Review Articles
Zygomycosis in
Immunocompromised non-Haematological Patients G. Petrikkos and M. Drogari-Apiranthitou
4st
Dept. of Internal Medicine, School of Medicine, National and
Kapodistrian University of Athens, "ΑΤΤΙΚΟΝ" Hospital, RΙΜΙΝΙ 1 –
Haidari , Athens - 12464. Greece
Published: March 15, 2011
Received: January 11, 2011
Accepted: January 16, 2011
Medit J Hemat Infect Dis 2011, 3: e2011012, DOI 10.4084/MJHID.2011.012
This article is available from: http://www.mjhid.org/article/view/7840
This is an Open Access article
distributed under the terms of
the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Abstract
Zygomycoses
caused by fungi of the mucorales order (mucormycoses) are emerging
fungal diseases with a high fatality rate. The most important
risk factors include neutropenia or functional neutropenia, diabetic
ketoacidosis, iron overload, major trauma, prolonged use of
corticosteroids, illicit intravenous drug (ID) use, neonatal
prematurity, malnourishment, and maybe a previous exposure to
antifungal agents with no activity against zygomycetes, such as
voriconazole and echinocandins.
A high index of suspicion is crucial for the diagnosis, as prompt and appropriate management can considerably reduce morbidity and mortality. Suspicion index can be increased through recognition of the differential patterns of clinical presentation. In the non- haematological immunocompromised patients, mucormycosis can manifest in various clinical forms, depending on the underlying condition: mostly as rhino-orbital or rhino-cerebral in diabetes patients, pulmonary infection in patients with malignancy or solid organ transplantation, disseminated infection in iron overloaded or deferoxamine treated patients, cerebral - with no sinus involvement - in ID users, gastrointestinal in premature infants or malnourishment, and cutaneous after direct inoculation in immunocompetent individuals with trauma or burns.
Treating a patient’s underlying medical condition and reducing immunosuppression are essential to therapy. Rapid correction of metabolic abnormalities is mandatory in cases such as uncontrolled diabetes, and corticosteroids or other immunosuppressive drugs should be discontinued where feasible. AmphotericinB or its newer and less toxic lipid formulations are the drugs of choice regarding antifungal chemotherapy, while extensive surgical debridement is essential to reduce infected and necrotic tissue. A high number of cases could be prevented through measures including diabetes control programmes and proper pre- and post-surgical hygiene.
A high index of suspicion is crucial for the diagnosis, as prompt and appropriate management can considerably reduce morbidity and mortality. Suspicion index can be increased through recognition of the differential patterns of clinical presentation. In the non- haematological immunocompromised patients, mucormycosis can manifest in various clinical forms, depending on the underlying condition: mostly as rhino-orbital or rhino-cerebral in diabetes patients, pulmonary infection in patients with malignancy or solid organ transplantation, disseminated infection in iron overloaded or deferoxamine treated patients, cerebral - with no sinus involvement - in ID users, gastrointestinal in premature infants or malnourishment, and cutaneous after direct inoculation in immunocompetent individuals with trauma or burns.
Treating a patient’s underlying medical condition and reducing immunosuppression are essential to therapy. Rapid correction of metabolic abnormalities is mandatory in cases such as uncontrolled diabetes, and corticosteroids or other immunosuppressive drugs should be discontinued where feasible. AmphotericinB or its newer and less toxic lipid formulations are the drugs of choice regarding antifungal chemotherapy, while extensive surgical debridement is essential to reduce infected and necrotic tissue. A high number of cases could be prevented through measures including diabetes control programmes and proper pre- and post-surgical hygiene.
Introduction
The term ‘Zygomycosis’ refers to a group of rare infections caused by hyaline filamentous fungi belonging to the class of Zygomycetes. This class is subdivided in two orders, Mucorales and Entomophthorales, both involved in human disease.[1]
Mucorales are saprobiotic organisms ubiquitous in nature; some members of this order are weak plant parasites or plant pathogens and opportunistic pathogens for man.[2] The vast majority of human zygomycotic disease is caused by genera of the Mucorales order, therefore, the term ‘mucormycosis’ is used interchangeably with the term ‘zygomycosis’ (the term ‘phycomycosis’ has also been used in the past). The most common genera confirmed in human disease are: Rhizopus, Mucor, Lichtheimia (formerly known as Absidia corymbifera or Mycocladus), Rhizomucor, Apophysomyces, Saksenaea, and Cunninghamella.[1]
For Mucorales the portals of entry in the human body are the respiratory tract through inhalation of fungal spores, the skin and less frequently the gut. They grow rapidly and produce wide hyaline, aseptate or poorly septated ribbon-like hyphae in tissues. They invade blood vessels and cause thrombosis and necrosis of the infected tissues. Disease in humans is limited to severely immunocompromised individuals, those with diabetic ketoacidosis, or those with burns or trauma. Infection usually progresses rapidly and the case fatality rate is very high. Depending on the underlying condition and the portal of entry they can cause rhinocerebral, pulmonary, cutaneous, gastrointestinal or even disseminated infection.[1]
Human infections caused by Entomophthorales, which are mainly insect pathogens, constitute a completely different clinical entity (entomopfthoramycoses). Genera involved in human disease are Conidiobolus and Basidiobolus. They affect mainly immunocompetent individuals, are prevalent in the tropics and do not disseminate.[3] In recent years the geographic distribution of basidiobolomycosis expanded and is reported also in immunocompromised hosts involving more tissues, but these cases are extremely rare.[4,5] Therefore, the present review focuses only on the zygomycotic infections caused by mucorales.
The most important risk factors for mucormycosis include prolonged neutropenia, diabetic ketoacidosis, iron overload and major trauma. Other important predisposing factors of mucormycosis include prolonged use of corticosteroids, illicit intravenous drug use, neonatal prematurity, malnourishment and broad-spectrum antimicrobial agents, as well as antifungal agents with no activity against zygomycetes, such as voriconazole and caspofungin.[6,7] The same may also be true for the other echinocandins, but data are lacking as they are not as widely used as caspofungin. Risk factors, mechanisms leading to mucormycosis and clinical presentation forms are illustrated in Table 1.
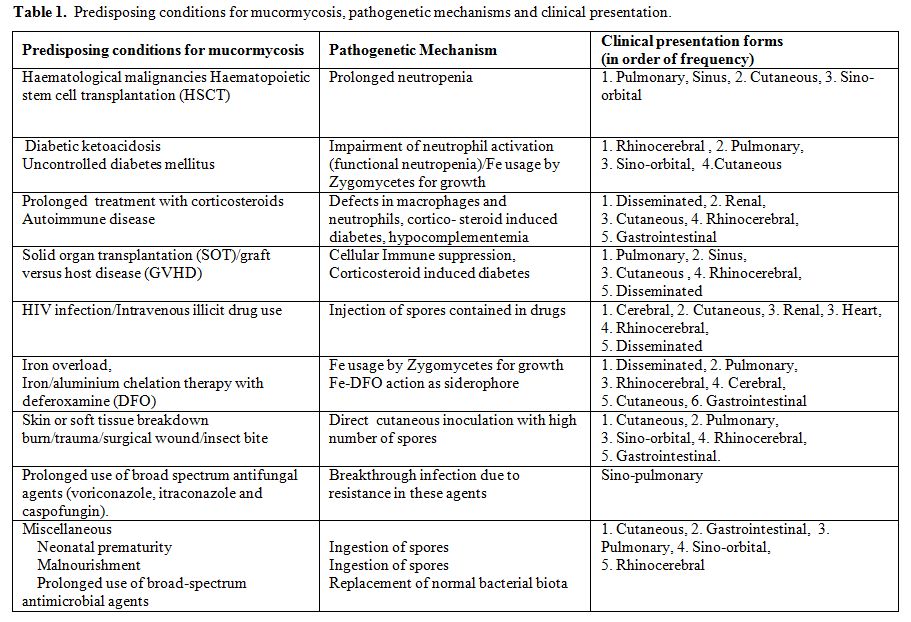
Table 1. Predisposing conditions for mucormycosis, pathogenetic mechanisms and clinical presentation.
The recent years, mucormycosis incidence is seemingly increasing, particularly in patients with haematological malignancies or haematopoietic stem cell transplantation, at least in western countries.[8,9,10] However, accurate estimation of the incidence is a challenge. Data from a global registry are not available yet and moreover there are difficulties in collecting well defined denominator data. Much information has been derived from the first most comprehensive study by Roden et al. 2005, [6] in which all data on zygomycosis recorded in the English language literature ever since the first report by Platauf in 1885,[11] were analyzed. More recent data from individual countries and institutions have also been reported [12-17] and percentages of clinical cases according to underlying condition are presented in Table2.
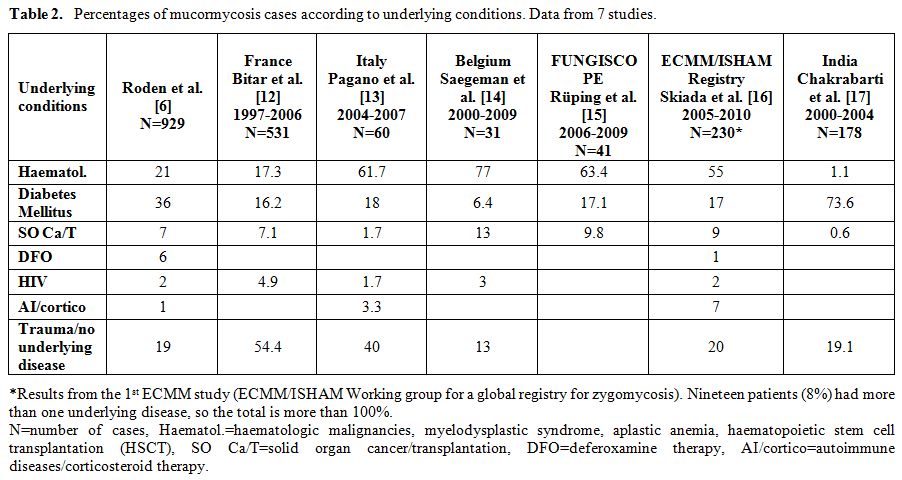
Table 2. Percentages of mucormycosis cases according to underlying conditions. Data from 7 studies.
Underlying Conditions
Diabetes – Ketoacidosis: Diabetes mellitus as a predisposing factor has been reported in 36–88% of all mucormycosis cases.[6,18-20] More susceptible are patients with uncontrolled hyperglycemia, particularly those with ketoacidosis.[21,22,23] Mucormycosis can also be observed in metabolically controlled diabetic patients24 and was found to be the first clinical manifestation of some patients with undiagnosed diabetes mellitus.[25] Type 1, type 2, and secondary diabetes mellitus have all been reported as risk factors in patients with mucormycosis.[26]
In the normal host protection from mucormycosis is provided by macrophages. They prevent the initiation of infection by phagocytosis and oxidative killing of the fungal spores. In case infection is established, neutrophils are activated and play a pivotal role in fungal killing. Activation happens in four phases:
a) neutrophils are chemotactically attracted to the hyphae,
b) they attach and
c) spread on the hyphae and finally,
d) using their oxidative cytotoxic system, neutrophils damage and kill the fungal elements without accompanying phagocytosis.
In diabetes, each of the four phases of neutrophil activation is impaired.[27] Hyperglycemia per se does not seem to play a major role in the pathogenesis of mucormycosis. Chemotaxis, the phagocytic functions (adherence and spreading), and finally the oxidative burst are all inhibited in the ketoacidotic state, essentially inducing functional neutropenia.[26-29]
The most common clinical presentation of mucormycosis in patients with diabetes mellitus is sinus disease (66%).6 Diabetes mellitus was recognized as a major risk factor for developing rhinocerebral mucormycosis very early on. The publication by Gregory et al. in 1943 on a case of uncontrolled diabetes was the first to describe fulminant rhinocerebral mucormycosis.[30]
Disease in diabetics can also present as pulmonary mucormycosis, a clinical presentation more common in neutropenic patients with malignancies or HSCT.[6]
The epidemiology in diabetic patients is variable. Roden et al.[6] showed that diabetic patients represented 36% of the 929 reported cases. They also reported a decreased incidence of mucormycosis in diabetic patients over time. This is in contrast to the increased prevalence of diabetes in the world.[31] The role of statins, used at least in the western world, has been speculated in an effort to explain this phenomenon.[32,33] In France, however, a population- based study of medical records of mucormycosis cases reported from 1997 to 2006 showed a 9% yearly increase in the annual incidence rate in the diabetic population.[12] Another retrospective study from the USA, reviewing cases with rhino-orbital-cerebral mucormycosis from two teaching tertiary-care hospitals revealed that 83% of cases were observed in diabetic patients and more importantly, 41% of them had no known history of their condition.[34] A striking finding in this study was that 56% of the patients were of Hispanic origin, a much larger proportion than that in the general population (≈25% expected incidence of Hispanic ethnicity based on demographic characteristics at the study hospitals). Data from a tertiary-care centre in India35 were also overwhelming, as 73.6% of cases were observed in patients with uncontrolled diabetes and in 42.7% of these cases diabetes was diagnosed for the first time. These findings also underline the association between diabetes and socioeconomic status. Low income individuals have the tendency not to seek medical attention as healthcare is unaffordable to them, unless complications manifest. Almost 80% of type 2 diabetes deaths occur in low- and middle-income countries (WHO Fact sheet: Diabetes, ref.36). With diabetes control programs in place, also many cases of mucormycosis could be prevented.
The overall mortality of diabetic patients with mucormycosis who undergo treatment is approximately 44%, and this is lower compared to other immunocompromising conditions.[6] This can be explained by the relatively easy management of acute complications of diabetes compared to the management of other conditions. This percentage can be further decreased with improved management. [37]
Besides diabetic ketoacidosis, chronic metabolic acidosis due to other causes, such as chronic renal failure with uremia, [38] chronic salicylate poisoning, [39] and methylmalonicaciduria,[40] has also been reported as a risk factor for mucormycosis.
Iron overload and Deferoxamine (DFO) iron/aluminium chelation therapy: Many microorganisms require iron to grow, and conditions of iron excess have been known to predispose to infection with certain bacteria.[41,42] Iron acquisition is a critical step in the pathogenetic mechanism of mucormycosis.[43]
Therapy with DFO, an iron chelator used for the treatment of iron and/or aluminum overload in dialysis patients, was found paradoxically to be a risk factor for angioinvasive mucormycosis.[44,45] Although the first dialysis patient with mucormycosis, reported in 1979 by Gluskin et al,[46] was not noted to be receiving DFO, subsequent cases of mucormycosis in patients undergoing dialysis have been in those receiving DFO for aluminum or iron excess.[45] A report of an international registry [47] showed that 78% of dialysis patients with mucormycosis were being treated with DFO. In addition to patients with renal failure, patients with hematologic disorders, all of whom were treated with DFO, have been reported with mucormycosis.[45] Subsequent research showed that DFO can act as a siderophore for Mucorales: DFO forms a Fe-DFO complex with iron, which then binds to unidentified receptors on the surface of the zygomycetes. The iron is subsequently liberated by the fungus and is likely transported into the fungal cell by a high-affinity iron permease (rFTR1).[43] The increased sensitivity of dialysis patients to DFO-related mucormycosis is explained by the pharmacokinetic changes during uremia, which lead to a prolonged accumulation of Fe-DFO after DFO administration.[48]
Besides DFO, iron overload per se, either transfusional or due to dyserythropoiesis has also been recognized as a risk factor for mucormycosis.[49–51] In vitro and in vivo studies have shown that iron and DFO can enhance both growth and pathogenicity of Rhizopus spp. In a study reviewing a series of five cases of invasive mucormycosis among 263 allogeneic bone marrow transplant recipients,[51] the association between severe iron overload and mucormycosis was demonstrated. The mean values of serum ferritin level, transferrin saturation, and number of transfused units of erythrocytes in the study group were significantly higher compared with the matched control group.
Patients with diabetic ketoacidosis have elevated levels of available serum iron, likely due to the release of iron from binding proteins in the presence of low pH. Iron is then internalized by the zygomycetes with the help of copper oxidase (Cu-oxidase) and the high affinity iron permease rFTR1. Therefore, the increased susceptibility of patients with diabetic ketoacidosis to mucormycosis is likely due, at least in part, to an elevation in available serum iron during diabetic ketoacidosis following proton-mediated dissociation of iron from transferrin.[43]
The most common presentation of mucormycosis in patients receiving DFO appears to be the disseminated form (44%) and is associated with high mortality, reaching 80%.[6,47]
In contrast to DFO, two other iron chelators, deferiprone and deferasirox, do not supply iron to the fungus. This could be either because of their different structures and smaller size, rendering them inaccessible to fungal iron uptake systems, or because they share higher affinity constants for iron.[52] Deferiprone and deferasirox were shown to have fungicidal activity against Zygomycetes in vitro. Further, both iron chelators were shown to effectively treat mucormycosis in animal models, and one has been successfully used as salvage therapy for a patient with rhinocerebral mucormycosis.[43]
Solid organ malignancies and solid organ transplantation (SOT): The incidence of mucormycosis in transplant recipients, including both hematopoietic stem cell transplant (HSCT) and solid organ transplant (SOT) patients has increased. Although global surveys of the incidence of mucormycosis are still lacking, data from individual countries and institutions show that mucormycosis in SOT is rare, but seems to be a concern because of the high mortality rate.
The estimated incidence ranges from 0.4 – 16%.[6,12,52,53] Data from the Transplant Associated Infection Surveillance Network (TRANSNET) showed that among 1063 solid organ transplant recipients mucormycosis represented 2% of invasive fungal infections. Neutropenia predisposing to mucormycosis [6] was notably absent in SOT recipients as were acidosis with or without hyperglycemia and use of DFO. In contrast, all patients were receiving immunosuppression and the overwhelming majority was on corticosteroids. Furthermore, dissemination to distant organs occurred more frequently after rejection and its treatment.
The lung seems to be the most common site of infection in SOT recipients with mucormycosis.[6,54] Dissemination occurred preferentially to cutaneous and soft tissues, and not the brain. Mycocladus (Lichtheimia) corymbifer as causative pathogen appeared to be associated with a higher risk for dissemination in SOT with pulmonary mucormycosis in one study.[55]
In a matched case-controlled study [54] SOT recipients with mucormycosis were prospectively studied. Renal failure, diabetes mellitus, and prior voriconazole and/or caspofungin use were associated with a higher risk, whereas tacrolimus was associated with a lower risk of mucormycosis. Liver transplant recipients were more likely to have disseminated disease and developed mucormycosis earlier after transplantation than did other SOT recipients (median, 0.8 versus 5.7 months).
HIV/AIDS - Intravenous drug abuse: Mucormycosis in HIV/AIDS patients is very rare. Antinori et al.[56] in a large retrospective study of 1630 autopsies of patients who died of AIDS during 1984 through 2002, found only 2 cases with mucormycosis. However, it can be the presenting opportunistic infection in AIDS.[57]
Recently, human immunodeficiency virus (HIV) infection has been recognized as a risk factor for mucormycosis, but most cases in HIV-infected patients are also associated with intravenous drug abuse.[58,59,60,61] Cerebral mucormycosis with basal ganglia involvement is the usual clinical presentation in such cases, regardless of previous exposure to HIV.[62] The disease may develop insidiously or may progress rapidly with a fulminant course.
Endocarditis as well as the rhinocerebral form can also occur. Merchant et al.[63] reported on a patient with rhinocerebral mucormycosis and also reviewed another 6 cases reported in the literature. It is of note that 3 of them had hyperglycemia or diabetic ketoacidosis.
Mucormycosis in the HIV+ can also present in the kidneys, the skin, the gastrointestinal tract, the respiratory tract, or may be disseminated.[64-75] How the fungus enters the body is not very clear. The fact that most of the infections occur at sites remote from the needle stick however, suggest that most probably this happens through spores contained in the illicit drugs.[1]
