Although not verified in vivo, intravenous immunoglobulin (IVIG) has been used therapeutically based on a report that IgG from healthy individuals inhibits the capacity of TTP plasma to agglutinate platelets in vitro.[183] A recent study described a response to the combination of plasma exchange and IVIG in a patient who was refractory to plasma exchange alone.[184] There are anecdotal reports of favorable responses of TTP to vincristine,[180,185,186] as well as other immunosuppressive therapies such as azathioprine, cyclophosphamid, and staphylococcal protein A immunoadsorption.[187,188] By analogy, there are case series of use of these agents in the setting of TA-TMA. Results were disappointing and difficult to interpret since all of the patients received concurrent therapy with plasma exchange.[128,139,189]
On-going studies for further investigation of the pathogenesis of TA-TMA involve medications that modulate the endothelial cell inflammatory response.[190] These agents include statins [190] and bosentan,[191] an endothelin receptor antagonist with protective effects in in vivo ischemia-reperfusion injury models. Anti-oxidant agents, such as nitric oxide donors which limit vascular injury caused by free-radicals, also may alter the course of the disease.
Conclusions
TA-TMA is an uncommon but devastating complication of HCT. Evidence suggests that it represents the final common pathway of multiple, frequently confounding variables such as conditioning regimens, use of calcineurin inhibitors, acute GVHD and opportunistic infections. The elevated blood concentrations of vWF antigen confirm endothelial cell injury. The typically incomplete responses and high mortality rates call for better therapeutic approaches. An alternative classification that takes into consideration the underlying pathophysiologic mechanisms is presented here and should limit diagnostic uncertainty. Accurate diagnosis is instrumental in designing future studies comparing management strategies and outcomes among different series. Treatment of TA-TMA consists of discontinuing offending agents and substituting calcineurin inhibitors with daclizumab or other immunosuppressives. Due to questionable efficacy and significant associated adverse events, plasma exchange, in general, is not recommended. Finally, monitoring production of endothelial cell microparticles or protein concentration changes of vWF, soluble thrombomodulin, and PAI-1, which occur in the setting of endothelial cell injury, may be useful in detecting early onset of TA-TMA. In line with these considerations, interventions directed at improving endothelial cell function, accelerating endothelial cell recovery from injury and preventing apoptosis of these cells are potential goals for future developmental therapies.
Acknowledgements
The authors thank Keith R. McCrae M.D. for his thorough review of the manuscript and excellent suggestions.
Allogeneic hematopoietic cell transplantation (HCT) is a useful therapeutic modality for a wide range of hematologic and non-hematologic conditions.[1-3] Peripheral blood progenitor cell collection, the new ‘gold standard’ in hematopoietic cell harvesting, and non-myeloablative peripheral blood progenitor cell transplantation, have reduced treatment-related mortality and enabled an increasing number of patients with comorbid conditions as well as older patients to receive therapy for conditions such as acute leukemia, myelodysplastic syndrome, multiple myeloma and lymphoma. The obstacles to successful HCT include the development of acute and chronic graft-versus-host disease (GVHD), opportunistic infections, and other complications, one of which is transplantation-associated thrombotic microangiopathy (TA-TMA).[4-6] The etiologies of this syndrome are diverse, and diagnosis of TA-TMA in this patient population requires a high degree of clinical suspicion. Moreover, management of TA-TMA remains a challenging task, mainly due to the poor response to therapeutic modalities that are beneficial in non-transplant-associated TMA.
Pathologic and clinical features
TMAs are defined by the association of microangiopathic hemolytic anemia, thrombocytopenia (platelet count < 100x109/L) and ischemic manifestations related to the formation of platelet-rich thrombi in the microcirculation.[7] TMAs include thrombotic thrombocytopenic purpura (TTP), and the hemolytic-uremic syndrome (HUS), and variants of these, which are characterized by ischemic manifestations involving the brain or gastrointestinal tract and/or kidneys, respectively.[8] TMA may be primary, or occur secondary to other disorders such as pregnancy, infections, autoimmune diseases and the post-HCT state.[9]
The clinical presentation of TMA invariably includes the presence of schistocytes on the peripheral blood film and consumptive thrombocytopenia. Surrogate markers include DAT (direct antiglobulin test)-negative hemolytic anemia, an elevated serum lactate dehydrogenase (LDH), decreased serum haptoglobin and indirect hyperbilirubinemia. Coagulation studies are usually normal. A “pentad” of signs and symptoms was traditionally associated with classic TTP: thrombocytopenia, microangiopathic hemolytic anemia (MAHA), neurologic abnormalities, renal abnormalities and fever. This complete set of symptoms occurs in only 40% of patients, and more than 70% have only the triad of MAHA, thrombocytopenia, and neurologic changes at the time of diagnosis.10 In current clinical practice, thrombocytopenia, schistocytosis, and an elevated serum LDH in the appropriate clinical setting provide sufficient criteria for the diagnosis.[7] The clinical manifestations of HUS are similar to TTP, although renal abnormalities, as opposed to neurologic dysfunction, often predominate.
Presentation of TA-TMA is similar to other forms of TMA; multiple contributing pathogenic factors have been implicated.[4,11] These include endothelial cell injury due to toxic conditioning regimens (high-dose chemotherapy and total-body irradiation [TBI]), cytomegalovirus (CMV) infection, the use of calcineurin inhibitors such as cyclosporine, and a possible graft-versus-host effect on the endothelium.[4,12-14] Because anemia, thrombocytopenia, renal impairment, and changes in mental status are common and may have multiple causes in the transplant population, diagnosis may be difficult.[15] This observation currently is motivating experts in the field to reformulate a classification of TMAs more focused on pathophysiologic mechanisms rather than clinical symptoms.[16, 17]
Diagnostic criteria
Until recently, there were no widely accepted criteria for the definition of hematopoietic progenitor cell TA-TMA. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) and the International Working Group separately formed toxicity committees to develop a consensus formulation of criteria for diagnosing clinically significant TA-TMA; these are listed in Table 1.[18,19]

Table 1: Diagnostic criteria for
transplantation-associated TMA
Incidence and risk factors
The reported proportion of patients developing a clinically significant TA-TMA syndrome has varied greatly. George and coworkers15 presented a review of published reports on TMA after allogeneic HCT. Twenty-eight different definitions of this syndrome have been used in the 35 reviewed reports. Reflecting the different definitions, the incidence of TA-TMA varied in these reports from 0.5 to 63.6% of HCT recipients, the median frequency of diagnosis being 7.9%. The mortality in the different series ranged from 0% to 100%; the overall mortality rate was 61%. Of the deceased patients, 35 autopsy reports were identified. Three of the autopsies attributed death to HUS due to observation of isolated renal TMA. The remaining 32 deaths were attributed to other causes, the most common being systemic infection, including CMV, Aspergillus species, adenovirus and human herpesvirus-6 (HHV-6). Eleven autopsies stated that there was no evidence of TTP-HUS.[15] In another review, Pettitt and Clark4 estimated that TA-TMA occurs in 14% and 7% of allogeneic and autologous transplant recipients, respectively. A more recent report of more than 4000 HCT recipients estimated the frequency of severe TA-TMA to be 0.5% and 0.13% of allogeneic and autologous recipients, respectively.[6] This varying incidence of TA-TMA among reported series likely reflects the level of physician awareness, the different diagnostic criteria, and the heterogeneity of the transplant population.
A variety of potential risk factors for the development of TA-TMA have been proposed (Table 2). Among the earliest reports, the use of cyclosporine (CsA) for the prevention of GVHD was recognized as a potential culprit.[20,21] Intensive immunosuppression with other inhibitors of the Ca2+-activated phosphatase, calcineurin (tacrolimus),[22-24] and (TBI)[25] have been associated with TA-TMA as well. In the following section we examine the pathophysiology and clinical characteristics of both primary and secondary TMAs. The heterogeneity in clinical background (idiopathic TTP vs. disease-associated TMA, including TA-TMA) and plasma concentration of markers (normal vs. decreased ADAMTS13 activity) is well emphasized and represents a barrier to the development of clear treatment guidelines.

Table 2: Potential risk factors for
development of TA-TMA
Pathophysiology
Idiopathic TTP: Moake and colleagues [26] were the first to describe the presence of very high molecular weight [so-called ultralarge (UL)] multimers of von Willebrand factor (vWF) in the plasma of a patient with recurrent TTP. Once released from stimulated endothelial cells, UL-vWF, not present in plasma from healthy individuals, promote excessive aggregation of platelets, primarily in the microvasculature. Some ULvWF multimers remain on the endothelial cell surface as long strings that adhere to platelets. The molecules responsible for the binding of UL-vWF to endothelial cells and platelets are believed to involve integrin αvβ3 and glycoprotein Ib (GpIb), respectively.[27] Microvascular thrombosis and hemolytic anemia occur particularly in high shear stress locations, such as the microvasculature, that results in unfolding of ULvWF multimers and exposure of platelet binding sites.[7,28]
Moake hypothesized that a deficiency of a vWF cleaving protease might be responsible for the presence of ULvWF, [26] but it was Furlan and colleagues [29] et al., Tsai and Lian [30] who first isolated a plasma metalloprotease that cleaved the peptide bond between the tyrosine 1605 and methionine 1606 in the central A2 domain of vWF. These investigators subsequently found a deficiency of this vWF-cleaving protease in a retrospective cohort of patients clinically diagnosed as having TTP.[29,30] The protease was characterized in 2001 by Zheng and coworkers31 as a new (the thirteenth) member of the ADAMTS (A Disintegrin And Metalloprotease with Thrombospondin 1 repeats) family of metalloproteases and was thus called ADAMTS13.[16,31-34] The primary role of ADAMTS13 is to regulate the multimeric structure of vWF by cleaving the most hemostatically active ULvWF multimers.[35] Failure of this regulatory mechanism causes the highly adhesive, unusually large multimers of vWF to accumulate in plasma, which may lead to the microvascular thrombosis, tissue ischemia and infarction which are characteristic of TTP.
The estimated annual incidence of idiopathic TTP is 3.7 to 11 cases per million.[36] In extremely rare cases, severe deficiency of ADAMTS13 (defined as <5% serum activity) is related to compound heterozygous or homozygous mutations of the ADAMTS13 gene (Upshaw-Shulman syndrome).[37-39] Defects in coding of the metalloprotease gene, located on chromosome 9q34, result in functionally deficient enzyme. In the vast majority of cases, severe ADAMTS13 deficiency is secondary to the development of anti-ADAMTS13 autoantibodies that can be detected in vitro.[29,30] Functional testing often is employed in which anti-ADAMTS13 autoantibodies are detected by their inhibitory effect on ADAMTS13 enzymatic activity.[40] More recently, physical methods of detection have been used and identify either immunoglobulin G (IgG) or IgM species via enzyme-linked immunosorbent assay (ELISA).[41,42] In more than 80% of acquired TTP, anti-ADAMTS13 antibodies are inhibitory IgG.[29,30] Less frequently, the mechanism for acquired TTP may be different, involving anti-ADAMTS13 noninhibitory IgG or IgM [41,42] autoantibodies that promote the clearance of ADAMTS13 from blood without inhibiting its activity.[41]
Adults with acquired idiopathic TTP require daily plasma exchange until neurologic symptoms have resolved and both a normal serum LDH and platelet count have been maintained for at least 2 to 3 days.[43-46] Plasma exchange is thought to remove antibodies against ADAMTS13, while replacing the deficient protease.[47,48] Plasma exchange results in the remission of TTP, which is usually fatal when untreated, in approximately 80%-90% of cases,[49-52] generally without persistent organ damage. Production of ADAMTS13 autoantibodies may also be suppressed by high-dose corticosteroid treatment, [52] although there is very little controlled data that demonstrates efficacy of steroids in the treatment of idiopathic TTP. Other therapies include use of the monoclonal antibody rituximab that is directed against the CD20 epitope on B lymphocytes; given weekly for 4-8 weeks, this agent will eliminate antibody-producing cells [53-57] and has been shown to induce remission in refractory TTP, and to reduce the otherwise high incidence of relapse in these patients.[56,58] Finally, splenectomy also has been shown to be effective in anecdotal cases of refractory TTP, and to reduce the incidence of relapse in small series.
Hemolytic-uremic syndrome (HUS): HUS refers to TMA that primarily affects the kidney, often causing oliguric or anuric renal failure.[59] HUS may present with a variety of manifestations, with one variant occurring after Escherichia coli O157:H7 gastroenteritis, primarily in children.[60] In adults, however, HUS occurs most commonly in association with pregnancy, with more than 90% of cases developing in the postpartum period.[61] The characteristic histologic lesion of HUS consists of vessel wall thickening, with swelling and detachment of the endothelial cells from the basement membrane. In HUS, microthrombi are rich in fibrin and contain relatively little vWF.[62] Their location is confined primarily to the kidneys and thus, renal failure is the dominant feature. HUS associated with renal failure in the absence of a diarrheal illness or other predisposing condition is commonly referred to as atypical HUS. It has been proposed that this group may, in part, consist of patients with complement system dysfunction owing to either a mutation of a complement-regulatory protein,[63] an antibody directed at one of these proteins,[64] or an activating mutation of a complement protein such as C3. Study of families with a history of HUS has implicated mutations in several proteins responsible for regulating the alternative complement pathway, namely complement factor H,[65] membrane cofactor protein (MCP), and factor I (IF),[66] as well as thrombomodulin.[67] Exposure to agents potentially toxic to the vascular endothelium (such as certain viruses, bacteria, toxins, immunocomplexes, and cytotoxic drugs) may initiate local intravascular thrombosis.[68] This action promotes C3bBb convertase formation and complement deposition within capillary vessels. Under normal conditions, however, factor H may effectively limit complement deposition and further extension of the process by modulating C3bBb activity.[69] In contrast, when the factor H bioavailability is reduced due to decreased activity or is congenitally defective, C3bBb convertase formation and complement deposition may become uncontrolled. As a result, the microangiopathic process is extended, leading to full-blown manifestations of the disease.
Secondary TMA: Secondary forms of TMA refer to a diverse group of disorders with frequently overlapping clinical features (Table 3). An alternative classification that takes into consideration the underlying pathophysiologic mechanisms (immune-mediated vs. nonimmune-mediated) is presented here (Table 4). Immune-mediated forms result from autoantibodies against ADAMTS13.[41,42,70] The second category (nonimmune-mediated) occurs from massive endothelial cell stimulation with consequent release of ULvWF multimers in amounts exceeding the system’s degradative ability, despite the presence of normal or only mildly reduced concentrations of ADAMTS13.[71] Distinction between these forms should limit diagnostic uncertainty and assist with management strategies, namely implementation of plasma exchange and use of immunosuppressive therapy.