Corticosteroids/Rheumatic diseases: Corticosteroid therapy is another primary risk factor that enhances a patient’s susceptibility to mucormycosis by causing either defects in macrophages and neutrophils or steroid-induced diabetes.[1]
Corticosteroids have a wide range of complex immunosuppressive effects, mainly by affecting cellular immunity, and increase host susceptibility to invasive fungal infections.[76] The mechanism by which the corticosteroids enhance susceptibility to developing mucormycosis is probably twofold. First, steroids suppress the normal inflammatory cell response that would otherwise occur, and second, they may induce a diabetic state.[77] In a recent study only 21% of patients with corticosteroid induced diabetes were receiving medication for diabetes.[34]
Of the few cases of mucormycosis in patients with lupus erythematosus (SLE) reported in English language literature[78-93] it appears that disease can present with any clinical form, but disseminated mucormycosis is usual in this group of patients. Mok et al.[78] reviewed all SLE cases published from 1970 through 2002 and reported a very high overall mortality of mucormycosis (88%). Additional predisposing factors for opportunistic infection included hypocomplementemia, nephrotic syndrome, uremia, leukopenia, and diabetes mellitus. The diagnosis often was made only at autopsy (63%). The cutaneous form appeared to have the best prognosis with combined medical and surgical treatment.[78]
Mucormycosis in other autoimmune diseases are scarce, but in cases such as in Wegener’s granulomatosis it may mimic disease relapse and remain underdiagnosed.[94]
No underlying disease: A considerable proportion of patients with mucormycosis have no apparent immune deficiency.[6] These patients have usually primary mucormycosis of the skin associated with burns, trauma, insect bites, tattooing and also complicating surgical wounds and catheter insertion sites. Recently, Skiada and Petrikkos reviewed 67 cases of cutaneous mucormycosis in English language literature, from 2004 -2008.[95] Most of the patients had an underlying immunocompromising condition such as haematologic malignancy, diabetes, or solid organ transplantation, but the immunocompetent represented a large proportion (40%).
Infection usually occurs through direct inoculation. It can be very invasive locally, penetrating the cutaneous and subcutaneous tissues into the adjacent fat, muscle, fascia and bone. In rare cases it can even disseminate to deep organs. Road traffic accidents, crush injuries, but also minor traumas such as those caused by plant thorns or insect bites, can lead to traumatic implantation of contaminated soil and subsequent mucormycosis. Major burn injury is another well described cause of cutaneous mucormycosis. An increased risk for developing mucormycosis in burn patients is the development of a condition called “burned stressed pseudodiabetes”, which is marked by hyperglycemia and glycosuria.[96,97]
In a new PubMed search from 2008 - 2010, we found 27 additional cases of cutaneous mucormycosis occurring in non immunocompromised hosts.[98-110] Risk factors in these cases included soil contaminated wounds, insect bites, burns, but the majority of cases (N=12) were iatrogenic, either after surgery or at injection sites. Infections with Mucorales occurring in nosocomial settings have been repeatedly reported and have even caused small hospital outbreaks in the USA, the UK and in Europe.[111] Sources of infection included contaminated bandages, Elastopad adhesive dressings, wooden tongue depressors and non-sterile karaya ostomy bags.
The clinical presentation is variable. The clinical signs initially are non specific, consisting of erythema and induration. Eventually the lesions may form blisters, pustules, or necrotic ulcerations. Cotton wool-like material may also be observed on the margins of the wound. The lesions may mimic pyoderma gangrenosum, bacterial synergistic gangrene or infections produced by Pseudomonas, Aspergillus, Histoplasma etc. Biopsy specimens for histology and cultures are necessary to establish diagnosis.
Strains involved are usually Rhizopus spp. (the majority), Lichtheimia corymbifera (syn. Absidia, Mycocladus), Apohysomyces elegans, Mucor spp., Cunninghamella bertholetiae and Saksenaea vasiformis. In the cases reported after 2008 the cultured species were R. arrhizus in 3 cases,[99,101] Lichtheimia spp.in [5,98,100,107,109] A. elegans in 4,[98] S. vasiformis in 3,[98,102,110] and Rhizomucor variabilis in 7.[103-104] The later, a non-thermophile species of the genus Rhizomucor, in contrast to other mucorales can cause large but slowly progressing opportunistic infections of the skin in immune-competent individuals. These fungi seem to have an increasing incidence in farmers from China.
The prognosis of mucormycosis in the immunocompetent is better than that in other patient groups but mortality remains high depending on the site, extension of infection and time of initiation of antifungal therapy. Iatrogenic cutaneous mucormycosis could easily be prevented with proper preoperative preparation and postoperative dressing of the surgical sites.
Diagnosis
The clinical diagnosis of mucormycosis among non haematological immunocompromised patients is notoriously difficult because of the similarity with aspergillosis or other mycoses caused by filamentous fungi. Diagnosis is usually delayed, as the majority of the physicians are not so much familiar with the disease. The leading signs and symptoms of mucormycosis are presented in Table 3.
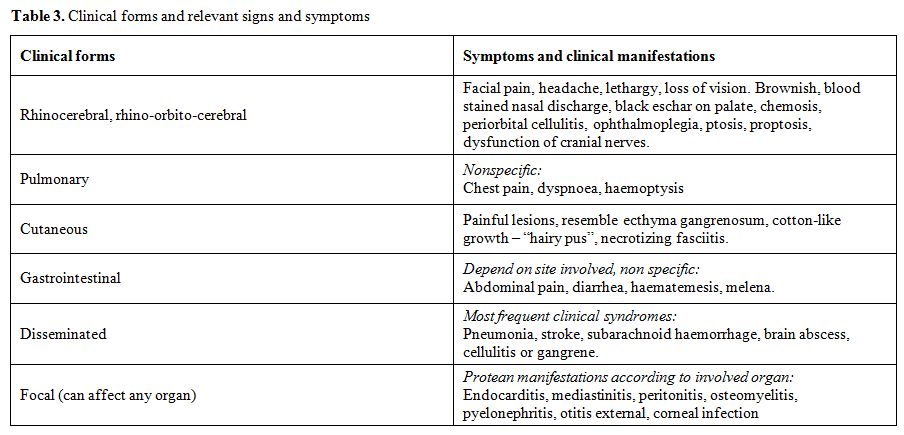
Table 3. Clinical forms and relevant signs and symptoms
When clinical presentation may suggest mucormycosis, imaging techniques are helpful, but cultures and histopathology are required for confirmation.
In case of rhinocerebral involvement, CT and particularly MRI are helpful in enabling an early detection of orbital, sinus, meningeal, bone, and cerebral lesions as well as intracranial vascular occlusion even before clinical signs develop. In pulmonary mucormycosis, lung biopsies (endoscopic, CT-guided or surgical) should be performed, depending on the radiological findings obtained by CT scans. CT guided percutaneous lung biopsy has been found highly efficient to early differentiate aspergillosis from mucormycosis in haematological patients. Whatever the initial clinical site involved, a sinus and chest CT are mandatory in addition to brain imaging. This is of major importance for the indicated therapeutic approach.[112]
The laboratory diagnosis of mucormycosis is challenging and requires expertise and proper sampling. Proper clinical samples include scrapings and aspirates from sinuses, nasal discharges, BAL, needle biopsies from pulmonary lesions, skin scrapings from cutaneous lesions and biopsy tissues. Zygomycetes are almost never isolated from blood and very rarely isolated from cultures of cerebrospinal fluid, sputum, urine or faeces, or swabs of infected areas.[113]
The significance of the isolates grown in cultures may be doubtful, especially when grown from samples of non sterile sites, as they are commonly encountered as contaminants. Histopathologic evidence of fungal invasion of tissue is required to confirm clinical or radiological diagnosis and/or reliability of culture.
The direct microscopic examination of biopsy material in 10%-20% KOH and/or calcofluor-white wet mount shows characteristic broad (6–15 μm in diameter), thin walled, mostly aseptate, ribbon-like hyaline hyphae with almost right –angle branching at irregular intervals. In tissue, Zygomycetes hyphae can be distinguished from the regularly septated hyphae of more common opportunistic molds such as Aspergillus spp. Histopathologic sections occasionally show folded, twisted, and compressed hyphae, which may be mistaken for septated hyphae. Sporangia, the reproductive hyphal structures that contain spores, are seen rarely in tissue in patients infected with zygomycetes. These hyphae are poorly stained with PAS and Gram stains but are very well seen by H&E, GMS, or Periodic-acid Schiff stain. Hyphae may be observed within necrotic tissue and signs of angioinvasion and infarction; neutrophilic infiltrates or granuloma formation may be present in patients who are not granulocytopenic or with more chronic infection, respectively.[114]
Identification of zygomycetes at the genus and species levels requires culture studies, because all members of this group are morphologically similar in tissue. Poor recovery of Zygomycetes may reflect limited septation of the hyphae, making the fungi more liable to damage resulting from tissue excessive grinding. Samples should be kept at room temperature until culture plating, as these fungi may not survive refrigerator temperatures, and excessive grinding should be avoided. The zygomycetes can be easily grown on conventional media like SDA with antibiotics, at temperatures 25o C to 37oC, without cycloheximide as this substance is inhibitory to most of them except A. elegans. If all other media fail, sterile bread without preservatives in a test tube may recover zygomycetes from clinical samples, since these fungi are commonly associated with bread.[115] The rapidly growing mycelia are described as fibrous or cotton-candy.
There are no reliable serologic or skin tests for mucormycosis and the recently introduced antigen tests for Aspergillus (galactomannan) and other fungal species (b-D-glucan) do not detect Zygomycetes because of the limited amount of galactomannan and glucan in their cell walls.[116]
When cultures are negative, molecular identification of zygomycetes from fresh frozen or paraffin embedded tissue can help to confirm diagnosis and identify the fungus to the genus and species level. Different techniques have been reported: DNA probes targeting the ribosomal 18S subunit, ITS1 sequencing after PCR with pan-fungal primers, 18S-targeted semi-nested PCR and real-time PCR targeting cytochrome b gene.[117-122] The identification to the species level of a strain isolated in culture and the identification of a zygomycete in tissue by PCR make the diagnosis much easier. However, at present, there is no standardized method available. Nevertheless, problems remain, since cultures are positive in only 50% of cases 6 and tissue for examination is not always available.
Sequencing of PCR products leads to a presumptive identification of the Zygomycetes, which provides some guidance in selecting antifungal therapy that would not have been available using histopathology alone. Molecular typing is most useful for characterizing zygomycete epidemiology in the setting of a cluster of nosocomial cases, outbreaks, or pseudo-outbreaks, in order to rule out clonal spread or a common infecting source.
Treatment
The prognosis of patients with zygomycosis due to Mucorales without treatment is very poor approaching 100%, depending on the underlying condition and form of mucormycosis.[123] Given the rapid development of the disease, early diagnosis and immediate initiation of treatment is critical for a favourable outcome.[124]
The therapeutic approach to mucormycosis is multimodal, with an equally important three-point strategy that includes (a) antifungal therapy, (b) surgical debridement and (c) correction of the underlying condition predisposing the patient to the disease. Moreover, management of co-morbid factors and adjunctive treatments may improve host response.
Because of the relative rarity of mucormycosis, prospective, comparative studies of antifungal agents and strategies have not been conducted. Therefore, the management of mucormycosis is so far based on the results of case series and case reports, animal model studies and in vitro susceptibility data.
The most frequently used antifungal agents to treat mucormycosis are listed in Table 4.
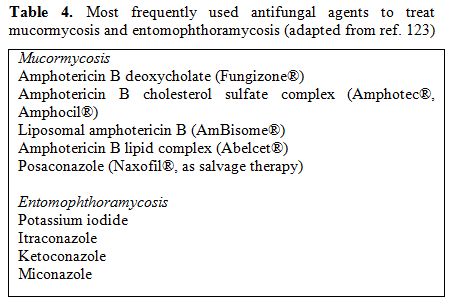
Table 4. Most frequently used antifungal agents to treat mucormycosis and entomophthoramycosis (adapted from ref. 123)
Amphotericin B has the most significant in-vitro activity against Zygomycetes and has been successfully used to treat mucormycosis for many years. Amphotericin B deoxycholate (d-AmB) is the only antifungal agent that has been approved by the US Food and Drug Administration for primary treatment of mucormycosis. However, this formulation has significant toxicity. The newer lipid formulations of amphotericin B that include liposomal AmB (L-AmB), AmB lipid complex (ABLC), and AmB colloidal dispersion (ABCD) are less nephrotoxic and have almost completely replaced amphotericin B deoxycholate in the recent years. It seems reasonable to recommend either L-AmB or ABLC as first-line treatment for mucormycosis.[125-128] The optimal daily dose, as well as the length of treatment, are both undefined yet. Starting dosages of 5–7.5 mg/kg/day for L-AMB and of 5mg/kg/day for ABLC, respectively, are commonly used for adults and children.[129]
Of the azoles only posaconazole has in vitro activity against Zygomycetes and has been shown to be active as salvage therapy.[130,131] Some physicians are using the combination of posaconazole with amphotericin B, but the efficiency of this approach has not been proven in clinical trials.
Zygomycetes are resistant to caspofungin and 5-flucytosine in vitro.[132,133] Itraconazole may have some activity against certain strains of Rhizomucor and Lichtheimia [134] and could be an option especially in cases of cutaneous infection. However, despite rare case reports,[135-138] data are insufficient to support its use as monotherapy of mucormycosis in clinical practice.
There is some evidence that combination of amphotericin B with caspofungin may be active, but these results are preliminary.[139]
Certain Mucorales species have however variable susceptibilities to amphotericin, with MICs ranging from <1 to 100 μg/ml and treatment may be unsuccessful. Moreover, the drug may not reach the target because of tissue infarction and thrombosis.[140] In order to increase oxygen concentration in tissues hyperbaric oxygen has been used as adjunctive therapy in limited studies, but evidence is insufficient to define the efficacy of this expensive intervention.[141]
Another option for the treatment of mucormycosis is the adjunctive use of iron-chelators deferasirox and deferiprone. A clinical trial is currently underway, testing the combination of deferasirox with liposomal amphotericin B.
Granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF and GM-CSF are routinely given to neutropenic patients with mucormycosis or another invasive fungal disease. These two cytokines have also be used in a limited number of cases of mucormycosis in non-neutropenic patients as adjunctive treatment with favorable outcomes.[142,143]
Treating a patient’s underlying medical condition and reducing immunosuppression are essential to therapy. Rapid correction of metabolic abnormalities is mandatory in uncontrolled diabetes. Corticosteroids should be discontinued, if feasible, and other immunosuppressive drugs should be tapered as much as possible.
Diabetics may have a more favourable outcome than non-diabetics. An overall survival rate of 60-77% for rhino-orbito-cerebral mucormycosis has been reported in diabetics, versus 20-34% in non-diabetics.144,145 Combined surgical and medical therapy leads to reduced mortality rates compared to medical treatment alone.6,146
Despite the progress in the treatment of mucormycosis, many problems are yet unsolved and the mortality of the disease is still high.
Conclusions
Depending on the underlying condition of immunocompromised non- haematological patients, mucormycosis can manifest in various clinical forms: mostly as rhino-orbital or rhino-cerebral in diabetes patients, pulmonary infection in patients with malignancy or solid organ transplantation, disseminated infection in iron overloaded or deferoxamine treated patients, cerebral - with no sinus involvement - in ID users, gastrointestinal in premature infants, and cutaneous after direct inoculation in the immunocompetent.
Although there are many unresolved issues concerning the epidemiology, diagnosis and treatment of mucormycosis, advances have been made. Knowledge of the differential patterns of clinical presentation can raise the suspicion index for these rare but highly lethal infections. This is very important since prompt and appropriate management can reduce mortality and morbidity considerably.
The term ‘Zygomycosis’ refers to a group of rare infections caused by hyaline filamentous fungi belonging to the class of Zygomycetes. This class is subdivided in two orders, Mucorales and Entomophthorales, both involved in human disease.[1]
Mucorales are saprobiotic organisms ubiquitous in nature; some members of this order are weak plant parasites or plant pathogens and opportunistic pathogens for man.[2] The vast majority of human zygomycotic disease is caused by genera of the Mucorales order, therefore, the term ‘mucormycosis’ is used interchangeably with the term ‘zygomycosis’ (the term ‘phycomycosis’ has also been used in the past). The most common genera confirmed in human disease are: Rhizopus, Mucor, Lichtheimia (formerly known as Absidia corymbifera or Mycocladus), Rhizomucor, Apophysomyces, Saksenaea, and Cunninghamella.[1]
For Mucorales the portals of entry in the human body are the respiratory tract through inhalation of fungal spores, the skin and less frequently the gut. They grow rapidly and produce wide hyaline, aseptate or poorly septated ribbon-like hyphae in tissues. They invade blood vessels and cause thrombosis and necrosis of the infected tissues. Disease in humans is limited to severely immunocompromised individuals, those with diabetic ketoacidosis, or those with burns or trauma. Infection usually progresses rapidly and the case fatality rate is very high. Depending on the underlying condition and the portal of entry they can cause rhinocerebral, pulmonary, cutaneous, gastrointestinal or even disseminated infection.[1]
Human infections caused by Entomophthorales, which are mainly insect pathogens, constitute a completely different clinical entity (entomopfthoramycoses). Genera involved in human disease are Conidiobolus and Basidiobolus. They affect mainly immunocompetent individuals, are prevalent in the tropics and do not disseminate.[3] In recent years the geographic distribution of basidiobolomycosis expanded and is reported also in immunocompromised hosts involving more tissues, but these cases are extremely rare.[4,5] Therefore, the present review focuses only on the zygomycotic infections caused by mucorales.
The most important risk factors for mucormycosis include prolonged neutropenia, diabetic ketoacidosis, iron overload and major trauma. Other important predisposing factors of mucormycosis include prolonged use of corticosteroids, illicit intravenous drug use, neonatal prematurity, malnourishment and broad-spectrum antimicrobial agents, as well as antifungal agents with no activity against zygomycetes, such as voriconazole and caspofungin.[6,7] The same may also be true for the other echinocandins, but data are lacking as they are not as widely used as caspofungin. Risk factors, mechanisms leading to mucormycosis and clinical presentation forms are illustrated in Table 1.