Table 3: Primary and secondary thrombotic microangiopathies
Table 4: Pathophysiologic
classification of primary and
secondary thrombotic microangiopathies.
The most common physiologic condition present in the immune-mediated forms, which is often associated with severe ADAMTS13 deficiency, is pregnancy.[29,36,72,73] Immune-mediated TMA in pregnancy should be distinguished from a number of pregnancy-associated TMAs such as preeclampsia, hemolysis with elevated liver enzymes and low platelets (HELLP) syndrome, acute fatty liver of pregnancy, and antiphospholipid syndrome.[74,75] Accurate diagnosis is essential since plasma exchange is indicated for pregnancy-associated TTP while fetal-placental delivery is therapeutic for HELLP syndrome.
The association between TTP and SLE has been well recognized in clinical and histologic reports.[76] Severe deficiency of ADAMTS13 activity is predominantly associated with the presence of inhibitory anti-ADAMTS13 IgG.
ADAMTS13 levels are not generally decreased with infections such as HIV, suggesting an alternative mechanism for TMA in these patients.[77,78] Cases of TMA associated with severe ADAMTS13 deficiency and inhibitory anti-ADAMTS13 IgG have been reported with influenza A,[79] legionella pneumonia [80] and brucellosis.[81]
Antibodies that inhibit plasma ADAMTS13 have also been demonstrated in patients with ticlopidine [82] or clopidogrel-associated TMA.[83] The immune dysregulation by these thienopyridine compounds might be analogous to the anti-RBC antibody ‘escape’, associated with the antihypertensive medication α-methyldopa.[84]
The most frequent concomitant conditions associated with TMA forms presenting with normal or mildly reduced levels of ADAMTS13 (greater than 10% serum activity) are malignant hypertension,17 metastatic tumors,85 solid organ transplantation, HCT (particularly allogeneic transplants), and the use of drugs such as cyclosporine, mitomycin, and α-interferon.[34]
Despite having some features in common, TA-TMA differs from de novo TTP in many aspects including the absence of severe ADAMTS13 deficiency, a different spectrum of clinical symptoms, poor response to plasma exchange, and the lack of evidence of systemic microthrombus formation.[15] Several small retrospective studies of TA-TMA encompassing a total of 33 HCT recipients also suggest that severe ADAMTS13 deficiency may be rare among this patient population.[86-88] Indeed, other prospective reports in HCT recipients suggest that the majority of these patients experience only a mild decrease in ADAMTS13 activity (usually after the cytotoxic conditioning) that can persist for weeks; however, severe ADAMTS13 deficiency was rare.[89,90] These data suggest that, unlike idiopathic TTP, ADAMTS13 deficiency is not the primary component of the pathophysiology of TA-TMA and other factors may play a more central role.
Role of endothelial cell injury: For a long time, many authorities have considered endothelial cell injury as the central and likely inciting factor that sustains the microangiopathic process in TMA, including the post-transplantation state. As early as 1942, Altschule [91] suggested that microvascular endothelial cell activation was the primary event causing platelet deposition in arterioles and capillaries with secondary “clearance of enormous numbers of platelets from the circulation”. Endothelial cells synthesize many substances involved in coagulation and fibrinolysis including vWF, thrombomodulin, tissue-type plasminogen activator (tPA), plasminogen activator inhibitor (PAI-1), protein S, prostacyclin (PGI2), and nitrous oxide (NO). Alterations in the concentration of these substances have been reported in idiopathic TTP and TA-TMA.[92,93] Whether these alterations represent an initiating effect or simply are reflective of endothelial cell injury, remains elusive. Levels of vWF antigen and soluble thrombomodulin were measured in patients with idiopathic TTP and TA-TMA.[94] vWF antigen and thrombomodulin levels were elevated in both patient groups compared to controls. Thrombomodulin concentrations were significantly higher in TA-TMA compared to idiopathic TTP, supporting a role for endothelial cell damage in the former. Gordon and colleagues [95] demonstrated that protein C deficiency correlated with thrombotic complications in patients undergoing HCT. Several groups have reported elevated plasma levels of fibrinogen, tPA, PAI-1, vWF antigen, thrombomodulin, and intercellular adhesion molecule 1 (ICAM-1).[96-100] Kanamori et al.[100] proposed that measurement of thrombomodulin levels on day 14 post-HCT may be useful in surveillance for TA-TMA. Cohen and colleagues [93] proposed that endothelial cell injury is pathognomonic of TA-TMA as well after they demonstrated absent endothelial cell PGI2 release and scanning electron microscopy (EM) evidence of endothelial cell damage with TMA after allogeneic HCT.
Risk factors and prognosis: Non-modifiable risk factors for development of TA-TMA include female gender, African American race, and older age.[36,122,123] Prior medical history of severe hepatic dysfunction and advanced primary disease also increase the risk of developing TA-TMA.[124,125] Treatment-related risk factors include: unrelated donor transplants; [88,122-124,126-128] HLA-mismatched donors;124 fludarabine-based non-myeloablative conditioning regimens;88,129 busulfan and TBI myeloablative conditioning. [25,123,130] The incidence of TA-TMA did not differ according to graft source, e.g. bone marrow versus peripheral blood.122 As mentioned above, use of calcineurin inhibitors such as cyclosporine,[14,126,131-133] tacrolimus,[24,130,132] and sirolimus[132,134,135] are also associated with the development of TA-TMA. Infections and the development of GVHD also increase the risk of developing TA-TMA.[14,21,123,124,128] In the HCT patients, non-transplantation etiologies of TMA such as idiopathic TTP and HUS should always be considered in the differential diagnosis, as they may coexist with the primary hematologic disease. In TA-TMA, poor prognostic indicators include: patient age > 18 years, a graft source from an unrelated or haploidentical donor,[123] at least five schistocytes per high-power field on peripheral film,[136] TA-TMA in the absence of sirolimus,[134] and nephropathy.[122] Specifically, Uderzo and coworkers[123] reported three factors statistically significant in predicting outcome of TA-TMA: adult age, unrelated or haploidentical graft source, and high TMA index (elevated LDH-platelet ratio). A retrospective cohort analysis of myeloablative allogeneic HCT recipients showed that sirolimus exposure constitutes a risk factor for the development of TA-TMA (10.8% in the sirolimus-exposed subjects vs. 4.2% in the non-sirolimus group)[134] but is also a favorable prognostic indicator in terms of TA-TMA overall survival (58.3% for TA-TMA related to sirolimus exposure vs. 11.1% in the non-sirolimus group)134 and renal recovery (92% vs. 78% respectively).[134] Martinez and colleagues136 reported lower one-year survival in patients with TA-TMA than in patients without TA-TMA (27 ± 18.1% for TA-TMA with high schistocyte counts; 53 ± 15% for TA-TMA with low schistocyte counts; vs. 78 ± 7% in patients without TA-TMA, p< 0.0001). A survey of the European Group for Blood and Marrow Transplantation (EBMT)122 conducted among forty-five centers included 406 patients transplanted, and reported an incidence of TA-TMA of 6.7%. The only factor predictive of resolution of TA-TMA was the absence of nephropathy.[122]
Therapeutic modalities
At the present time there is no consensus on what constitutes appropriate therapy for patients with TA-TMA. Initial attempts should focus on the following: i) eliminating possible causative conditions such as treating underlying infections and controlling acute GVHD; and ii) pharmacologic therapy with medications such as daclizumab, defibrotide and rituximab. The rationale for use of these agents is based on empirical benefit.
Eliminating Risk Factors and Consideration of Plasma Exchange: Cyclosporine, tacrolimus and sirolimus should be discontinued immediately and replaced with alternative immunosuppressive medications. Corticosteroids, mycophenolate mofetil, azathioprine and methotrexate can be used as appropriate alternatives. Withdrawal of cyclosporine with initiation of plasma exchange/apheresis has shown response rates of up to 63%.[137] In all reported cases cyclosporine was discontinued at the time of diagnosis of TA-TMA, and the effect of this intervention in isolation cannot be determined as patients went on to have therapeutic plasma exchange.
Unlike the situation in idiopathic TTP, responses to plasma exchange alone are suboptimal in TA-TMA. Reported response rates vary between 0-49%,[138,139] compared with 78-91% in patients with idiopathic TTP.[45,140] Further, rise in platelet count, the usual marker for response to plasma exchange, cannot be relied upon in TA-TMA because platelet engraftment may not yet have occurred. In addition, plasma exchange procedures are associated with a significant number of complications, including systemic infections, catheter thrombosis, bleeding, pneumothorax, pericardial tamponade, and with plasma infusion, serum sickness and anaphylaxis.
Based on the incomplete responses and high complication rates, we do not advocate use of this procedure but rather alternative therapeutic approaches as discussed below.
Daclizumab: Daclizumab is a humanized monoclonal anti-CD25 antibody, which targets the chain of the IL-2 receptor.[141] This agent is 90% humanized, retaining only 10% of the original murine compartments in the critical hypervariable segments for binding specificity. Daclizumab has been used to decrease the incidence of acute rejection in solid organ transplants including renal,[142] hepatic, cardiac,[143,144] and lung transplantation.[143,145] Daclizumab also has been used successfully in T-cell mediated autoimmune diseases such as multiple sclerosis,[146-148] pure red cell aplasia,[149] and aplastic anemia.[150]
In the HCT setting, intravenous daclizumab (with a serum half-life of 20 days) has been used to prevent or treat acute GVHD;[151] more recently, this agent has been used for the treatment of TA-TMA. Adverse effects include an increased risk for bacterial, candida and aspergillosis infections, as well as CMV reactivation. Through its effect on depleting alloreactive T-cells, daclizumab can substitute for a calcineurin inhibitor. Wolff et al.[132]used daclizumab at an initial loading dose of 2mg/kg and then 1mg/kg weekly. Nine of 13 affected patients attained complete remission after therapy.[132] Four of the patients who had a complete remission from TMA also had complete resolution of active GVHD. A fifth, complete remitter patient from both TMA and GVHD died of primary disease relapse; the remaining eight patients died from infections, GVHD or multiorgan failure.[132] The long half-life and potent immunosuppressive effect make this agent a promising treatment modality that merits further investigation.
Defibrotide: Defibrotide is a large, single-stranded polydeoxyribonucleotide, derived from porcine mucosa by controlled depolymerization. It has been found to have potent anti-thrombotic, anti-ischemic, anti-inflammatory, and thrombolytic properties, without significant systemic anticoagulant effects.[127,152-158] This drug exerts its properties by inhibition of TNFα-mediated endothelial cell apoptosis in vitro,148 decreasing the activity of PAI-1 and increasing endogenous tissue plasminogen activator (tPA) function.[159]
Hepatic veno-occlusive disease (VOD) is a potentially lethal complication of both allogeneic and autologous HCT,[156,160,161] especially after prior exposure to the immunoconjugate gemtuzumab ozogamicin.[162] In some studies, the incidence of hepatic VOD after HCT approaches 20% with mortality ranging from 7% to 50%.[163] The pathogenesis of VOD involves injury to the sinusoidal endothelial cells, leading to occlusion of small vessels with fibrin deposition and disruption of hepatic function. Previous attempts at therapy using either heparin or tPA have been unsuccessful.[164,165] Defibrotide therapy has improved outcomes for hepatic VOD that develops after HCT (30% to 60% CR rate).[166-170] Given the similarities in pathophysiology with TA-TMA, including loss of small vessel endothelial cell integrity, Corti and coworkers[155] reported that 3 of 12 affected patients with TA-TMA given oral defibrotide achieved partial remission, while 5 of 12 patients achieved a complete response.[155] Because the effects of defibrotide are exerted locally within the vascular bed, it is usually well tolerated with no significant systemic effects on coagulation such as seen during treatment with tPA.
Rituximab: Rituximab, discussed above, has been used with increasing frequency for the treatment of various hematologic and rheumatologic disorders including idiopathic TTP,[171-173] acquired coagulation factor inhibitors,[174,175] antiphospholipid antibody syndrome,[173] systemic lupus erythematosus176 and rheumatoid arthritis.[177,178] Rituximab use in relapsed, refractory TTP is linked to its ability to eliminate antibodies to ADAMTS13.171 Au et al.[179] treated five TA-TMA patients refractory to a week of plasma exchange and prednisolone with rituximab 375mg/m2/week for four doses. Four attained complete remission; two patients recovered after receiving two weeks of rituximab.[179] At a median follow-up of 305 days, 3 of 4 responders remained in remission [179] but the fourth responder died of sepsis. The only non-responder died of multi-organ failure after three weeks.[179] ADAMTS13 antigen levels were marginal or low either post-HCT or at the onset of TMA and did not change significantly after rituximab-induced remission.[179] It remains unclear whether these patients actually had TA-TMA (or TTP), thus making the use of rituximab in this setting uncertain. In addition, there are no established guidelines for recommending duration of rituximab treatment. Given the lack of reliable markers for remission (serum ADAMTS13 activity and anti-ADAMTS13 antibody levels), maintenance rituximab therapy cannot be recommended.
Other modalities and future directions: Single agent response rates for the antiplatelet agents aspirin and dipyridamole approximate 10%,[49,180] a result indistinguishable from the natural history of TTP. Antiplatelet agents have not been convincingly shown to increase the response to plasma exchange [45,51,181] and may promote bleeding in the setting of acute thrombocytopenia and invasive procedures.[51,182] Hence, their use as first-line treatment of TMA, including TA-TMA, cannot be recommended. Although not verified in vivo, intravenous immunoglobulin (IVIG) has been used therapeutically based on a report that IgG from healthy individuals inhibits the capacity of TTP plasma to agglutinate platelets in vitro.[183] A recent study described a response to the combination of plasma exchange and IVIG in a patient who was refractory to plasma exchange alone.[184] There are anecdotal reports of favorable responses of TTP to vincristine,[180,185,186] as well as other immunosuppressive therapies such as azathioprine, cyclophosphamid, and staphylococcal protein A immunoadsorption.[187,188] By analogy, there are case series of use of these agents in the setting of TA-TMA. Results were disappointing and difficult to interpret since all of the patients received concurrent therapy with plasma exchange.[128,139,189]
On-going studies for further investigation of the pathogenesis of TA-TMA involve medications that modulate the endothelial cell inflammatory response.[190] These agents include statins [190] and bosentan,[191] an endothelin receptor antagonist with protective effects in in vivo ischemia-reperfusion injury models. Anti-oxidant agents, such as nitric oxide donors which limit vascular injury caused by free-radicals, also may alter the course of the disease.
Conclusions
TA-TMA is an uncommon but devastating complication of HCT. Evidence suggests that it represents the final common pathway of multiple, frequently confounding variables such as conditioning regimens, use of calcineurin inhibitors, acute GVHD and opportunistic infections. The elevated blood concentrations of vWF antigen confirm endothelial cell injury. The typically incomplete responses and high mortality rates call for better therapeutic approaches. An alternative classification that takes into consideration the underlying pathophysiologic mechanisms is presented here and should limit diagnostic uncertainty. Accurate diagnosis is instrumental in designing future studies comparing management strategies and outcomes among different series. Treatment of TA-TMA consists of discontinuing offending agents and substituting calcineurin inhibitors with daclizumab or other immunosuppressives. Due to questionable efficacy and significant associated adverse events, plasma exchange, in general, is not recommended. Finally, monitoring production of endothelial cell microparticles or protein concentration changes of vWF, soluble thrombomodulin, and PAI-1, which occur in the setting of endothelial cell injury, may be useful in detecting early onset of TA-TMA. In line with these considerations, interventions directed at improving endothelial cell function, accelerating endothelial cell recovery from injury and preventing apoptosis of these cells are potential goals for future developmental therapies.
Acknowledgements
The authors thank Keith R. McCrae M.D. for his thorough review of the manuscript and excellent suggestions.
References
- Burt RK, Loh Y, Pearce W, Beohar N, Barr
WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of
blood-derived and marrow-derived stem cells for nonmalignant diseases.
JAMA 2008 Feb 27;299:925-36.

- Blaise D, Bay JO, Faucher C, Michallet M,
Boiron JM, Choufi B, Cahn JY, Gratecos N, Sotto JJ, Francois S, Fleury
J, Mohty M, Chabannon C, Bilger K, Gravis G, Viret F, Braud AC, Bardou
VJ, Maraninchi D, Viens P. Reduced-intensity preparative regimen and
allogeneic stem cell transplantation for advanced solid tumors. Blood
2004 Jan 15;103:435-41.

- Copelan EA. Hematopoietic stem-cell
transplantation. N Engl J Med 2006 Apr 27;354:1813-26.

- Pettitt AR, Clark RE. Thrombotic
microangiopathy following bone marrow transplantation. Bone Marrow
Transplant 1994 Oct;14:495-504.

- Kwaan HC. Miscellaneous secondary
thrombotic microangiopathy. Semin Hematol 1987 Jul;24:141-7.

- Iacopino P, Pucci G, Arcese W, Bosi A,
Falda M, Locatelli F, Marenco P, Miniero R, Morabito F, Rossetti F,
Sica S, Uderzo C, Bacigalupo A. Severe thrombotic microangiopathy: an
infrequent complication of bone marrow transplantation. Gruppo Italiano
Trapianto Midollo Osseo (GITMO). Bone Marrow Transplant 1999
Jul;24:47-51.

- Moake JL. Thrombotic microangiopathies. N
Engl J Med 2002 Aug 22;347:589-600.

- George JN, Vesely SK, Terrell DR. The
Oklahoma Thrombotic Thrombocytopenic Purpura-Hemolytic Uremic Syndrome
(TTP-HUS) Registry: a community perspective of patients with clinically
diagnosed TTP-HUS. Semin Hematol 2004 Jan;41:60-7.

- Sadler JE. Thrombotic thrombocytopenic
purpura: a moving target. Hematology Am Soc Hematol Educ Program
2006:415-20.

- Ridolfi RL, Bell WR. Thrombotic
thrombocytopenic purpura. Report of 25 cases and review of the
literature. Medicine (Baltimore) 1981 Nov;60:413-28.

- Schriber JR, Herzig GP.
Transplantation-associated thrombotic thrombocytopenic purpura and
hemolytic uremic syndrome. Semin Hematol 1997 Apr;34:126-33.

- Zeigler ZR, Shadduck RK, Nemunaitis J,
Andrews DF, Rosenfeld CS. Bone marrow transplant-associated thrombotic
microangiopathy: a case series. Bone Marrow Transplant 1995
Feb;15:247-53.

- Maslo C, Peraldi MN, Desenclos JC,
Mougenot B, Cywiner-Golenzer C, Chatelet FP, Jacomet C, Rondeau E,
Rozenbaum W, Sraer JD. Thrombotic microangiopathy and cytomegalovirus
disease in patients infected with human immunodeficiency virus. Clin
Infect Dis 1997 Mar;24:350-5.

- Holler E, Kolb HJ, Hiller E, Mraz W,
Lehmacher W, Gleixner B, Seeber C, Jehn U, Gerhartz HH, Brehm G, et al.
Microangiopathy in patients on cyclosporine prophylaxis who developed
acute graft-versus-host disease after HLA-identical bone marrow
transplantation. Blood 1989 May 15;73:2018-24.

- George JN, Li X, McMinn JR, Terrell DR,
Vesely SK, Selby GB. Thrombotic thrombocytopenic purpura-hemolytic
uremic syndrome following allogeneic HPC transplantation: a diagnostic
dilemma. Transfusion 2004 Feb;44:294-304.

- Sadler JE, Moake JL, Miyata T, George JN.
Recent advances in thrombotic thrombocytopenic purpura. Hematology Am
Soc Hematol Educ Program 2004:407-23.

- Tsai HM. Advances in the pathogenesis,
diagnosis, and treatment of thrombotic thrombocytopenic purpura. J Am
Soc Nephrol 2003 Apr;14:1072-81.

- Ho VT, Cutler C, Carter S, Martin P, Adams
R, Horowitz M, Ferrara J, Soiffer R, Giralt S. Blood and marrow
transplant clinical trials network toxicity committee consensus
summary: thrombotic microangiopathy after hematopoietic stem cell
transplantation. Biol Blood Marrow Transplant 2005 Aug;11:571-5.

- Ruutu T, Barosi G, Benjamin RJ, Clark RE,
George JN, Gratwohl A, Holler E, Iacobelli M, Kentouche K, Lammle B,
Moake JL, Richardson P, Socie G, Zeigler Z, Niederwieser D, Barbui T.
Diagnostic criteria for hematopoietic stem cell transplant-associated
microangiopathy: results of a consensus process by an International
Working Group. Haematologica 2007 Jan;92:95-100.

- Powles RL, Clink HM, Spence D, Morgenstern
G, Watson JG, Selby PJ, Woods M, Barrett A, Jameson B, Sloane J, Lawler
SD, Kay HE, Lawson D, McElwain TJ, Alexander P. Cyclosporin A to
prevent graft-versus-host disease in man after allogeneic bone-marrow
transplantation. Lancet 1980 Feb 16;1:327-9.

- Fuge R, Bird JM, Fraser A, Hart D, Hunt L,
Cornish JM, Goulden N, Oakhill A, Pamphilon DH, Steward CG, Marks DI.
The clinical features, risk factors and outcome of thrombotic
thrombocytopenic purpura occurring after bone marrow transplantation.
Br J Haematol 2001 Apr;113:58-64.

- Pham PT, Peng A, Wilkinson AH, Gritsch HA,
Lassman C, Pham PC, Danovitch GM. Cyclosporine and
tacrolimus-associated thrombotic microangiopathy. Am J Kidney Dis 2000
Oct;36:844-50.

- Trimarchi HM, Truong LD, Brennan S,
Gonzalez JM, Suki WN. FK506-associated thrombotic microangiopathy:
report of two cases and review of the literature. Transplantation 1999
Feb 27;67:539-44.

- Sarkodee-Adoo C, Sotirescu D,
Sensenbrenner L, Rapoport AP, Cottler-Fox M, Tricot G, Ruehle K,
Meisenberg B. Thrombotic microangiopathy in blood and marrow transplant
patients receiving tacrolimus or cyclosporine A. Transfusion 2003
Jan;43:78-84.

- Chappell ME, Keeling DM, Prentice HG,
Sweny P. Haemolytic uraemic syndrome after bone marrow transplantation:
an adverse effect of total body irradiation? Bone Marrow Transplant
1988 Jul;3:339-47.

- Moake JL, Rudy CK, Troll JH, Weinstein MJ,
Colannino NM, Azocar J, Seder RH, Hong SL, Deykin D. Unusually large
plasma factor VIII:von Willebrand factor multimers in chronic relapsing
thrombotic thrombocytopenic purpura. N Engl J Med 1982 Dec 2;307:1432-5.

- Huang J, Roth R, Heuser JE, Sadler JE.
Integrin alpha(v)beta(3) on human endothelial cells binds von
Willebrand factor strings under fluid shear stress. Blood 2009 Feb
12;113:1589-97.

- Dong JF, Moake JL, Nolasco L, Bernardo A,
Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez
JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand
factor multimers on the endothelial surface under flowing conditions.
Blood 2002 Dec 1;100:4033-9.

- Furlan M, Robles R, Galbusera M, Remuzzi
G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U,
Solenthaler M, Lammle B. von Willebrand factor-cleaving protease in
thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome.
N Engl J Med 1998 Nov 26;339:1578-84.

- Tsai HM, Lian EC. Antibodies to von
Willebrand factor-cleaving protease in acute thrombotic
thrombocytopenic purpura. N Engl J Med 1998 Nov 26;339:1585-94.

- Zheng X, Chung D, Takayama TK, Majerus EM,
Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving
protease (ADAMTS13), a metalloprotease involved in thrombotic
thrombocytopenic purpura. J Biol Chem 2001 Nov 2;276:41059-63.

- Hovinga JA, Studt JD, Alberio L, Lammle B.
von Willebrand factor-cleaving protease (ADAMTS-13) activity
determination in the diagnosis of thrombotic microangiopathies: the
Swiss experience. Semin Hematol 2004 Jan;41:75-82.

- Matsumoto M, Yagi H, Ishizashi H, Wada H,
Fujimura Y. The Japanese experience with thrombotic thrombocytopenic
purpura-hemolytic uremic syndrome. Semin Hematol 2004 Jan;41:68-74.

- Tsai HM. Current concepts in thrombotic
thrombocytopenic purpura. Annu Rev Med 2006 57:419-36.

- Levy GG, Motto DG, Ginsburg D. ADAMTS13
turns 3. Blood 2005 Jul 1;106:11-7.

- Terrell DR, Williams LA, Vesely SK, Lammle
B, Hovinga JA, George JN. The incidence of thrombotic thrombocytopenic
purpura-hemolytic uremic syndrome: all patients, idiopathic patients,
and patients with severe ADAMTS-13 deficiency. J Thromb Haemost 2005
Jul;3:1432-6.

- Levy GG, Nichols WC, Lian EC, Foroud T,
McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R,
Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira
EE, Upshaw JD, Jr., Ginsburg D, Tsai HM. Mutations in a member of the
ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature
2001 Oct 4;413:488-94.

- Kokame K, Miyata T. Genetic defects
leading to hereditary thrombotic thrombocytopenic purpura. Semin
Hematol 2004 Jan;41:34-40.

- Schneppenheim R, Budde U, Hassenpflug W,
Obser T. Severe ADAMTS-13 deficiency in childhood. Semin Hematol 2004
Jan;41:83-9.

- Miyata T, Kokame K, Banno F. Measurement
of ADAMTS13 activity and inhibitors. Curr Opin Hematol 2005
Sep;12:384-9.

- Scheiflinger F, Knobl P, Trattner B,
Plaimauer B, Mohr G, Dockal M, Dorner F, Rieger M. Nonneutralizing IgM
and IgG antibodies to von Willebrand factor-cleaving protease
(ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura.
Blood 2003 Nov 1;102:3241-3.