Table 1. Predisposing conditions for mucormycosis, pathogenetic mechanisms and clinical presentation.
The recent years, mucormycosis incidence is seemingly increasing, particularly in patients with haematological malignancies or haematopoietic stem cell transplantation, at least in western countries.[8,9,10] However, accurate estimation of the incidence is a challenge. Data from a global registry are not available yet and moreover there are difficulties in collecting well defined denominator data. Much information has been derived from the first most comprehensive study by Roden et al. 2005, [6] in which all data on zygomycosis recorded in the English language literature ever since the first report by Platauf in 1885,[11] were analyzed. More recent data from individual countries and institutions have also been reported [12-17] and percentages of clinical cases according to underlying condition are presented in Table2.

Table 2. Percentages of mucormycosis cases according to underlying conditions. Data from 7 studies.
Underlying Conditions
Diabetes – Ketoacidosis: Diabetes mellitus as a predisposing factor has been reported in 36–88% of all mucormycosis cases.[6,18-20] More susceptible are patients with uncontrolled hyperglycemia, particularly those with ketoacidosis.[21,22,23] Mucormycosis can also be observed in metabolically controlled diabetic patients24 and was found to be the first clinical manifestation of some patients with undiagnosed diabetes mellitus.[25] Type 1, type 2, and secondary diabetes mellitus have all been reported as risk factors in patients with mucormycosis.[26]
In the normal host protection from mucormycosis is provided by macrophages. They prevent the initiation of infection by phagocytosis and oxidative killing of the fungal spores. In case infection is established, neutrophils are activated and play a pivotal role in fungal killing. Activation happens in four phases:
a) neutrophils are chemotactically attracted to the hyphae,
b) they attach and
c) spread on the hyphae and finally,
d) using their oxidative cytotoxic system, neutrophils damage and kill the fungal elements without accompanying phagocytosis.
In diabetes, each of the four phases of neutrophil activation is impaired.[27] Hyperglycemia per se does not seem to play a major role in the pathogenesis of mucormycosis. Chemotaxis, the phagocytic functions (adherence and spreading), and finally the oxidative burst are all inhibited in the ketoacidotic state, essentially inducing functional neutropenia.[26-29]
The most common clinical presentation of mucormycosis in patients with diabetes mellitus is sinus disease (66%).6 Diabetes mellitus was recognized as a major risk factor for developing rhinocerebral mucormycosis very early on. The publication by Gregory et al. in 1943 on a case of uncontrolled diabetes was the first to describe fulminant rhinocerebral mucormycosis.[30]
Disease in diabetics can also present as pulmonary mucormycosis, a clinical presentation more common in neutropenic patients with malignancies or HSCT.[6]
The epidemiology in diabetic patients is variable. Roden et al.[6] showed that diabetic patients represented 36% of the 929 reported cases. They also reported a decreased incidence of mucormycosis in diabetic patients over time. This is in contrast to the increased prevalence of diabetes in the world.[31] The role of statins, used at least in the western world, has been speculated in an effort to explain this phenomenon.[32,33] In France, however, a population- based study of medical records of mucormycosis cases reported from 1997 to 2006 showed a 9% yearly increase in the annual incidence rate in the diabetic population.[12] Another retrospective study from the USA, reviewing cases with rhino-orbital-cerebral mucormycosis from two teaching tertiary-care hospitals revealed that 83% of cases were observed in diabetic patients and more importantly, 41% of them had no known history of their condition.[34] A striking finding in this study was that 56% of the patients were of Hispanic origin, a much larger proportion than that in the general population (≈25% expected incidence of Hispanic ethnicity based on demographic characteristics at the study hospitals). Data from a tertiary-care centre in India35 were also overwhelming, as 73.6% of cases were observed in patients with uncontrolled diabetes and in 42.7% of these cases diabetes was diagnosed for the first time. These findings also underline the association between diabetes and socioeconomic status. Low income individuals have the tendency not to seek medical attention as healthcare is unaffordable to them, unless complications manifest. Almost 80% of type 2 diabetes deaths occur in low- and middle-income countries (WHO Fact sheet: Diabetes, ref.36). With diabetes control programs in place, also many cases of mucormycosis could be prevented.
The overall mortality of diabetic patients with mucormycosis who undergo treatment is approximately 44%, and this is lower compared to other immunocompromising conditions.[6] This can be explained by the relatively easy management of acute complications of diabetes compared to the management of other conditions. This percentage can be further decreased with improved management. [37]
Besides diabetic ketoacidosis, chronic metabolic acidosis due to other causes, such as chronic renal failure with uremia, [38] chronic salicylate poisoning, [39] and methylmalonicaciduria,[40] has also been reported as a risk factor for mucormycosis.
Iron overload and Deferoxamine (DFO) iron/aluminium chelation therapy: Many microorganisms require iron to grow, and conditions of iron excess have been known to predispose to infection with certain bacteria.[41,42] Iron acquisition is a critical step in the pathogenetic mechanism of mucormycosis.[43]
Therapy with DFO, an iron chelator used for the treatment of iron and/or aluminum overload in dialysis patients, was found paradoxically to be a risk factor for angioinvasive mucormycosis.[44,45] Although the first dialysis patient with mucormycosis, reported in 1979 by Gluskin et al,[46] was not noted to be receiving DFO, subsequent cases of mucormycosis in patients undergoing dialysis have been in those receiving DFO for aluminum or iron excess.[45] A report of an international registry [47] showed that 78% of dialysis patients with mucormycosis were being treated with DFO. In addition to patients with renal failure, patients with hematologic disorders, all of whom were treated with DFO, have been reported with mucormycosis.[45] Subsequent research showed that DFO can act as a siderophore for Mucorales: DFO forms a Fe-DFO complex with iron, which then binds to unidentified receptors on the surface of the zygomycetes. The iron is subsequently liberated by the fungus and is likely transported into the fungal cell by a high-affinity iron permease (rFTR1).[43] The increased sensitivity of dialysis patients to DFO-related mucormycosis is explained by the pharmacokinetic changes during uremia, which lead to a prolonged accumulation of Fe-DFO after DFO administration.[48]
Besides DFO, iron overload per se, either transfusional or due to dyserythropoiesis has also been recognized as a risk factor for mucormycosis.[49–51] In vitro and in vivo studies have shown that iron and DFO can enhance both growth and pathogenicity of Rhizopus spp. In a study reviewing a series of five cases of invasive mucormycosis among 263 allogeneic bone marrow transplant recipients,[51] the association between severe iron overload and mucormycosis was demonstrated. The mean values of serum ferritin level, transferrin saturation, and number of transfused units of erythrocytes in the study group were significantly higher compared with the matched control group.
Patients with diabetic ketoacidosis have elevated levels of available serum iron, likely due to the release of iron from binding proteins in the presence of low pH. Iron is then internalized by the zygomycetes with the help of copper oxidase (Cu-oxidase) and the high affinity iron permease rFTR1. Therefore, the increased susceptibility of patients with diabetic ketoacidosis to mucormycosis is likely due, at least in part, to an elevation in available serum iron during diabetic ketoacidosis following proton-mediated dissociation of iron from transferrin.[43]
The most common presentation of mucormycosis in patients receiving DFO appears to be the disseminated form (44%) and is associated with high mortality, reaching 80%.[6,47]
In contrast to DFO, two other iron chelators, deferiprone and deferasirox, do not supply iron to the fungus. This could be either because of their different structures and smaller size, rendering them inaccessible to fungal iron uptake systems, or because they share higher affinity constants for iron.[52] Deferiprone and deferasirox were shown to have fungicidal activity against Zygomycetes in vitro. Further, both iron chelators were shown to effectively treat mucormycosis in animal models, and one has been successfully used as salvage therapy for a patient with rhinocerebral mucormycosis.[43]
Solid organ malignancies and solid organ transplantation (SOT): The incidence of mucormycosis in transplant recipients, including both hematopoietic stem cell transplant (HSCT) and solid organ transplant (SOT) patients has increased. Although global surveys of the incidence of mucormycosis are still lacking, data from individual countries and institutions show that mucormycosis in SOT is rare, but seems to be a concern because of the high mortality rate.
The estimated incidence ranges from 0.4 – 16%.[6,12,52,53] Data from the Transplant Associated Infection Surveillance Network (TRANSNET) showed that among 1063 solid organ transplant recipients mucormycosis represented 2% of invasive fungal infections. Neutropenia predisposing to mucormycosis [6] was notably absent in SOT recipients as were acidosis with or without hyperglycemia and use of DFO. In contrast, all patients were receiving immunosuppression and the overwhelming majority was on corticosteroids. Furthermore, dissemination to distant organs occurred more frequently after rejection and its treatment.
The lung seems to be the most common site of infection in SOT recipients with mucormycosis.[6,54] Dissemination occurred preferentially to cutaneous and soft tissues, and not the brain. Mycocladus (Lichtheimia) corymbifer as causative pathogen appeared to be associated with a higher risk for dissemination in SOT with pulmonary mucormycosis in one study.[55]
In a matched case-controlled study [54] SOT recipients with mucormycosis were prospectively studied. Renal failure, diabetes mellitus, and prior voriconazole and/or caspofungin use were associated with a higher risk, whereas tacrolimus was associated with a lower risk of mucormycosis. Liver transplant recipients were more likely to have disseminated disease and developed mucormycosis earlier after transplantation than did other SOT recipients (median, 0.8 versus 5.7 months).
HIV/AIDS - Intravenous drug abuse: Mucormycosis in HIV/AIDS patients is very rare. Antinori et al.[56] in a large retrospective study of 1630 autopsies of patients who died of AIDS during 1984 through 2002, found only 2 cases with mucormycosis. However, it can be the presenting opportunistic infection in AIDS.[57]
Recently, human immunodeficiency virus (HIV) infection has been recognized as a risk factor for mucormycosis, but most cases in HIV-infected patients are also associated with intravenous drug abuse.[58,59,60,61] Cerebral mucormycosis with basal ganglia involvement is the usual clinical presentation in such cases, regardless of previous exposure to HIV.[62] The disease may develop insidiously or may progress rapidly with a fulminant course.
Endocarditis as well as the rhinocerebral form can also occur. Merchant et al.[63] reported on a patient with rhinocerebral mucormycosis and also reviewed another 6 cases reported in the literature. It is of note that 3 of them had hyperglycemia or diabetic ketoacidosis.
Mucormycosis in the HIV+ can also present in the kidneys, the skin, the gastrointestinal tract, the respiratory tract, or may be disseminated.[64-75] How the fungus enters the body is not very clear. The fact that most of the infections occur at sites remote from the needle stick however, suggest that most probably this happens through spores contained in the illicit drugs.[1]
Corticosteroids/Rheumatic diseases: Corticosteroid therapy is another primary risk factor that enhances a patient’s susceptibility to mucormycosis by causing either defects in macrophages and neutrophils or steroid-induced diabetes.[1]
Corticosteroids have a wide range of complex immunosuppressive effects, mainly by affecting cellular immunity, and increase host susceptibility to invasive fungal infections.[76] The mechanism by which the corticosteroids enhance susceptibility to developing mucormycosis is probably twofold. First, steroids suppress the normal inflammatory cell response that would otherwise occur, and second, they may induce a diabetic state.[77] In a recent study only 21% of patients with corticosteroid induced diabetes were receiving medication for diabetes.[34]
Of the few cases of mucormycosis in patients with lupus erythematosus (SLE) reported in English language literature[78-93] it appears that disease can present with any clinical form, but disseminated mucormycosis is usual in this group of patients. Mok et al.[78] reviewed all SLE cases published from 1970 through 2002 and reported a very high overall mortality of mucormycosis (88%). Additional predisposing factors for opportunistic infection included hypocomplementemia, nephrotic syndrome, uremia, leukopenia, and diabetes mellitus. The diagnosis often was made only at autopsy (63%). The cutaneous form appeared to have the best prognosis with combined medical and surgical treatment.[78]
Mucormycosis in other autoimmune diseases are scarce, but in cases such as in Wegener’s granulomatosis it may mimic disease relapse and remain underdiagnosed.[94]
No underlying disease: A considerable proportion of patients with mucormycosis have no apparent immune deficiency.[6] These patients have usually primary mucormycosis of the skin associated with burns, trauma, insect bites, tattooing and also complicating surgical wounds and catheter insertion sites. Recently, Skiada and Petrikkos reviewed 67 cases of cutaneous mucormycosis in English language literature, from 2004 -2008.[95] Most of the patients had an underlying immunocompromising condition such as haematologic malignancy, diabetes, or solid organ transplantation, but the immunocompetent represented a large proportion (40%).
Infection usually occurs through direct inoculation. It can be very invasive locally, penetrating the cutaneous and subcutaneous tissues into the adjacent fat, muscle, fascia and bone. In rare cases it can even disseminate to deep organs. Road traffic accidents, crush injuries, but also minor traumas such as those caused by plant thorns or insect bites, can lead to traumatic implantation of contaminated soil and subsequent mucormycosis. Major burn injury is another well described cause of cutaneous mucormycosis. An increased risk for developing mucormycosis in burn patients is the development of a condition called “burned stressed pseudodiabetes”, which is marked by hyperglycemia and glycosuria.[96,97]
In a new PubMed search from 2008 - 2010, we found 27 additional cases of cutaneous mucormycosis occurring in non immunocompromised hosts.[98-110] Risk factors in these cases included soil contaminated wounds, insect bites, burns, but the majority of cases (N=12) were iatrogenic, either after surgery or at injection sites. Infections with Mucorales occurring in nosocomial settings have been repeatedly reported and have even caused small hospital outbreaks in the USA, the UK and in Europe.[111] Sources of infection included contaminated bandages, Elastopad adhesive dressings, wooden tongue depressors and non-sterile karaya ostomy bags.
The clinical presentation is variable. The clinical signs initially are non specific, consisting of erythema and induration. Eventually the lesions may form blisters, pustules, or necrotic ulcerations. Cotton wool-like material may also be observed on the margins of the wound. The lesions may mimic pyoderma gangrenosum, bacterial synergistic gangrene or infections produced by Pseudomonas, Aspergillus, Histoplasma etc. Biopsy specimens for histology and cultures are necessary to establish diagnosis.
Strains involved are usually Rhizopus spp. (the majority), Lichtheimia corymbifera (syn. Absidia, Mycocladus), Apohysomyces elegans, Mucor spp., Cunninghamella bertholetiae and Saksenaea vasiformis. In the cases reported after 2008 the cultured species were R. arrhizus in 3 cases,[99,101] Lichtheimia spp.in [5,98,100,107,109] A. elegans in 4,[98] S. vasiformis in 3,[98,102,110] and Rhizomucor variabilis in 7.[103-104] The later, a non-thermophile species of the genus Rhizomucor, in contrast to other mucorales can cause large but slowly progressing opportunistic infections of the skin in immune-competent individuals. These fungi seem to have an increasing incidence in farmers from China.
The prognosis of mucormycosis in the immunocompetent is better than that in other patient groups but mortality remains high depending on the site, extension of infection and time of initiation of antifungal therapy. Iatrogenic cutaneous mucormycosis could easily be prevented with proper preoperative preparation and postoperative dressing of the surgical sites.
Diagnosis
The clinical diagnosis of mucormycosis among non haematological immunocompromised patients is notoriously difficult because of the similarity with aspergillosis or other mycoses caused by filamentous fungi. Diagnosis is usually delayed, as the majority of the physicians are not so much familiar with the disease. The leading signs and symptoms of mucormycosis are presented in Table 3.