- Rieger M, Mannucci PM, Kremer Hovinga JA,
Herzog A, Gerstenbauer G, Konetschny C, Zimmermann K, Scharrer I,
Peyvandi F, Galbusera M, Remuzzi G, Bohm M, Plaimauer B, Lammle B,
Scheiflinger F. ADAMTS13 autoantibodies in patients with thrombotic
microangiopathies and other immunomediated diseases. Blood 2005 Aug
15;106:1262-7.

- Bukowski RM, Hewlett JS, Harris JW,
Hoffman GC, Battle JD, Jr., Silverblatt E, Yang IY. Exchange
transfusions in the treatment of thrombotic thrombocytopenic purpura.
Semin Hematol 1976 Jul;13:219-32.

- Byrnes JJ, Khurana M. Treatment of
thrombotic thrombocytopenic purpura with plasma. N Engl J Med 1977 Dec
22;297:1386-9.

- Rock GA, Shumak KH, Buskard NA, Blanchette
VS, Kelton JG, Nair RC, Spasoff RA. Comparison of plasma exchange with
plasma infusion in the treatment of thrombotic thrombocytopenic
purpura. Canadian Apheresis Study Group. N Engl J Med 1991 Aug
8;325:393-7.

- Brunskill SJ, Tusold A, Benjamin S,
Stanworth SJ, Murphy MF. A systematic review of randomized controlled
trials for plasma exchange in the treatment of thrombotic
thrombocytopenic purpura. Transfus Med 2007 Feb;17:17-35.

- Scott EA, Puca KE, Pietz BC, Duchateau BK,
Friedman KD. Comparison and stability of ADAMTS13 activity in
therapeutic plasma products. Transfusion 2007 Jan;47:120-5.

- Rock G, Shumak KH, Sutton DM, Buskard NA,
Nair RC. Cryosupernatant as replacement fluid for plasma exchange in
thrombotic thrombocytopenic purpura. Members of the Canadian Apheresis
Group. Br J Haematol 1996 Aug;94:383-6.

- Kwaan HC, Soff GA. Management of
thrombotic thrombocytopenic purpura and hemolytic uremic syndrome.
Semin Hematol 1997 Apr;34:159-66.

- Lankford KV, Hillyer CD. Thrombotic
thrombocytopenic purpura: new insights in disease pathogenesis and
therapy. Transfus Med Rev 2000 Jul;14:244-57.

- George JN. How I treat patients with
thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Blood
2000 Aug 15;96:1223-9.

- Allford SL, Hunt BJ, Rose P, Machin SJ.
Guidelines on the diagnosis and management of the thrombotic
microangiopathic haemolytic anaemias. Br J Haematol 2003 Feb;120:556-73.

- Rock G. The management of thrombotic
thrombocytopenic purpura in 2005. Semin Thromb Hemost 2005
Dec;31:709-16.

- Hull MJ, Eichbaum QG. Efficacy of
rituximab and concurrent plasma exchange in the treatment of thrombotic
thrombocytopenic purpura. Clin Adv Hematol Oncol 2006 Mar;4:210-4;
discussion 7-8.

- Darabi K, Berg AH. Rituximab can be
combined with daily plasma exchange to achieve effective B-cell
depletion and clinical improvement in acute autoimmune TTP. Am J Clin
Pathol 2006 Apr;125:592-7.

- Fakhouri F, Vernant JP, Veyradier A, Wolf
M, Kaplanski G, Binaut R, Rieger M, Scheiflinger F, Poullin P, Deroure
B, Delarue R, Lesavre P, Vanhille P, Hermine O, Remuzzi G, Grunfeld JP.
Efficiency of curative and prophylactic treatment with rituximab in
ADAMTS13-deficient thrombotic thrombocytopenic purpura: a study of 11
cases. Blood 2005 Sep 15;106:1932-7.

- Heidel F, Lipka DB, von Auer C, Huber C,
Scharrer I, Hess G. Addition of rituximab to standard therapy improves
response rate and progression-free survival in relapsed or refractory
thrombotic thrombocytopenic purpura and autoimmune haemolytic anaemia.
Thromb Haemost 2007 Feb;97:228-33.

- Ling HT, Field JJ, Blinder MA. Sustained
response with rituximab in patients with thrombotic thrombocytopenic
purpura: a report of 13 cases and review of the literature. Am J
Hematol 2009 Jul;84:418-21.

- Kaplan BS, Meyers KE, Schulman SL. The
pathogenesis and treatment of hemolytic uremic syndrome. J Am Soc
Nephrol 1998 Jun;9:1126-33.

- Karmali MA, Petric M, Lim C, Fleming PC,
Arbus GS, Lior H. The association between idiopathic hemolytic uremic
syndrome and infection by verotoxin-producing Escherichia coli. J
Infect Dis 1985 May;151:775-82.

- McRae KR, Cines DB, Sadler JE. THROMBOTIC THROMBOCYTOPENIC PURPURA AND THE HEMOLYTIC UREMIC SYNDROME. In: Hoffman R, Benz EJJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, et al., editors. Hematology: Basic Principles and Practice. Philadelphia: Churchill Livingstone Elsevier; 2008. p. 2099 - 112.
- Hosler GA, Cusumano AM, Hutchins GM.
Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome are
distinct pathologic entities. A review of 56 autopsy cases. Arch Pathol
Lab Med 2003 Jul;127:834-9.

- Noris M, Remuzzi G. Atypical
hemolytic-uremic syndrome. N Engl J Med 2009 Oct 22;361:1676-87.

- Dragon-Durey MA, Loirat C, Cloarec S,
Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Fremeaux-Bacchi V.
Anti-Factor H autoantibodies associated with atypical hemolytic uremic
syndrome. J Am Soc Nephrol 2005 Feb;16:555-63.

- Noris M, Ruggenenti P, Perna A, Orisio S,
Caprioli J, Skerka C, Vasile B, Zipfel PF, Remuzzi G.
Hypocomplementemia discloses genetic predisposition to hemolytic uremic
syndrome and thrombotic thrombocytopenic purpura: role of factor H
abnormalities. Italian Registry of Familial and Recurrent Hemolytic
Uremic Syndrome/Thrombotic Thrombocytopenic Purpura. J Am Soc Nephrol
1999 Feb;10:281-93.

- Caprioli J, Noris M, Brioschi S, Pianetti
G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S,
Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK,
Kavanagh D, Atkinson JP, Remuzzi G. Genetics of HUS: the impact of MCP,
CFH, and IF mutations on clinical presentation, response to treatment,
and outcome. Blood 2006 Aug 15;108:1267-79.

- Delvaeye M, Noris M, De Vriese A, Esmon
CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts
D, Zoja C, Remuzzi G, Conway EM. Thrombomodulin mutations in atypical
hemolytic-uremic syndrome. N Engl J Med 2009 Jul 23;361:345-57.

- Kanso AA, Abou Hassan N, Badr K. Microvascular and Macrovascular Diseases of the Kidney. In: Brenner BM, editor. Brenner: Brenner and Rector's The Kidney. 8th ed. Philadelphia: Saunders, Elsevier; 2007. p. 1147 - 73.
- Zipfel PF, Hellwage J, Friese MA, Hegasy
G, Jokiranta ST, Meri S. Factor H and disease: a complement regulator
affects vital body functions. Mol Immunol 1999 Mar-Apr;36:241-8.

- Klaus C, Plaimauer B, Studt JD, Dorner F,
Lammle B, Mannucci PM, Scheiflinger F. Epitope mapping of ADAMTS13
autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood
2004 Jun 15;103:4514-9.

- van der Plas RM, Schiphorst ME, Huizinga
EG, Hene RJ, Verdonck LF, Sixma JJ, Fijnheer R. von Willebrand factor
proteolysis is deficient in classic, but not in bone marrow
transplantation-associated, thrombotic thrombocytopenic purpura. Blood
1999 Jun 1;93:3798-802.

- George JN. Clinical practice. Thrombotic
thrombocytopenic purpura. N Engl J Med 2006 May 4;354:1927-35.

- Mannucci PM, Canciani MT, Forza I, Lussana
F, Lattuada A, Rossi E. Changes in health and disease of the
metalloprotease that cleaves von Willebrand factor. Blood 2001 Nov
1;98:2730-5.

- Stavrou E, McCrae KR. Immune
thrombocytopenia in pregnancy. Hematol Oncol Clin North Am 2009
Dec;23:1299-316.

- Coppo P, Veyradier A. Thrombotic
microangiopathies: towards a pathophysiology-based classification.
Cardiovasc Hematol Disord Drug Targets 2009 Mar;9:36-50.

- Devinsky O, Petito CK, Alonso DR. Clinical
and neuropathological findings in systemic lupus erythematosus: the
role of vasculitis, heart emboli, and thrombotic thrombocytopenic
purpura. Ann Neurol 1988 Apr;23:380-4.

- Becker S, Fusco G, Fusco J, Balu R,
Gangjee S, Brennan C, Feinberg J. HIV-associated thrombotic
microangiopathy in the era of highly active antiretroviral therapy: an
observational study. Clin Infect Dis 2004 Nov 1;39 Suppl 5:S267-75.

- Gruszecki AC, Wehrli G, Ragland BD, Reddy
VV, Nabell L, Garcia-Hernandez A, Marques MB. Management of a patient
with HIV infection-induced anemia and thrombocytopenia who presented
with thrombotic thrombocytopenic purpura. Am J Hematol 2002
Mar;69:228-31.

- Kosugi N, Tsurutani Y, Isonishi A, Hori Y,
Matsumoto M, Fujimura Y. Influenza A infection triggers thrombotic
thrombocytopenic purpura by producing the anti-ADAMTS13 IgG inhibitor.
Intern Med 49:689-93.

- Talebi T, Fernandez-Castro G, Montero AJ,
Stefanovic A, Lian E. A Case of Severe Thrombotic Thrombocytopenic
Purpura With Concomitant Legionella Pneumonia: Review of Pathogenesis
and Treatment. Am J Ther Mar 8.

- Akbayram S, Dogan M, Peker E, Akgun C,
Oner AF, Caksen H. Thrombotic Thrombocytopenic Purpura in a Case of
Brucellosis. Clin Appl Thromb Hemost Mar 8.

- Tsai HM, Rice L, Sarode R, Chow TW, Moake
JL. Antibody inhibitors to von Willebrand factor metalloproteinase and
increased binding of von Willebrand factor to platelets in
ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern
Med 2000 May 16;132:794-9.

- Bennett CL, Connors JM, Carwile JM, Moake
JL, Bell WR, Tarantolo SR, McCarthy LJ, Sarode R, Hatfield AJ, Feldman
MD, Davidson CJ, Tsai HM. Thrombotic thrombocytopenic purpura
associated with clopidogrel. N Engl J Med 2000 Jun 15;342:1773-7.

- Zakarija A, Kwaan HC, Moake JL, Bandarenko
N, Pandey DK, McKoy JM, Yarnold PR, Raisch DW, Winters JL, Raife TJ,
Cursio JF, Luu TH, Richey EA, Fisher MJ, Ortel TL, Tallman MS, Zheng
XL, Matsumoto M, Fujimura Y, Bennett CL. Ticlopidine- and
clopidogrel-associated thrombotic thrombocytopenic purpura (TTP):
review of clinical, laboratory, epidemiological, and pharmacovigilance
findings (1989-2008). Kidney Int Suppl 2009 Feb:S20-4.

- Fontana S, Gerritsen HE, Kremer Hovinga J,
Furlan M, Lammle B. Microangiopathic haemolytic anaemia in
metastasizing malignant tumours is not associated with a severe
deficiency of the von Willebrand factor-cleaving protease. Br J
Haematol 2001 Apr;113:100-2.

- Arai S, Allan C, Streiff M, Hutchins GM,
Vogelsang GB, Tsai HM. Von Willebrand factor-cleaving protease activity
and proteolysis of von Willebrand factor in bone marrow
transplant-associated thrombotic microangiopathy. Hematol J 2001
2:292-9.

- Allford SL, Bird JM, Marks DI. Thrombotic
thrombocytopenic purpura following stem cell transplantation. Leuk
Lymphoma 2002 Oct;43:1921-6.

- Elliott MA, Nichols WL, Jr., Plumhoff EA,
Ansell SM, Dispenzieri A, Gastineau DA, Gertz MA, Inwards DJ, Lacy MQ,
Micallef IN, Tefferi A, Litzow M. Posttransplantation thrombotic
thrombocytopenic purpura: a single-center experience and a contemporary
review. Mayo Clin Proc 2003 Apr;78:421-30.

- Kentouche K, Zintl F, Angerhaus D, Fuchs
D, Hermann J, Schneppenheim R, Budde U. von Willebrand factor-cleaving
protease (ADAMTS13) in the course of stem cell transplantation. Semin
Thromb Hemost 2006 Mar;32:98-104.

- Peyvandi F, Siboni SM, Lambertenghi
Deliliers D, Lavoretano S, De Fazio N, Moroni B, Lambertenghi Deliliers
G, Mannuccio Mannucci P. Prospective study on the behaviour of the
metalloprotease ADAMTS13 and of von Willebrand factor after bone marrow
transplantation. Br J Haematol 2006 Jul;134:187-95.

- Altschule MD. A Rare Type of Acute Thrombotic Thrombocytopenic Purpura: Widespread Formation of Platelet Thrombi in Capillaries. N Engl J Med 1942 227:477-79.
- Takahashi H, Hanano M, Wada K, Tatewaki W,
Niwano H, Tsubouchi J, Nakano M, Nakamura T, Shibata A. Circulating
thrombomodulin in thrombotic thrombocytopenic purpura. Am J Hematol
1991 Nov;38:174-7.

- Cohen H, Bull HA, Seddon A, Enayat MS,
Hill FG, Woolf N, Machin SJ. Vascular endothelial cell function and
ultrastructure in thrombotic microangiopathy following allogeneic bone
marrow transplantation. Eur J Haematol 1989 Sep;43:207-14.

- Zeigler ZR, Rosenfeld CS, Andrews DF, 3rd,
Nemunaitis J, Raymond JM, Shadduck RK, Kramer RE, Gryn JF, Rintels PB,
Besa EC, George JN. Plasma von Willebrand Factor Antigen (vWF:AG) and
thrombomodulin (TM) levels in Adult Thrombotic Thrombocytopenic
Purpura/Hemolytic Uremic Syndromes (TTP/HUS) and bone marrow
transplant-associated thrombotic microangiopathy (BMT-TM). Am J Hematol
1996 Dec;53:213-20.

- Gordon BG, Haire WD, Stephens LC, Kotulak
GD, Kessinger A. Protein C deficiency following hematopoietic stem cell
transplantation: optimization of intravenous vitamin K dose. Bone
Marrow Transplant 1993 Jul;12:73-6.

- Testa S, Manna A, Porcellini A, Maffi F,
Morstabilini G, Denti N, Macchi S, Rosti G, Porcellini G, Cassi D,
Ferrari L. Increased plasma level of vascular endothelial glycoprotein
thrombomodulin as an early indicator of endothelial damage in bone
marrow transplantation. Bone Marrow Transplant 1996 Aug;18:383-8.

- Richard S, Seigneur M, Blann A, Adams R,
Renard M, Puntous M, Boiron JM, Amiral J, Reiffers J, Boisseau M.
Vascular endothelial lesion in patients undergoing bone marrow
transplantation. Bone Marrow Transplant 1996 Nov;18:955-9.

- Salat C, Holler E, Kolb HJ, Pihusch R,
Reinhardt B, Hiller E. Endothelial cell markers in bone marrow
transplant recipients with and without acute graft-versus-host disease.
Bone Marrow Transplant 1997 May;19:909-14.

- Nurnberger W, Michelmann I, Burdach S,
Gobel U. Endothelial dysfunction after bone marrow transplantation:
increase of soluble thrombomodulin and PAI-1 in patients with multiple
transplant-related complications. Ann Hematol 1998 Feb;76:61-5.

- Kanamori H, Maruta A, Sasaki S, Yamazaki
E, Ueda S, Katoh K, Tamura T, Otsuka-Aoba M, Taguchi J, Harano H, Ogawa
K, Mohri H, Okubo T, Matsuzaki M, Watanabe S, Koharazawa H, Fujita H,
Kodama F. Diagnostic value of hemostatic parameters in bone marrow
transplant-associated thrombotic microangiopathy. Bone Marrow
Transplant 1998 Apr;21:705-9.

- Laurence J, Mitra D, Steiner M,
Staiano-Coico L, Jaffe E. Plasma from patients with idiopathic and
human immunodeficiency virus-associated thrombotic thrombocytopenic
purpura induces apoptosis in microvascular endothelial cells. Blood
1996 Apr 15;87:3245-54.

- Goon PK, Boos CJ, Lip GY. Circulating
endothelial cells: markers of vascular dysfunction. Clin Lab 2005
51:531-8.

- Mallat Z, Benamer H, Hugel B, Benessiano
J, Steg PG, Freyssinet JM, Tedgui A. Elevated levels of shed membrane
microparticles with procoagulant potential in the peripheral
circulating blood of patients with acute coronary syndromes.
Circulation 2000 Feb 29;101:841-3.

- Jimenez JJ, Jy W, Mauro LM, Soderland C,
Horstman LL, Ahn YS. Endothelial cells release phenotypically and
quantitatively distinct microparticles in activation and apoptosis.
Thromb Res 2003 Feb 15;109:175-80.

- Casciola-Rosen L, Rosen A, Petri M,
Schlissel M. Surface blebs on apoptotic cells are sites of enhanced
procoagulant activity: implications for coagulation events and
antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci U
S A 1996 Feb 20;93:1624-9.

- Shet AS, Aras O, Gupta K, Hass MJ, Rausch
DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains
tissue factor-positive microparticles derived from endothelial cells
and monocytes. Blood 2003 Oct 1;102:2678-83.

- Jimenez JJ, Jy W, Mauro LM, Horstman LL,
Soderland C, Ahn YS. Endothelial microparticles released in thrombotic
thrombocytopenic purpura express von Willebrand factor and markers of
endothelial activation. Br J Haematol 2003 Dec;123:896-902.

- Pihusch V, Rank A, Steber R, Pihusch M,
Pihusch R, Toth B, Hiller E, Kolb HJ. Endothelial cell-derived
microparticles in allogeneic hematopoietic stem cell recipients.
Transplantation 2006 May 27;81:1405-9.

- Nomura S, Ishii K, Kanazawa S, Inami N,
Uoshima N, Ishida H, Yoshihara T, Kitayama H, Hayashi K. Significance
of elevation in cell-derived microparticles after allogeneic stem cell
transplantation: transient elevation of platelet-derived microparticles
in TMA/TTP. Bone Marrow Transplant 2005 Nov;36:921-2.

- Hoorn CM, Wagner JG, Petry TW, Roth RA.
Toxicity of mitomycin C toward cultured pulmonary artery endothelium.
Toxicol Appl Pharmacol 1995 Jan;130:87-94.

- Kohn S, Fradis M, Podoshin L, Ben-David
J, Zidan J, Robinson E. Endothelial injury of capillaries in the stria
vascularis of guinea pigs treated with cisplatin and gentamicin.
Ultrastruct Pathol 1997 May-Jun;21:289-99.

- Nagaya S, Wada H, Oka K, Tanigawa M,
Tamaki S, Tsuzi K, Miyanishi E, Wakita Y, Minami N, Deguchi K, et al.
Hemostatic abnormalities and increased vascular endothelial cell
markers in patients with red cell fragmentation syndrome induced by
mitomycin C. Am J Hematol 1995 Dec;50:237-43.

- Oner AF, Gurgey A, Kirazli S, Okur H,
Tunc B. Changes of hemostatic factors in children with acute
lymphoblastic leukemia receiving combined chemotherapy including high
dose methylprednisolone and L-asparaginase. Leuk Lymphoma 1999
Apr;33:361-4.

- Chow AY, Chin C, Dahl G, Rosenthal DN.
Anthracyclines cause endothelial injury in pediatric cancer patients: a
pilot study. J Clin Oncol 2006 Feb 20;24:925-8.

- Fajardo LF. The pathology of ionizing
radiation as defined by morphologic patterns. Acta Oncol 2005 44:13-22.

- Takatsuka H, Wakae T, Mori A, Okada M,
Fujimori Y, Takemoto Y, Okamoto T, Kanamaru A, Kakishita E. Endothelial
damage caused by cytomegalovirus and human herpesvirus-6. Bone Marrow
Transplant 2003 Mar;31:475-9.