Table 3. Clinical forms and relevant signs and symptoms
When clinical presentation may suggest mucormycosis, imaging techniques are helpful, but cultures and histopathology are required for confirmation.
In case of rhinocerebral involvement, CT and particularly MRI are helpful in enabling an early detection of orbital, sinus, meningeal, bone, and cerebral lesions as well as intracranial vascular occlusion even before clinical signs develop. In pulmonary mucormycosis, lung biopsies (endoscopic, CT-guided or surgical) should be performed, depending on the radiological findings obtained by CT scans. CT guided percutaneous lung biopsy has been found highly efficient to early differentiate aspergillosis from mucormycosis in haematological patients. Whatever the initial clinical site involved, a sinus and chest CT are mandatory in addition to brain imaging. This is of major importance for the indicated therapeutic approach.[112]
The laboratory diagnosis of mucormycosis is challenging and requires expertise and proper sampling. Proper clinical samples include scrapings and aspirates from sinuses, nasal discharges, BAL, needle biopsies from pulmonary lesions, skin scrapings from cutaneous lesions and biopsy tissues. Zygomycetes are almost never isolated from blood and very rarely isolated from cultures of cerebrospinal fluid, sputum, urine or faeces, or swabs of infected areas.[113]
The significance of the isolates grown in cultures may be doubtful, especially when grown from samples of non sterile sites, as they are commonly encountered as contaminants. Histopathologic evidence of fungal invasion of tissue is required to confirm clinical or radiological diagnosis and/or reliability of culture.
The direct microscopic examination of biopsy material in 10%-20% KOH and/or calcofluor-white wet mount shows characteristic broad (6–15 μm in diameter), thin walled, mostly aseptate, ribbon-like hyaline hyphae with almost right –angle branching at irregular intervals. In tissue, Zygomycetes hyphae can be distinguished from the regularly septated hyphae of more common opportunistic molds such as Aspergillus spp. Histopathologic sections occasionally show folded, twisted, and compressed hyphae, which may be mistaken for septated hyphae. Sporangia, the reproductive hyphal structures that contain spores, are seen rarely in tissue in patients infected with zygomycetes. These hyphae are poorly stained with PAS and Gram stains but are very well seen by H&E, GMS, or Periodic-acid Schiff stain. Hyphae may be observed within necrotic tissue and signs of angioinvasion and infarction; neutrophilic infiltrates or granuloma formation may be present in patients who are not granulocytopenic or with more chronic infection, respectively.[114]
Identification of zygomycetes at the genus and species levels requires culture studies, because all members of this group are morphologically similar in tissue. Poor recovery of Zygomycetes may reflect limited septation of the hyphae, making the fungi more liable to damage resulting from tissue excessive grinding. Samples should be kept at room temperature until culture plating, as these fungi may not survive refrigerator temperatures, and excessive grinding should be avoided. The zygomycetes can be easily grown on conventional media like SDA with antibiotics, at temperatures 25o C to 37oC, without cycloheximide as this substance is inhibitory to most of them except A. elegans. If all other media fail, sterile bread without preservatives in a test tube may recover zygomycetes from clinical samples, since these fungi are commonly associated with bread.[115] The rapidly growing mycelia are described as fibrous or cotton-candy.
There are no reliable serologic or skin tests for mucormycosis and the recently introduced antigen tests for Aspergillus (galactomannan) and other fungal species (b-D-glucan) do not detect Zygomycetes because of the limited amount of galactomannan and glucan in their cell walls.[116]
When cultures are negative, molecular identification of zygomycetes from fresh frozen or paraffin embedded tissue can help to confirm diagnosis and identify the fungus to the genus and species level. Different techniques have been reported: DNA probes targeting the ribosomal 18S subunit, ITS1 sequencing after PCR with pan-fungal primers, 18S-targeted semi-nested PCR and real-time PCR targeting cytochrome b gene.[117-122] The identification to the species level of a strain isolated in culture and the identification of a zygomycete in tissue by PCR make the diagnosis much easier. However, at present, there is no standardized method available. Nevertheless, problems remain, since cultures are positive in only 50% of cases 6 and tissue for examination is not always available.
Sequencing of PCR products leads to a presumptive identification of the Zygomycetes, which provides some guidance in selecting antifungal therapy that would not have been available using histopathology alone. Molecular typing is most useful for characterizing zygomycete epidemiology in the setting of a cluster of nosocomial cases, outbreaks, or pseudo-outbreaks, in order to rule out clonal spread or a common infecting source.
Treatment
The prognosis of patients with zygomycosis due to Mucorales without treatment is very poor approaching 100%, depending on the underlying condition and form of mucormycosis.[123] Given the rapid development of the disease, early diagnosis and immediate initiation of treatment is critical for a favourable outcome.[124]
The therapeutic approach to mucormycosis is multimodal, with an equally important three-point strategy that includes (a) antifungal therapy, (b) surgical debridement and (c) correction of the underlying condition predisposing the patient to the disease. Moreover, management of co-morbid factors and adjunctive treatments may improve host response.
Because of the relative rarity of mucormycosis, prospective, comparative studies of antifungal agents and strategies have not been conducted. Therefore, the management of mucormycosis is so far based on the results of case series and case reports, animal model studies and in vitro susceptibility data.
The most frequently used antifungal agents to treat mucormycosis are listed in Table 4.