- Matsuda Y, Hara J, Miyoshi H, Osugi Y,
Fujisaki H, Takai K, Ohta H, Tanaka-Taya K, Yamanishi K, Okada S.
Thrombotic microangiopathy associated with reactivation of human
herpesvirus-6 following high-dose chemotherapy with autologous bone
marrow transplantation in young children. Bone Marrow Transplant 1999
Oct;24:919-23.

- Biedermann BC, Sahner S, Gregor M,
Tsakiris DA, Jeanneret C, Pober JS, Gratwohl A. Endothelial injury
mediated by cytotoxic T lymphocytes and loss of microvessels in chronic
graft versus host disease. Lancet 2002 Jun 15;359:2078-83.

- Rosenthal RA, Chukwuogo NA, Ocasio VH,
Kahng KU. Cyclosporine inhibits endothelial cell prostacyclin
production. J Surg Res 1989 Jun;46:593-6.

- Voss BL, Hamilton KK, Samara EN, McKee
PA. Cyclosporine suppression of endothelial prostacyclin generation. A
possible mechanism for nephrotoxicity. Transplantation 1988
Apr;45:793-6.

- Fortin MC, Raymond MA, Madore F, Fugere
JA, Paquet M, St-Louis G, Hebert MJ. Increased risk of thrombotic
microangiopathy in patients receiving a cyclosporin-sirolimus
combination. Am J Transplant 2004 Jun;4:946-52.

- Ruutu T, Hermans J, Niederwieser D,
Gratwohl A, Kiehl M, Volin L, Bertz H, Ljungman P, Spence D, Verdonck
LF, Prentice HG, Bosi A, Du Toit CE, Brinch L, Apperley JF. Thrombotic
thrombocytopenic purpura after allogeneic stem cell transplantation: a
survey of the European Group for Blood and Marrow Transplantation
(EBMT). Br J Haematol 2002 Sep;118:1112-9.

- Uderzo C, Bonanomi S, Busca A, Renoldi M,
Ferrari P, Iacobelli M, Morreale G, Lanino E, Annaloro C, Volpe AD,
Alessandrino P, Longoni D, Locatelli F, Sangalli H, Rovelli A. Risk
factors and severe outcome in thrombotic microangiopathy after
allogeneic hematopoietic stem cell transplantation. Transplantation
2006 Sep 15;82:638-44.

- Roy V, Rizvi MA, Vesely SK, George JN.
Thrombotic thrombocytopenic purpura-like syndromes following bone
marrow transplantation: an analysis of associated conditions and
clinical outcomes. Bone Marrow Transplant 2001 Mar;27:641-6.

- Takatsuka H, Nakajima T, Nomura K,
Okikawa Y, Wakae T, Toda A, Itoi H, Okada M, Misawa M, Hara H, Ogawa H.
Changes of clotting factors (7, 9 and 10) and hepatocyte growth factor
in patients with thrombotic microangiopathy after bone marrow
transplantation. Clin Transplant 2006 Sep-Oct;20:640-3.

- Paquette RL, Tran L, Landaw EM.
Thrombotic microangiopathy following allogeneic bone marrow
transplantation is associated with intensive graft-versus-host disease
prophylaxis. Bone Marrow Transplant 1998 Aug;22:351-7.

- Uderzo C, Fumagalli M, De Lorenzo P,
Busca A, Vassallo E, Bonanomi S, Lanino E, Dini G, Varotto S, Messina
C, Miniero R, Valsecchi MG, Balduzzi A. Impact of thrombotic
thrombocytopenic purpura on leukemic children undergoing bone marrow
transplantation. Bone Marrow Transplant 2000 Nov;26:1005-9.

- Daly AS, Hasegawa WS, Lipton JH, Messner
HA, Kiss TL. Transplantation-associated thrombotic microangiopathy is
associated with transplantation from unrelated donors, acute
graft-versus-host disease and venoocclusive disease of the liver.
Transfus Apher Sci 2002 Aug;27:3-12.

- Shimoni A, Yeshurun M, Hardan I, Avigdor
A, Ben-Bassat I, Nagler A. Thrombotic microangiopathy after allogeneic
stem cell transplantation in the era of reduced-intensity conditioning:
The incidence is not reduced. Biol Blood Marrow Transplant 2004
Jul;10:484-93.

- Nakamae H, Yamane T, Hasegawa T, Nakamae
M, Terada Y, Hagihara K, Ohta K, Hino M. Risk factor analysis for
thrombotic microangiopathy after reduced-intensity or myeloablative
allogeneic hematopoietic stem cell transplantation. Am J Hematol 2006
Jul;81:525-31.

- Shulman H, Striker G, Deeg HJ, Kennedy M,
Storb R, Thomas ED. Nephrotoxicity of cyclosporin A after allogeneic
marrow transplantation: glomerular thromboses and tubular injury. N
Engl J Med 1981 Dec 3;305:1392-5.

- Wolff D, Wilhelm S, Hahn J, Gentilini C,
Hilgendorf I, Steiner B, Kahl C, Junghanss C, Hartung G, Casper J,
Uharek L, Holler E, Freund M. Replacement of calcineurin inhibitors
with daclizumab in patients with transplantation-associated
microangiopathy or renal insufficiency associated with
graft-versus-host disease. Bone Marrow Transplant 2006 Sep;38:445-51.

- Kersting S, Koomans HA, Hene RJ, Verdonck
LF. Acute renal failure after allogeneic myeloablative stem cell
transplantation: retrospective analysis of incidence, risk factors and
survival. Bone Marrow Transplant 2007 Mar;39:359-65.

- Cutler C, Henry NL, Magee C, Li S, Kim
HT, Alyea E, Ho V, Lee SJ, Soiffer R, Antin JH. Sirolimus and
thrombotic microangiopathy after allogeneic hematopoietic stem cell
transplantation. Biol Blood Marrow Transplant 2005 Jul;11:551-7.

- Couriel DR, Saliba R, Escalon MP, Hsu Y,
Ghosh S, Ippoliti C, Hicks K, Donato M, Giralt S, Khouri IF, Hosing C,
de Lima MJ, Andersson B, Neumann J, Champlin R. Sirolimus in
combination with tacrolimus and corticosteroids for the treatment of
resistant chronic graft-versus-host disease. Br J Haematol 2005
Aug;130:409-17.

- Martinez MT, Bucher C, Stussi G, Heim D,
Buser A, Tsakiris DA, Tichelli A, Gratwohl A, Passweg JR.
Transplant-associated microangiopathy (TAM) in recipients of allogeneic
hematopoietic stem cell transplants. Bone Marrow Transplant 2005
Dec;36:993-1000.

- Worel N, Greinix HT, Leitner G,
Mitterbauer M, Rabitsch W, Rosenmayr A, Hocker P, Kalhs P.
ABO-incompatible allogeneic hematopoietic stem cell transplantation
following reduced-intensity conditioning: close association with
transplant-associated microangiopathy. Transfus Apher Sci 2007
Jun;36:297-304.

- Daly AS, Xenocostas A, Lipton JH.
Transplantation-associated thrombotic microangiopathy: twenty-two years
later. Bone Marrow Transplant 2002 Dec;30:709-15.

- Teruya J, Styler M, Verde S, Topolsky D,
Crilley P. Questionable efficacy of plasma exchange for thrombotic
thrombocytopenic purpura after bone marrow transplantation. J Clin
Apher 2001 16:169-74.

- Rock G, Shumak K, Kelton J, Blanchette
VS, Buskard N, Nair R, Spasoff R. Thrombotic thrombocytopenic purpura:
outcome in 24 patients with renal impairment treated with plasma
exchange. Canadian Apheresis Study Group. Transfusion 1992 Oct;32:710-4.

- Goebel J, Stevens E, Forrest K, Roszman
TL. Daclizumab (Zenapax) inhibits early interleukin-2 receptor signal
transduction events. Transpl Immunol 2000 Nov;8:153-9.

- Wiseman LR, Faulds D. Daclizumab: a
review of its use in the prevention of acute rejection in renal
transplant recipients. Drugs 1999 Dec;58:1029-42.

- Kobashigawa J, David K, Morris J, Chu AH,
Steffen BJ, Gotz VP, Gordon RD. Daclizumab is associated with decreased
rejection and no increased mortality in cardiac transplant patients
receiving MMF, cyclosporine, and corticosteroids. Transplant Proc 2005
Mar;37:1333-9.

- Hershberger RE, Starling RC, Eisen HJ,
Bergh CH, Kormos RL, Love RB, Van Bakel A, Gordon RD, Popat R, Cockey
L, Mamelok RD. Daclizumab to prevent rejection after cardiac
transplantation. N Engl J Med 2005 Jun 30;352:2705-13.

- Vincenti F, Nashan B, Light S.
Daclizumab: outcome of phase III trials and mechanism of action. Double
Therapy and the Triple Therapy Study Groups. Transplant Proc 1998
Aug;30:2155-8.

- Martin R. Humanized anti-CD25 antibody
treatment with daclizumab in multiple sclerosis. Neurodegener Dis 2008
5:23-6.

- Bielekova B, Richert N, Howard T, Blevins
G, Markovic-Plese S, McCartin J, Frank JA, Wurfel J, Ohayon J, Waldmann
TA, McFarland HF, Martin R. Humanized anti-CD25 (daclizumab) inhibits
disease activity in multiple sclerosis patients failing to respond to
interferon beta. Proc Natl Acad Sci U S A 2004 Jun 8;101:8705-8.

- Rose JW, Burns JB, Bjorklund J, Klein J,
Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and
remitting multiple sclerosis: MRI and clinical results. Neurology 2007
Aug 21;69:785-9.

- Sloand EM, Scheinberg P, Maciejewski J,
Young NS. Brief communication: Successful treatment of pure red-cell
aplasia with an anti-interleukin-2 receptor antibody (daclizumab). Ann
Intern Med 2006 Feb 7;144:181-5.

- Maciejewski JP, Sloand EM, Nunez O, Boss
C, Young NS. Recombinant humanized anti-IL-2 receptor antibody
(daclizumab) produces responses in patients with moderate aplastic
anemia. Blood 2003 Nov 15;102:3584-6.