Table 4. Most frequently used antifungal agents to treat mucormycosis and entomophthoramycosis (adapted from ref. 123)
Amphotericin B has the most significant in-vitro activity against Zygomycetes and has been successfully used to treat mucormycosis for many years. Amphotericin B deoxycholate (d-AmB) is the only antifungal agent that has been approved by the US Food and Drug Administration for primary treatment of mucormycosis. However, this formulation has significant toxicity. The newer lipid formulations of amphotericin B that include liposomal AmB (L-AmB), AmB lipid complex (ABLC), and AmB colloidal dispersion (ABCD) are less nephrotoxic and have almost completely replaced amphotericin B deoxycholate in the recent years. It seems reasonable to recommend either L-AmB or ABLC as first-line treatment for mucormycosis.[125-128] The optimal daily dose, as well as the length of treatment, are both undefined yet. Starting dosages of 5–7.5 mg/kg/day for L-AMB and of 5mg/kg/day for ABLC, respectively, are commonly used for adults and children.[129]
Of the azoles only posaconazole has in vitro activity against Zygomycetes and has been shown to be active as salvage therapy.[130,131] Some physicians are using the combination of posaconazole with amphotericin B, but the efficiency of this approach has not been proven in clinical trials.
Zygomycetes are resistant to caspofungin and 5-flucytosine in vitro.[132,133] Itraconazole may have some activity against certain strains of Rhizomucor and Lichtheimia [134] and could be an option especially in cases of cutaneous infection. However, despite rare case reports,[135-138] data are insufficient to support its use as monotherapy of mucormycosis in clinical practice.
There is some evidence that combination of amphotericin B with caspofungin may be active, but these results are preliminary.[139]
Certain Mucorales species have however variable susceptibilities to amphotericin, with MICs ranging from <1 to 100 μg/ml and treatment may be unsuccessful. Moreover, the drug may not reach the target because of tissue infarction and thrombosis.[140] In order to increase oxygen concentration in tissues hyperbaric oxygen has been used as adjunctive therapy in limited studies, but evidence is insufficient to define the efficacy of this expensive intervention.[141]
Another option for the treatment of mucormycosis is the adjunctive use of iron-chelators deferasirox and deferiprone. A clinical trial is currently underway, testing the combination of deferasirox with liposomal amphotericin B.
Granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF and GM-CSF are routinely given to neutropenic patients with mucormycosis or another invasive fungal disease. These two cytokines have also be used in a limited number of cases of mucormycosis in non-neutropenic patients as adjunctive treatment with favorable outcomes.[142,143]
Treating a patient’s underlying medical condition and reducing immunosuppression are essential to therapy. Rapid correction of metabolic abnormalities is mandatory in uncontrolled diabetes. Corticosteroids should be discontinued, if feasible, and other immunosuppressive drugs should be tapered as much as possible.
Diabetics may have a more favourable outcome than non-diabetics. An overall survival rate of 60-77% for rhino-orbito-cerebral mucormycosis has been reported in diabetics, versus 20-34% in non-diabetics.144,145 Combined surgical and medical therapy leads to reduced mortality rates compared to medical treatment alone.6,146
Despite the progress in the treatment of mucormycosis, many problems are yet unsolved and the mortality of the disease is still high.
Conclusions
Depending on the underlying condition of immunocompromised non- haematological patients, mucormycosis can manifest in various clinical forms: mostly as rhino-orbital or rhino-cerebral in diabetes patients, pulmonary infection in patients with malignancy or solid organ transplantation, disseminated infection in iron overloaded or deferoxamine treated patients, cerebral - with no sinus involvement - in ID users, gastrointestinal in premature infants, and cutaneous after direct inoculation in the immunocompetent.
Although there are many unresolved issues concerning the epidemiology, diagnosis and treatment of mucormycosis, advances have been made. Knowledge of the differential patterns of clinical presentation can raise the suspicion index for these rare but highly lethal infections. This is very important since prompt and appropriate management can reduce mortality and morbidity considerably.
References
- Ribes J.A., Vanover-Sams C. L. and Baker D.
J. Zygomycetes in Human Disease. Clinical Microbiology Reviews, April
2000, p. 236-301, Vol. 13, No. 2.

- De Hoog, J. Guarro, J. Gené and M.J. Figueras, Atlas of clinical fungi (2nd ed.), Centraalbureau voor Schimmelcultures / Universitat Rovira i Virgili, Utrecht 2000. Pp. 81-123.
- Drouhet E, Ravisse P. Entomophthoromycosis.
Curr Top Med Mycol. 1993;5:215-45.

- Dworzack, D. L., A. S. Pollok, G. R.
Hodges, W. C. Barnes,
L. Ajello and A. Padhye. 1978. Zygomycosis of the maxillary sinus and
palate caused by Basidiobolus haptosporus. Arch. Intern. Med.
138:1274–1276. doi:10.1001/archinte.138.8.1274
PMid:567045

- Roy, A. D., and H. M. Cameroon. 1972.
Rhinophycomycosis
entomophthorae occurring in a chimpanzee in the wild in East Africa.
Am. J. Trop. Med. Hyg. 21:234–237. PMid:5062225

- Roden MM, Zaoutis TE, Buchanan WL, Knudsen
TA, Sarkisova
TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP,
Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929
reported cases. Clin Infect Dis. 2005 Sep 1;41:634-53. Epub 2005 Jul
29. doi:10.1086/432579
PMid:16080086

- Ramos A, Cuervas-Mons V, Noblejas A, Baños
I, Duran P,
Marcos R, Sánchez-Turrión V, Jiménez M, Arellano B, Corbacho C,
Sánchez-Romero I. Breakthrough rhinocerebral mucormycosis in a liver
transplant patient receiving caspofungin. Transplant Proc. 2009
Jun;41:1972-5. doi:10.1016/j.transproceed.2009.01.077
PMid:19545771

- Spellberg B, Edwards J Jr, Ibrahim A. Novel
perspectives on
mucormycosis: pathophysiology, presentation, and management. Clin
Microbiol Rev 2005;18:556–69 doi:10.1128/CMR.18.3.556-569.2005
PMid:16020690 PMCid:1195964

- Prabhu RM, Patel R. Mucormycosis and
entomophthoramycosis: a
review of the clinical manifestations, diagnosis and treatment. Clin
Microbiol Infect 2004 Mar;10 Suppl
1:31-47.doi:10.1111/j.1470-9465.2004.00843.x PMid:14748801

- Chakrabarti A, Das A, Mandal J, et al. The
rising trend of
invasive zygomycosis in patients with uncontrolled diabetes mellitus.
Med Mycol 2006; 44:335-42. doi:10.1080/13693780500464930
PMid:16772227

- Platauf, A. P. (1885). Mycosis mucorina. Virchows Archiv: An International Journal of Pathology 102, 543–64.doi:10.1007/BF01932420
- Bitar D, Van Cauteren D, Lanternier F,
Dannaoui E, Che D,
Dromer F, Desenclos JC, Lortholary O. Increasing incidence of
zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis. 2009
Sep;15:1395-401. doi:10.3201/eid1509.090334
PMid:19788806
PMCid:2819884

- Zygomycosis in Italy: a survey of
FIMUA-ECMM (Federazione
Italiana di Micopatologia Umana ed Animale and European Confederation
of Medical Mycology). Pagano L, Valentini CG, Posteraro B, Girmenia C,
Ossi C, Pan A, Candoni A, Nosari A, Riva M, Cattaneo C, Rossini F,
Fianchi L, Caira M, Sanguinetti M, Gesu GP, Lombardi G, Vianelli N,
Stanzani M, Mirone E, Pinsi G, Facchetti F, Manca N, Savi L, Mettimano
M, Selva V, Caserta I, Scarpellini P, Morace G, D'Arminio Monforte A,
Grossi P, Giudici D, Tortorano AM, Bonini A, Ricci L, Picardi M,
Rossano F, Fanci R, Pecile P, Fumagalli L, Ferrari L, Capecchi PL,
Romano C, Busca A, Barbui A, Garzia M, Minniti RR, Farina G, Montagna
MT, Bruno F, Morelli O, Chierichini A, Placanica PM, Castagnola E,
Bandettini R, Giordano S, Monastero R, Tosti ME, Rossi MR, Spedini P,
Piane R, Nucci M, Pallavicini F, Bassetti M, Cristini F, LA Sorda M,
Viviani M. J Chemother. 2009 Jun;21:322-9. PMid:19567354

- Saegeman V, Maertens J, Meersseman W,
Spriet I, Verbeken E,
Lagrou K. Increasing incidence of mucormycosis in university hospital,
Belgium. Emerg Infect Dis. 2010 Sep;16:1456-8. doi:10.3201/eid1609.100276 PMid:20735932

- Rüping MJ, Heinz WJ, Kindo AJ, Rickerts V,
Lass-Flörl C,
Beisel C, Herbrecht R, Roth Y, Silling G, Ullmann AJ, Borchert K,
Egerer G, Maertens J, Maschmeyer G, Simon A, Wattad M, Fischer G,
Vehreschild JJ, Cornely OA. Forty-one recent cases of invasive
zygomycosis from a global clinical registry. J Antimicrob Chemother.
2010 Feb;65:296-302. doi:10.1093/jac/dkp430 PMid:20008047

- Skiada A, Pagano L, Groll A, Zimmerli S,
Dupont B, Lagrou
K, Lass-Florl C, Bouza E, Klimko N, Gaustad P, Richardson M, Hamal P,
Akova M, Meis JF, Rodriguez-Tudela JL, Roilides E, Mitrousia-Ziouva A,
Petrikkos G; for the European Confederation of Medical Mycology Working
Group on Zygomycosis. Zygomycosis in Europe: Analysis of 230 cases
accrued by the registry of the European Confederation of Medical
Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007.
Clin Microbiol Infect. 2011 Jan 2. doi:
10.1111/j.1469-0691.2010.03456.x. [Epub ahead of print] doi:10.1111/j.1469-0691.2010.03456.x
PMid:21199154

- Chakrabarti A, Chatterjee SS, Das A, Panda
N, Shivaprakash
MR, Kaur A, Varma SC, Singhi S, Bhansali A, Sakhuja V. Invasive
zygomycosis in India: experience in a tertiary care hospital. Postgrad
Med J. 2009 Nov;85(1009):573-81.doi:10.1136/pgmj.2008.076463
PMid:19892892

- Nithyanandam S, Jacob MS, Battu RR, Thomas
RK, Correa MA,
D’Souza O (2003) Rhino-orbito-cerebral mucormycosis. A retrospective
analysis of clinical features and treatment outcomes. Indian J
Ophthalmol 51:231–236 PMid:14601848

- Joshi N, Caputo GM, Weitekamp MR, Karchmer
AW (1999)
Infections in patients with diabetes mellitus. N Engl J Med
341:1906–1912. doi:10.1056/NEJM199912163412507 PMid:10601511

- M. Chayakulkeeree . M. A. Ghannoum . J. R.
Perfect.
Zygomycosis: the re-emerging fungal infection. Eur J Clin Microbiol
Infect Dis. 2006; 25:215-29.doi:10.1007/s10096-006-0107-1
PMid:6756909

- Sugar AM (2005) Agents of mucormycosis and related species. In: Mandell GL, Bennett JE, Dolin R (eds). Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Elsevier Churchill Livingstone, Philadelphia, pp 2973–2984.
- Gonzalez CE, Rinaldi MG, Sugar AM (2002)
Zygomycosis. Infect Dis Clin North Am 16:895–914. doi:10.1016/S0891-5520(02)00037-5

- Greenberg RN, Scott LJ, Vaughn HH, Ribes
JA (2004).
Zygomycosis (mucormycosis): emerging clinical importance and new
treatments. Curr Opin Infect Dis
17:517–525.doi:10.1097/00001432-200412000-00003
PMid:15640705

- Helderman JH, Cooper HS, Mann J (1974)
Chronic phycomycosis in a controlled diabetic. Ann Intern Med 80:419.
PMid:4816193

- Bhansali A, Bhadada S, Sharma A, Suresh V,
Gupta A, Singh
P, Chakarbarti A, Dash RJ (2004) Presentation and outcome of
rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad
Med J 80:670–674. doi:10.1136/pgmj.2003.016030 PMid:15537854
PMCid:1743145

- Bagdade, J. D. 1976. Phagocytic and
microbiocidal function in diabetes mellitus. Acta Endocrinol. 83:27–33.

- Bybee, J. D., and D. E. Rogers. 1964. The
phagocytic
activity of polymorphonuclear leukocytes obtained from patients with
diabetes mellitus. J. Lab. Clin. Med. 64:1–13. PMid:14192564

- Mowat, A. G., and J. Baum. 1971.
Chemotaxis of
polymorphonuclear leukocytes from patients with diabetes mellitus. N.
Engl. J. Med. 284:621–627. doi:10.1056/NEJM197103252841201 PMid:5545603

- Sheldon, W. H., and H. Bauer. 1959. The
development of the
acute inflammatory response to experimental cutaneous mucormycosis in
normal and diabetic rabbits. J. Exp. Med.
110:845–852. doi:10.1084/jem.110.6.845
PMid:14445772 PMCid:2137039

- Gregory, J. E., A. Golden, and W. Haymaker. 1943. Mucormycosis of the central nervous system: a report of three cases. Bull. Johns Hopkins Hosp.73:405–419.
- Ludvigsson J. Why diabetes incidence
increases--a unifying
theory. Ann N Y Acad Sci. 2006 Oct;1079:374-82. doi:10.1196/annals.1375.058 PMid:17130582

- Kontoyiannis DP. Decrease in the number of
reported cases
of zygomycosis among patients with diabetes mellitus: a hypothesis.
Clin Infect Dis. 2007 Apr 15;44:1089-90. doi:10.1086/512817
PMid:17366455

- Chamilos G, Lewis RE, Kontoyiannis DP.
Lovastatin has
significant activity against zygomycetes and interacts synergistically
with voriconazole. Antimicrob Agents Chemother. 2006 Jan;50:96-103. doi:10.1128/AAC.50.1.96-103.2006
PMid:16377673 PMCid:1346800

- Reed C, Bryant R, Ibrahim AS, Edwards J
Jr, Filler SG,
Goldberg R, Spellberg B. Combination polyene-caspofungin treatment of
rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008 Aug
1;47:364-71. doi:10.1086/589857
PMid:18558882 PMCid:2793535

- Chakrabarti A, Das A, Mandal J,
Shivaprakash MR, George VK,
Tarai B, Rao P, Panda N, Verma SC, Sakhuja V. The rising trend of
invasive zygomycosis in patients with uncontrolled diabetes mellitus.
Med Mycol. 2006 Jun;44:335-42. doi:10.1080/13693780500464930

- PMid:16772227 http://www.who.int/mediacentre/factsheets/fs312/en/index.html
- Perlroth J, Choi B, Spellberg B.
Nosocomial fungal
infections: epidemiology, diagnosis, and treatment. Med Mycol. 2007
Jun;45:321-46. doi:10.1080/13693780701218689
PMid:17510856

- Gupta KL, Radotra BD, Sakhuja V, Banerjee
AK, Chugh KS
(1989) Mucormycosis in patients with renal failure. Ren Fail 11:195–199
doi:10.3109/08860228909054931
PMid:2485482

- Espinoza CG, Halkias DG (1983) Pulmonary
mucormycosis as a
complication of chronic salicylate poisoning. Am J Clin Pathol
80:508–511 PMid:6624716

- Lewis LL, Hawkins HK, Edwards MS (1990)
Disseminated
mucormycosis in an infant with methylmalonicaciduria. Pediatr Infect
Dis J 9:851–854. doi:10.1097/00006454-199011000-00016

- Bullen J J: The significance of iron in
infection. Rev Infect Dis 1981; 3: 1127- 1138. doi:10.1093/clinids/3.6.1127

- Payne SM: Iron and virulence in the family
Enterobacteriaceae. CRC Crit Rev Microbio] 1988; 16: 81-111. doi:10.3109/10408418809104468 PMid:3067977

- Ibrahim AS, Spellberg B, Edwards J Jr.
Iron acquisition: a
novel perspective on mucormycosis pathogenesis and treatment. Curr Opin
Infect Dis. 2008 Dec;21:620-5. doi:10.1097/QCO.0b013e3283165fd1
PMid:18978530 PMCid:2773686

- Artis WM, Fountain JA, Delcher HK, Jones
HE: A mechanism of
susceptibility to mucormycosis in diabetic ketoacidosis: transferrin
and iron availability. Diabetes 1982; 31: 1109-1114. doi:10.2337/diabetes.31.12.1109 PMid:6816646

- Daly AL, Velazquez LA, Bradley SF,
Kauffman CA.
Mucormycosis: association with deferoxamine therapy. Am J Med. 1989
Oct;87:468-71.doi:10.1016/S0002-9343(89)80836-8

- Gluskin, M., M. P. Solomon, B. Gold, M. L.
Corrado, and J.
Berger. 1979. Mucormycosis slough of nasal floor and palate in the
anephric patient. J. Am. Dent. Assoc. 98:224-227.PMid:284067

- Boelaert JR, Fenves AZ, Coburn JW.
Deferoxamine therapy and
mucormycosis in dialysis patients: report of an international registry.
Am J Kidney Dis. 1991 Dec;18:660-7.PMid:1962650

- Boelaert, J. R., J. Van Cutsem, M. De
Locht, Y. J.
Schneider, and R. R. Crichton. 1994. Deferoxamine augments growth and
pathogenicity of Rhizopus, while hydroxypyridinone chelators have no
effect. Kidney Int. 45:667–671.doi:10.1038/ki.1994.89
PMid:8196268

- Sugar AM (1992) Mucormycosis. Clin Infect Dis 14(Suppl 1): S126–S129.
- McNab AA, McKelvie P (1997) Iron overload
is a risk factor for zygomycosis. Arch Ophthalmol
115:919–921.PMid:9230837

- Maertens J, Demuynck H, Verbeken EK,
Zachee P, Verhoef GE,
Vandenberghe P, Boogaerts MA (1999) Mucormycosis in allogeneic bone
marrow transplant recipients: report of five cases and review of the
role of iron overload in the pathogenesis. Bone Marrow Transplant
24:307–312. doi:10.1038/sj.bmt.1701885 PMid:10455371

- Symeonidis AS. The role of iron and iron
chelators in
zygomycosis. Clin Microbiol Infect. 2009 Oct;15 Suppl
5:26-32.doi:10.1111/j.1469-0691.2009.02976.x
PMid:19754753

- Almyroudis NG, Sutton DA, Linden P,
Rinaldi MG, Fung J,
Kusne S. Zygomycosis in solid organ transplant recipients in a tertiary
transplant center and review of the literature. Am J Transplant. 2006
Oct;6:2365-74. doi:10.1111/j.1600-6143.2006.01496.x
PMid:16925570