- Vincenti F, Kirkman R, Light S,
Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H,
Gaston R, Backman L, Burdick J. Interleukin-2-receptor blockade with
daclizumab to prevent acute rejection in renal transplantation.
Daclizumab Triple Therapy Study Group. N Engl J Med 1998 Jan
15;338:161-5.

- Palmer KJ, Goa KL. Defibrotide. A review
of its pharmacodynamic and pharmacokinetic properties, and therapeutic
use in vascular disorders. Drugs 1993 Feb;45:259-94.

- Richardson PG, Elias AD, Krishnan A,
Wheeler C, Nath R, Hoppensteadt D, Kinchla NM, Neuberg D, Waller EK,
Antin JH, Soiffer R, Vredenburgh J, Lill M, Woolfrey AE, Bearman SI,
Iacobelli M, Fareed J, Guinan EC. Treatment of severe veno-occlusive
disease with defibrotide: compassionate use results in response without
significant toxicity in a high-risk population. Blood 1998 Aug
1;92:737-44.

- Zager RA. Acute renal failure in the
setting of bone marrow transplantation. Kidney Int 1994 Nov;46:1443-58.

- Corti P, Uderzo C, Tagliabue A, Della
Volpe A, Annaloro C, Tagliaferri E, Balduzzi A. Defibrotide as a
promising treatment for thrombotic thrombocytopenic purpura in patients
undergoing bone marrow transplantation. Bone Marrow Transplant 2002
Mar;29:542-3.

- DeLeve LD, Shulman HM, McDonald GB. Toxic
injury to hepatic sinusoids: sinusoidal obstruction syndrome
(veno-occlusive disease). Semin Liver Dis 2002 Feb;22:27-42.

- Rio B, Andreu G, Nicod A, Arrago JP,
Dutrillaux F, Samama M, Zittoun R. Thrombocytopenia in venocclusive
disease after bone marrow transplantation or chemotherapy. Blood 1986
Jun;67:1773-6.

- Pescador R, Porta R, Ferro L. An
integrated view of the activities of defibrotide. Semin Thromb Hemost
1996 22 Suppl 1:71-5.

- Falanga A, Vignoli A, Marchetti M, Barbui
T. Defibrotide reduces procoagulant activity and increases fibrinolytic
properties of endothelial cells. Leukemia 2003 Aug;17:1636-42.

- Lazarus HM, Gottfried MR, Herzig RH,
Phillips GL, Weiner RS, Sarna GP, Fay J, Wolff SN, Sudilovsky O, Gale
RP, Herzig GP. Veno-occlusive disease of the liver after high-dose
mitomycin C therapy and autologous bone marrow transplantation. Cancer
1982 May 1;49:1789-95.

- Kumar S, DeLeve LD, Kamath PS, Tefferi A.
Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after
hematopoietic stem cell transplantation. Mayo Clin Proc 2003
May;78:589-98.

- Saviola A, Luppi M, Potenza L, Morselli
M, Ferrari A, Riva G, Torelli G. Late occurrence of hepatic
veno-occlusive disease following gemtuzumab ozogamicin: successful
treatment with defibrotide. Br J Haematol 2003 Nov;123:752-3.

- Rollins BJ. Hepatic veno-occlusive
disease. Am J Med 1986 Aug;81:297-306.

- Bearman SI, Shuhart MC, Hinds MS,
McDonald GB. Recombinant human tissue plasminogen activator for the
treatment of established severe venocclusive disease of the liver after
bone marrow transplantation. Blood 1992 Nov 15;80:2458-62.

- Bearman SI, Lee JL, Baron AE, McDonald
GB. Treatment of hepatic venocclusive disease with recombinant human
tissue plasminogen activator and heparin in 42 marrow transplant
patients. Blood 1997 Mar 1;89:1501-6.

- Bulley SR, Strahm B, Doyle J, Dupuis LL.
Defibrotide for the treatment of hepatic veno-occlusive disease in
children. Pediatr Blood Cancer 2007 Jun 15;48:700-4.

- Ho VT, Linden E, Revta C, Richardson PG.
Hepatic veno-occlusive disease after hematopoietic stem cell
transplantation: review and update on the use of defibrotide. Semin
Thromb Hemost 2007 Jun;33:373-88.

- Sayer HG, Will U, Schilling K, Vogt T,
Wollina K, Hoffken K. Hepatic veno-occlusive disease (VOD) with
complete occlusion of liver venules after tandem autologous stem cell
transplantation-- successful treatment with high-dose
methylprednisolone and defibrotide. J Cancer Res Clin Oncol 2002
Mar;128:148-52.

- Sucak GT, Aki ZS, Yagci M, Yegin ZA,
Ozkurt ZN, Haznedar R. Treatment of sinusoidal obstruction syndrome
with defibrotide: a single-center experience. Transplant Proc 2007
Jun;39:1558-63.

- Richardson PG, Murakami C, Jin Z, Warren
D, Momtaz P, Hoppensteadt D, Elias AD, Antin JH, Soiffer R, Spitzer T,
Avigan D, Bearman SI, Martin PL, Kurtzberg J, Vredenburgh J, Chen AR,
Arai S, Vogelsang G, McDonald GB, Guinan EC. Multi-institutional use of
defibrotide in 88 patients after stem cell transplantation with severe
veno-occlusive disease and multisystem organ failure: response without
significant toxicity in a high-risk population and factors predictive
of outcome. Blood 2002 Dec 15;100:4337-43.

- Reddy PS, Deauna-Limayo D, Cook JD,
Ganguly SS, Blecke C, Bodensteiner DC, Skikne BS, Sahud MA. Rituximab
in the treatment of relapsed thrombotic thrombocytopenic purpura. Ann
Hematol 2005 Apr;84:232-5.

- Ahmad A, Aggarwal A, Sharma D, Dave HP,
Kinsella V, Rick ME, Schechter GP. Rituximab for treatment of
refractory/relapsing thrombotic thrombocytopenic purpura (TTP). Am J
Hematol 2004 Oct;77:171-6.

- George JN, Woodson RD, Kiss JE, Kojouri
K, Vesely SK. Rituximab therapy for thrombotic thrombocytopenic
purpura: a proposed study of the Transfusion Medicine/Hemostasis
Clinical Trials Network with a systematic review of rituximab therapy
for immune-mediated disorders. J Clin Apher 2006 Apr;21:49-56.

- Barnett B, Kruse-Jarres R, Leissinger CA.
Current management of acquired factor VIII inhibitors. Curr Opin
Hematol 2008 Sep;15:451-5.

- Franchini M, Lippi G. Acquired factor
VIII inhibitors. Blood 2008 Jul 15;112:250-5.

- Kalunian K, Joan TM. New directions in
the treatment of systemic lupus erythematosus. Curr Med Res Opin 2009
Jun;25:1501-14.

- Benucci M, Saviola G, Baiardi P, Manfredi
M. Cost-effectiveness treatment with Rituximab in patients with
rheumatoid arthritis in real life. Rheumatol Int May 16.

- Lee YH, Bae SC, Song GG. The efficacy and
safety of rituximab for the treatment of active rheumatoid arthritis: a
systematic review and meta-analysis of randomized controlled trials.
Rheumatol Int May 16.

- Au WY, Ma ES, Lee TL, Ha SY, Fung AT, Lie
AK, Kwong YL. Successful treatment of thrombotic microangiopathy after
haematopoietic stem cell transplantation with rituximab. Br J Haematol
2007 Jun;137:475-8.

- Ruggenenti P, Remuzzi G. The
pathophysiology and management of thrombotic thrombocytopenic purpura.
Eur J Haematol 1996 Apr;56:191-207.

- Bell WR, Braine HG, Ness PM, Kickler TS.
Improved survival in thrombotic thrombocytopenic purpura-hemolytic
uremic syndrome. Clinical experience in 108 patients. N Engl J Med 1991
Aug 8;325:398-403.

- Rosove MH, Ho WG, Goldfinger D.
Ineffectiveness of aspirin and dipyridamole in the treatment of
thrombotic thrombocytopenic purpura. Ann Intern Med 1982 Jan;96:27-33.

- Siddiqui FA, Lian EC. Novel
platelet-agglutinating protein from a thrombotic thrombocytopenic
purpura plasma. J Clin Invest 1985 Oct;76:1330-7.

- Moore JC, Arnold DM, Leber BF, Clare R,
Molnar GJ, Kelton JG. Intravenous immunoglobulin as an adjunct to
plasma exchange for the treatment of chronic thrombotic
thrombocytopenic purpura. Vox Sang 2007 Aug;93:173-5.

- Levin M, Grunwald HW. Use of vincristine
in refractory thrombotic thrombocytopenic purpura. Acta Haematol 1991
85:37-40.

- Mazzei C, Pepkowitz S, Klapper E,
Goldfinger D. Treatment of thrombotic thrombocytopenic purpura: a role
for early vincristine administration. J Clin Apher 1998 13:20-2.

- Gaddis TG, Guthrie TH, Jr., Drew MJ,
Sahud M, Howe RB, Mittelman A. Treatment of plasma refractory
thrombotic thrombocytopenic purpura with protein A immunoabsorption. Am
J Hematol 1997 Jun;55:55-8.

- Kasper S, Neurath MF, Huber C, Theobald
M, Scharrer I. Protein A immunoadsorption therapy for refractory,
mitomycin C-associated thrombotic microangiopathy. Transfusion 2007
Jul;47:1263-7.

- Zeigler ZR, Shadduck RK, Nath R, Andrews
DF, 3rd. Pilot study of combined cryosupernatant and protein A
immunoadsorption exchange in the treatment of grade 3-4 bone marrow
transplant-associated thrombotic microangiopathy. Bone Marrow
Transplant 1996 Jan;17:81-6.

- Chello M, Goffredo C, Patti G, Candura D,
Melfi R, Mastrobuoni S, Di Sciascio G, Covino E. Effects of
atorvastatin on arterial endothelial function in coronary bypass
surgery. Eur J Cardiothorac Surg 2005 Dec;28:805-10.

- Bohm F, Settergren M, Gonon AT, Pernow J.
The endothelin-1 receptor antagonist bosentan protects against
ischaemia/reperfusion-induced endothelial dysfunction in humans. Clin
Sci (Lond) 2005 Apr;108:357-63.