- Singh N, Aguado JM, Bonatti H, Forrest G,
Gupta KL, Safdar
N, John GT, Pursell KJ, Muñoz P, Patel R, Fortun J, Martin-Davila P,
Philippe B, Philit F, Tabah A, Terzi N, Chatelet V, Kusne S, Clark N,
Blumberg E, Julia MB, Humar A, Houston S, Lass-Flörl C, Johnson L,
Dubberke ER, Barron MA, Lortholary O. Zygomycosis in solid organ
transplant recipients: a prospective, matched case-control study to
assess risks for disease and outcome. J Infect Dis. 2009 Sep
15;200:1002-11. doi:10.1086/605445
PMid:19659439

- Sun HY, Aguado JM, Bonatti H, Forrest G,
Gupta KL, Safdar
N, John GT, Pursell KJ, Muñoz P, Patel R, Fortun J, Martin-Davila P,
Philippe B, Philit F, Tabah A, Terzi N, Chatelet V, Kusne S, Clark N,
Blumberg E, Julia MB, Humar A, Houston S, Lass-Florl C, Johnson L,
Dubberke ER, Barron MA, Lortholary O, Singh N; Zygomycosis Transplant
Study Group. Pulmonary zygomycosis in solid organ transplant recipients
in the current era. Am J Transplant. 2009 Sep;9:2166-71. doi:10.1111/j.1600-6143.2009.02754.x
PMid:19681829

- Antinori S, Nebuloni M, Magni C, Fasan M,
Adorni F, Viola
A, Corbellino M, Galli M, Vago G, Parravicini C, Ridolfo AL. Trends in
the postmortem diagnosis of opportunistic invasive fungal infections in
patients with AIDS: a retrospective study of 1,630 autopsies performed
between 1984 and 2002. Am J Clin Pathol. 2009 Aug;132:221-7. doi:10.1309/AJCPRAAE8LZ7DTNE PMid:19605816

- Moraru RA, Grossman ME. Palatal necrosis
in an AIDS
patient: a case of mucormycosis. Cutis. 2000 Jul;66:15-8.
Review.PMid:10916685

- Van den Saffele JK, Boelaert JR.
Zygomycosis in
HIV-positive patients: a review of the literature. Mycoses. 1996
Mar-Apr;39:77-84. doi:10.1111/j.1439-0507.1996.tb00106.x
PMid:8766998

- Nagy-Agren SE, Chu P, Smith GJ, Waskin HA,
Altice FL.
Zygomycosis (mucormycosis) and HIV infection: report of three cases and
review. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Dec
1;10:441-9. doi:10.1097/00042560-199512000-00007
PMid:7583440

- Sanchez MR, Ponge-Wilson I, Moy JA,
Rosenthal S.
Zygomycosis and HIV infection. J Am Acad Dermatol. 1994 May;30(5 Pt
2):904-8. doi:10.1016/S0190-9622(94)70110-5

- Blatt SP, Lucey DR, DeHoff D, Zellmer RB
(1991).
Rhinocerebral zygomycosis in a patient with AIDS. J Infect Dis
164:215–216.PMid:2056212

- Hopkins RJ, Rothman M, Fiore A, Goldblum
SE. Cerebral
mucormycosis associated with intravenous drug use: three case reports
and review. Clin Infect Dis. 1994 Dec;19:1133-7.PMid:7888545

- Merchant S, Reichman R, Koval CE.
Rhinocerebral zygomycosis
in an HIV-infected man during therapy with an investigational CCR5
inhibitor. AIDS. 2007 Jul
31;21:1666-9.doi:10.1097/QAD.0b013e328274257b PMid:17630574

- Parra-Ruiz J, Peña-Monje A, Tomas-Jimenez
C, Antelo-Lorenzo
R, Escobar-Lara T, Hernández-Quero J. Septic arthritis due to Absidia
corymbifera in a patient with HIV-1 infection. Infection. 2008
Jun;36:279-81. Epub 2007 Dec 14. doi:10.1007/s15010-007-6297-3
PMid:18084717

- Bongiovanni M, Ranieri R, Ferrari D,
Codecà C, Tartaro T,
Uziel L. Prolonged survival of an HIV-infected subject with severe
lymphoproliferative disease and rhinocerebral mucormycosis. J
Antimicrob Chemother. 2007 Jul;60:192-3. doi:10.1093/jac/dkm148
PMid:17496057

- Samant JS, Namgoong SH, Parveen T, Katner
HP.
Cytomegalovirus vasculitis and mucormycosis coinfection in late-stage
HIV/AIDS. Am J Med Sci. 2007
Feb;333:122-4.doi:10.1097/00000441-200702000-00011 PMid:17301593

- Boumis E, Chinello P, Conte A, Noto P,
Cicalini S, Grillo
LR, Petrosillo N. Rhino-orbital zygomycosis secondary to diabetic
ketoacidosis in an HIV-positive patient: case report and literature
review. AIDS. 2006 Jan
2;20:136-8.doi:10.1097/01.aids.0000198079.32207.5a PMid:16327338

- Samanta TK, Biswas J, Gopal L, Kumarasamy
N, Solomon S.
Panophthalmitis due to rhizopus in an AIDS patient: a
clinicopathological study. Indian J Ophthalmol. 2001 Mar;49:49-51.
PMid:15887716

- Virally ML, Riveline JP, Virally J,
Chevojon P, Regnard JF,
Belmekki A, Devidas A. Pulmonary mucormycosis in a diabetic patient
with HIV. Diabetes Care. 2002
Nov;25:2105.doi:10.2337/diacare.25.11.2105 PMid:12401766

- Guardia JA, Bourgoignie J, Diego J. Renal
mucormycosis in
the HIV patient. Am J Kidney Dis. 2000
May;35:E24.doi:10.1016/S0272-6386(00)70289-7

- Carvalhal GF, Machado MG, Pompeo A,
Saldanha L, Sabbaga E,
Arap S. Mucormycosis presenting as a renal mass in a patient with the
human immunodeficiency virus. J Urol. 1997 Dec;158:2230-1. doi:10.1016/S0022-5347(01)68208-9

- Blázquez R, Pinedo A, Cosín J, Miralles P,
Lacruz C, Bouza
E. Nonsurgical cure of isolated cerebral mucormycosis in an intravenous
drug user. Eur J Clin Microbiol Infect Dis. 1996 Jul;15:598-9. doi:10.1007/BF01709370
PMid:6756909

- Santos J, Espigado P, Romero C, Andreu J,
Rivero A, Pineda
JA. Isolated renal mucormycosis in two AIDS patients. Eur J Clin
Microbiol Infect Dis. 1994 May;13:430-2.doi:10.1007/BF01972004
PMid:6756909

- [No authors listed] Case records of the
Massachusetts
General Hospital. Weekly clinicopathological exercises. Case 52-1990. A
31-year-old HIV-seropositive women with a cerebral lesion seven years
after treatment of carcinoma of the cervix. N Engl J Med. 1990 Dec
27;323:1823-33. doi:10.1056/NEJM199012273232608 PMid:2247121

- Smith AG, Bustamante CI, Gilmor GD.
Zygomycosis
(absidiomycosis) in an AIDS patient. Absidiomycosis in AIDS.
Mycopathologia. 1989 Jan;105:7-10. doi:10.1007/BF00443823
PMid:2739694

- Chamilos G, Lewis RE, Hu J, Xiao L, Zal T,
Gilliet M,
Halder G, Kontoyiannis DP. Drosophila melanogaster as a model host to
dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci U S
A. 2008 Jul 8;105:9367-72. doi:10.1073/pnas.0709578105
PMid:18583479 PMCid:2453716

- McNulty, J. S. 1982. Rhinocerebral
mucormycosis: predisposing factors. Laryngoscope 92:1140–1143.
PMid:7132514

- Mok CC, Que TL, Tsui EY, Lam WY.
Mucormycosis in systemic
lupus erythematosus. Semin Arthritis Rheum. 2003 Oct;33:115-24. doi:10.1016/S0049-0172(03)00081-7

- Arce-Salinas CA, Pérez-Silva E.
Mucormycosis complications
in systemic lupus erythematosus. Lupus.
2010;19:985-8.doi:10.1177/0961203309357574
PMid:20064915

- Shenoi S, Emery HM. Successful treatment
of invasive
gastric mucormycosis in a child with systemic lupus erythematosus.
Lupus. 2010 Apr;19:646-9. doi:10.1177/0961203309349117
PMid:19939907

- Peel T, Daffy J, Thursky K, Stanley P,
Buising K.
Posaconazole as first line treatment for disseminated zygomycosis.
Mycoses. 2008 Nov;51:542-5. doi:10.1111/j.1439-0507.2008.01499.x
PMid:18422921

- Yu J, Li RY. Primary renal zygomycosis due
to Rhizopus
oryzae. Med Mycol. 2006 Aug;44:461-6. doi:10.1080/13693780500338951
PMid:16882613

- Fujimoto A, Nagao K, Tanaka K, Yamagami J,
Udagawa SI,
Sugiura M. The first case of cutaneous mucormycosis caused by Rhizopus
azygosporus. Br J Dermatol. 2005
Aug;153:428-30.doi:10.1111/j.1365-2133.2005.06593.x PMid:16086761

- Alsuwaida K. Primary cutaneous
mucormycosis complicating
the use of adhesive tape to secure the endotracheal tube. Can J
Anaesth. 2002 Oct;49:880-2. doi:10.1007/BF03017426
PMid:12374722

- Sungkanuparph S, Sathapatayavongs B,
Kunachak S,
Luxameechanporn T, Cheewaruangroj W. Treatment of invasive fungal
sinusitis with liposomal amphotericin B: a report of four cases. J Med
Assoc Thai. 2001 Apr;84:593-601.PMid:11460976

- Liu MF, Chen FF, Hsiue TR, Liu CC.
Disseminated zygomycosis
simulating cerebrovascular disease and pulmonary alveolar haemorrhage
in a patient with systemic lupus erythematosus. Clin Rheumatol.
2000;19:311-4. doi:10.1007/s100670070052
PMid:10941815

- Hosseini M, Lee J. Gastrointestinal
mucormycosis mimicking
ischemic colitis in a patient with systemic lupus erythematosus. Am J
Gastroenterol. 1998
Aug;93:1360-2.doi:10.1111/j.1572-0241.1998.00417.x
PMid:9707066

- Dickinson M, Kalayanamit T, Yang CA,
Pomper GJ, Franco-Webb
C, Rodman D Cutaneous zygomycosis (mucormycosis) complicating
endotracheal intubation: diagnosis and successful treatment. Chest.
1998 Jul;114:340-2. doi:10.1378/chest.114.1.340
PMid:9674495

- Fingerote RJ, Seigel S, Atkinson MH,
Lewkonia RM.
Disseminated zygomycosis associated with systemic lupus erythematosus.
J Rheumatol. 1990 Dec;17:1692-4.PMid:2084248

- Bloxham CA, Carr S, Ryan DW, Kesteven PJ,
Bexton RS,
Griffiths ID, Richards J. Disseminated zygomycosis and systemic lupus
erythematosus. Intensive Care Med.
1990;16:201-7 doi:10.1007/BF01724803
PMid:2351780

- Wong KL, Tai YT, Loke SL, Woo EK, Wong WS,
Chan MK, Ma JT.
Disseminated zygomycosis masquerading as cerebral lupus erythematosus.
Am J Clin Pathol. 1986 Oct;86:546-9.PMid:3766468

- Yu J, Li RY. Primary renal zygomycosis due
to Rhizopus
oryzae. Med Mycol. 2006 Aug;44:461-6. doi:10.1080/13693780500338951
PMid:16882613

- Arce-Salinas CA, Pérez-Silva E.
Mucormycosis complications
in systemic lupus erythematosus. Lupus. 2010;19:985-8. doi:10.1177/0961203309357574
PMid:20064915

- Nogueira EL, Ind PW, Friedland JS, Salama
AD. Mucormycosis
may mimic disease relapse in Wegener's granulomatosis. J Rheumatol.
2010 Jun;37:1364-5. doi:10.3899/jrheum.091423 PMid:20516047

- Skiada A, Petrikkos G. Cutaneous
zygomycosis. Clin
Microbiol Infect. 2009 Oct;15 Suppl 5:41-5. doi:10.1111/j.1469-0691.2009.02979.x
PMid:19754756

- Arney, G. K., E. Pearsons, and A. B.
Sutherland. 1960. Burn
stress diabetes. Ann. Surg. 152:77–90. doi:10.1097/00000658-196007000-00012
PMid:13794351 PMCid:1613595

- Rabin, E. R., G. D. Lundberg, and E. T.
Mitchell. 1961.
Mucormycosis in severely burned patients. N. Engl. J. Med.
264:1286–1289. doi:10.1056/NEJM196106222642504
PMid:13738817

- Chander J, Kaur J, Attri A, Mohan H.
Primary cutaneous
zygomycosis from a tertiary care centre in north-west India. Indian J
Med Res. 2010 Jun;131:765-70. PMid:20571164

- Lumbang WA, Caufield BA. Vesicular
eruption on the arm of an infant. Dermatol Online J. 2010 May
15;16:13.PMid:20492830

- Woo PC, Lau SK, Ngan AH, Tung ET, Leung
SY, To KK, Cheng
VC, Yuen KY. Lichtheimia hongkongensis sp. nov., a novel Lichtheimia
spp. associated with rhinocerebral, gastrointestinal, and cutaneous
mucormycosis. Diagn Microbiol Infect Dis. 2010 Mar;66:274-84. doi:10.1016/j.diagmicrobio.2009.10.009
PMid:20159375

- Tilak R, Raina P, Gupta SK, Tilak V,
Prakash P, Gulati AK.
Cutaneous zygomycosis: a possible postoperative complication in
immunocompetent individuals. Indian J Dermatol Venereol Leprol. 2009
Nov-Dec;75:596-9. doi:10.4103/0378-6323.57722
PMid:19915241

- Stewardson AJ, Holmes NE, Ellis DH,
Howden BP. Cutaneous
zygomycosis caused by Saksenaea vasiformis following water-related
wound in a 24-year-old immunocompetent woman. Mycoses. 2009
Nov;52:547-9. doi:10.1111/j.1439-0507.2008.01648.x
PMid:19682315

- Lu XL, Liu ZH, Shen YN, She XD, Lu GX,
Zhan P, Fu MH, Zhang
XL, Ge YP, Liu WD. Primary cutaneous zygomycosis caused by rhizomucor
variabilis: a new endemic zygomycosis? A case report and review of 6
cases reported from China. Clin Infect Dis. 2009 Aug 1;49:e39-43. doi:10.1086/600817 PMid:19566442

- Zhao Y, Zhang Q, Li L, Zhu J, Kang K,
Chen L. Primary
cutaneous mucormycosis caused by Rhizomucor variabilis in an
immunocompetent patient. Mycopathologia. 2009 Nov;168:243-7. doi:10.1007/s11046-009-9219-3
PMid:19562506

- Saravia-Flores M, Guaran DM, Argueta V.
Mycoses. Invasive
cutaneous infection caused by Apophysomyces elegans associated with a
spider bite. 2010 May;53:259-61.

- Piazza RC, Thomas WL, Stawski WS, Ford
RD. Mucormycosis of
the face. J Burn Care Res. 2009
May-Jun;30:520-3.doi:10.1097/BCR.0b013e3181a28d2f

- Almaslamani M, Taj-Aldeen SJ,
Garcia-Hermoso D, Dannaoui E,
Alsoub H, Alkhal A. An increasing trend of cutaneous zygomycosis caused
by Mycocladus corymbifer (formerly Absidia corymbifera): report of two
cases and review of primary cutaneous Mycocladus infections. Med Mycol.
2009;47:532-8.doi:10.1080/13693780802595746
PMid:19184768

- Chew HH, Abuzeid A, Singh D, Tai CC.
Surgical wound
mucormycosis necessitating hand amputation: a case report. J Orthop
Surg (Hong Kong). 2008 Aug;16:267-9.

- Chen CK, Wan SH, Kou SK. A rare cutaneous
fungal infection
complicating bacterial necrotising fasciitis. Hong Kong Med J. 2008
Aug;14:314-6. PMid:18685166

- Lechevalier P, Hermoso DG, Carol A,
Bonacorsi S, Ferkdadji
L, Fitoussi F, Lortholary O, Bourrillon A, Faye A, Dannaoui E,
Angoulvant F. Molecular diagnosis of Saksenaea vasiformis cutaneous
infection after scorpion sting in an immunocompetent adolescent. J Clin
Microbiol. 2008 Sep;46:3169-72. doi:10.1128/JCM.00052-08
PMid:18632909 PMCid:2546755

- Antoniadou A. Outbreaks of zygomycosis in
hospitals. Clin
Microbiol Infect. 2009 Oct;15 Suppl
5:55-9.doi:10.1111/j.1469-0691.2009.02982.x
PMid:19754759

- Lass-Flörl C, Resch G, Nachbaur D, Mayr
A, Gastl G,
Auberger J, et al. The value of computed tomography-guided percutaneous
lung biopsy for diagnosis of invasive fungal infection in
immunocompromised patients. Clin Infect Dis
2007;45:e101-4.doi:10.1086/521245 PMid:17806041

- Greenberg RN, Scott LJ, Vaughn HH, et al.
Zygomycosis
(mucormycosis): emerging clinical importance and new treatments. Curr
Opin Infect Dis 2004; 17:517–25.doi:10.1097/00001432-200412000-00003
PMid:15640705

- Chander J, Textbook of Medical Mycology; New Delhi :Mehta Publishers;2008.
- Lass-Flörl C. Zygomycosis: conventional
laboratory diagnosis. 2009 Oct;15 Suppl 5:60-5.

- Kurukularatne C, Garcia-Diaz J, Kemmerly
S, Reed D, et al.
Beyond Rhizopus and Mucor: Hurricane Katrina stirs up Synephalastrum in
New Orleans. Presented at Focus on Fungal Infections 16. Las Vegas,
March 8–10, 2006.

- Dannaoui E. Molecular tools for
identification of
Zygomycetes and the diagnosis of zygomycosis. Clin Microb Infect. 2009;
15 (Suppl. 5): 66–70.

- Bialek R, Konrad F, Kern J, et al. PCR
based identification
and discrimination of agents of mucormycosis and aspergillosis in
paraffin wax embedded tissue. J Clin Pathol 2005;58: 1180–4.

- Kobayashi M, Togitani K, Machida H, et
al. Molecular
polymerase chain reaction diagnosis of pulmonary mucormycosis caused by
Cunninghamella bertholletiae. Respirology 2004; 9:397–401.

- Rickerts V, Just-Nubling G, Konrad F, et
al. Diagnosis of
invasive aspergillosis and mucormycosis in immunocompromised patients
by seminested PCR assay of tissue samples. Eur J Clin Microbiol Infect
Dis 2006;25:8–13.

- Rickerts V, Mousset S, Lambrecht E,
Tintelnot K,
Schwerdtfeger R, Presterl E, et al. Comparison of histopathological
analysis, culture, and polymerase chain reaction assays to detect
invasive mold infections from biopsy specimens. Clin Infect Dis 2007
15;44:1078-83.

- Dannaoui E, Schwarz P, Slany M, Loeffler
J, Jorde AT,
Cuenca-Estrella M, et al. Molecular detection and identification of
zygomycetes species from paraffin-embedded tissues in a murine model of
disseminated zygomycosis: a collaborative European Society of Clinical
Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study
Group (EFISG) evaluation J Clin Microbiol, 2010; 48: 2043 - 2046.

- Prabhu R. M. and Patel R.
Mucormycosis and
entomophthoramycosis: a review of the clinical manifestations,
diagnosis and treatment. Clin Microbiol Infect 2004; 10 (Suppl. 1): 31–7

- Chamilos G, Lewis RE, Kontoyiannis DP.
Delaying
amphotericin B–based frontline therapy significantly increases
mortality among patients with hematologic malignancy who have
zygomycosis. Clin Infect Dis 2008; 47:503–9.

- Herbrecht R, Letscher-Bru V, Bowden RA et
al. Treatment of
21 cases of invasive mucormycosis with amphotericin B colloidal
dispersion. Eur J Clin Microbiol Infect Dis 2001; 20: 460–6.

- Ma B, Seymour JF, Januszewicz H, Slavin
MA. Cure of
pulmonary Rhizomucor pusillus infection in a patient with hairy-cell
leukemia. Role of liposomal amphotericin B and GM-CSF. Leuk Lymphoma
2001; 42: 1393–9.

- Roy V, Ali LI, Carter TH, Selby GB.
Successful non-surgical
treatment of disseminated polymicrobial fungal infection in a patient
with pancytopenia and graft-versushost disease. J Infect 2000; 41:
273–5.

- Larkin JA, Montero JA. Efficacy and
safety of amphotericin
B lipid complex for zygomycosis. Infect Med 2003; 20: 201–6.Bigby TD,
Serota ML, Tierney LM Jr, Matthay MA. Clinical spectrum of pulmonary
mucormycosis. Chest 1986; 89: 435–9.

- Spellberg B, Walsh T, Kontoyiannis DP, et
al.: Recent
advances in the management of mucormycosis: from bench to bedside. Clin
Infect Dis 2009, 48:1743-1751

- Greenberg RN, Mullane K, van Burik JA,
Raad I, Abzug MJ,
Anstead G, Herbrecht R, Langston A, Marr KA, Schiller G, Schuster M,
Wingard JR, Gonzalez CE, Revankar SG, Corcoran G, Kryscio RJ, Hare R.
Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents
Chemother. 2006 Jan;50:126-33.

- van Burik JA, Hare RS, Solomon HF,
Corrado ML, Kontoyiannis
DP. Posaconazole is effective as salvage therapy in zygomycosis:
a retrospective summary of 91 cases. Clin Infect Dis. 2006 Apr
1;42:e61-5.

- Gomez-Lopez A, Cuenca-Estrella M, Monzon
A,
Rodriguez-Tudela JL. In vitro susceptibilities of clinical isolates of
Zygomycota to amphotericin B, flucytosine, itraconazole,and
voriconazole. J Antimicrob Chemother 2001; 48: 919–21.

- Deresinski SC, Stevens DA. Caspofungin.
Clin Infect Dis 2003; 36: 1445–57.

- Alastruey-Izquierdo A, Castelli MV,
Cuesta I, Zaragoza O,
Monzón A, Mellado E, Rodríguez-Tudela JL. In vitro activity of
antifungals against Zygomycetes. Clin Microbiol Infect. 2009 Oct;15
Suppl 5:71-6. doi:10.1111/j.1469-0691.2009.02984.x
PMid:19754762

- Eisen DP, Robson J. Complete resolution
of pulmonary
Rhizopus oryzae infection with itraconazole treatment: more evidence of
the utility of azoles for zygomycosis. Mycoses 2004; 47(3-4):159-162. doi:10.1111/j.1439-0507.2004.00959.x
PMid:15078434

- Liao WQ, Yao ZR, Li ZQ, Xu H, Zhao J.
Pyoderma gangrenosum
caused by Rhizopus arrhizus. Mycoses 1995; 38(1-2):75-77. doi:10.1111/j.1439-0507.1995.tb00011.x
PMid:7637685

- Parthiban K, Gnanaguruvelan S, Janaki C,
Sentamilselvi G,
Boopalraj JM. Rhinocerebral zygomycosis. Mycoses 1998; 41(1-2):51-53. doi:10.1111/j.1439-0507.1998.tb00376.x
PMid:9610134

- Zhao Y, Zhang Q, Li L, Zhu J, Kang K,
Chen L. Primary
cutaneous mucormycosis caused by Rhizomucor variabilis in an
immunocompetent patient. Mycopathologia 2009.

- Ibrahim AS, Bowman JC, Avanessian V,
Brown K, Spellberg B,
Edwards JE, Jr. et al. Caspofungin inhibits Rhizopus oryzae
1,3-beta-D-glucan synthase, lowers burden in brain measured by
quantitative PCR, and improves survival at a low but not a high dose
during murine disseminated zygomycosis. Antimicrob Agents Chemother
2005; 49:721-727. doi:10.1128/AAC.49.2.721-727.2005
PMid:15673756
PMCid:547300

- Garey KW, Pendland SL, Huynh VT, Bunch
TH, Jensen GM,
Pursell KJ. Cunninghamella bertholletiae infection in a bone marrow
transplant patient: amphotericin lung penetration, MIC determinations,
and review of the literature. Pharmacotherapy 2001; 21: 855–60. doi:10.1592/phco.21.9.855.34560 PMid:11444582

- John BV, Chamilos G, Kontoyiannis DP.
Hyperbaric oxygen as
an adjunctive treatment for zygomycosis. Clin Microbiol Infect
2005;11:515-517 doi:10.1111/j.1469-0691.2005.01170.x PMid:15966968

- Liles WC, Huang JE, van Burik JA, Bowden
RA, Dale DC.
Granulocyte colony-stimulating factor administered in vivo augments
neutrophil-mediated activity against opportunistic fungal pathogens. J
Infect Dis 1997; 175: 1012-1015.doi:10.1086/513961 PMid:9086172

- Garcia-Diaz JB, Palau L, Pankey GA.
Resolution of
rhinocerebral Zygomycosis associated with adjuvant administration of
granulocyte-macrophage colony-stimulating factor. Clin Infect Dis 2001;
32: 145-150. doi:10.1086/320767 PMid:11360225

- Yohai RA, Bullock JD, Aziz AA, Markert
RJ. Survival factors
in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol 1994; 39: 3–22.
doi:10.1016/S0039-6257(05)80041-4

- Blitzer A, Lawson W, Meyers BR, Biller
HF. Patient survival
factors in paranasal sinus mucormycosis. Laryngoscope 1980; 90: 635–48.
doi:10.1288/00005537-198004000-00010
PMid:7359982

- Sun HY, Forrest G, Gupta KL, Aguado JM,
Lortholary O, Julia
MB, et al. Rhino-orbito-cerebral zygomycosis in solid organ transplant
recipients. Transplantation
2010;15:85-92.doi:10.1097/TP.0b013e3181dde8fc PMid:20626095
