Perspectives
Epidemiology of Therapy-Related Myeloid Neoplasms After Treatment for Pediatric Acute Lymphoblastic Leukemia in the Nordic Countries
Kjeld Schmiegelow
Faculty
of Medicine, Institute of Gynecology, Obstetrics and Pediatrics, and
the Department of Pediatrics, the University Hospital Rigshospitalet,
Copenhagen, Denmark.
Correspondence
to: Kjeld Schmiegelow, professor M.D., Dr.Med.Sci., The
Department of
Pediatrics, The Juliane Marie Center 5704, University Hospital
Rigshospitalet,
Blegdamsvej 9, 2100 Copenhagen, Denmark. Telephone: +45 3545 1357, Fax:
+45
3545 4524, E-mail: kschmiegelow@rh.dk
Published: May 16, 2011
Received: March 28, 2011
Accepted: May 3, 2011
Mediterr J Hematol Infect Dis 2011, 3: e2011020, DOI 10.4084/MJHID.2011.020
This article is available from: http://www.mjhid.org/article/view/8283
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Of
1614 Nordic children with ALL that were treated according to the NOPHO
ALL92 protocol, 20 developed an SMN (cumulative risk at 12 years:
1.6%). Sixteen of the twenty SMNs were acute myeloid leukemias or
myelodysplasias, and 9 of these had either monosomy 7 (n=7) or 7q
deletions (n=2). In Cox multivariate analysis longer duration of oral
MTX/6MP maintenance therapy (p=0.02; being longest for standard risk
patients) and presence of high-hyperdiploidy (p=0.07) were related to
an increased risk of SMN. In 524 patients we determined the erythrocyte
activity of thiopurine methyltransferase (TPMT), which methylates 6MP
and its metabolites, and thus reduces cellular levels of cytotoxic
6-thioguanine nucleotides. The TPMT activity was significantly lower in
those that did compared to those that did not develop an SMN (Median:
12.1 vs 18.1 IU/ml; p=0.02). Among 427 TPMT wild type patients, those
who developed SMN received higher 6MP doses than the remaining (69.7 vs
60.4 mg/m2, p=0.03), which may reflect increased levels of methylated
metabolites that inhibit purine de novo synthesis and thus enhance
incorporation of 6-thioguanine nucleotides into DNA. In conclusion, the
duration and intensity of 6MP/MTX maintenance therapy of childhood ALL
may influence the risk of SMN.
Introduction
One of the most devastating complications to the therapy of childhood acute lymphoblastic leukemia (ALL) is the development of second cancers (SMN), a complication that in general has a poor prognosis.[1-5] Although on average it occurs in 2% of all patients depending on the therapy given, the published cumulative incidences vary from less than 1% to almost 10%.[6-8] Furthermore, since the overall survival of childhood ALL now approaches 85%, SMN now encompass 15-20% of all deaths after childhood ALL therapy.
The incidence of SMN will be influenced not only by the treatment modalities applied but also by the duration of follow-up, since SMN may emerge several decades from the initial ALL treatment. Furthermore, some centres actively screen for subclinical cancers,
e.g. meningeomas [9] and thyroid cancers.[10] Finally, the strategies or reporting of SMNs differ widely between collaborative groups ranging from no-news-are-good-news to follow-up by regular questionnaires or systematic exchange of data with national or regional cancer registries, which in the Nordic countries are population-based and enforced by legislation and by exchange of data with the National hospital discharge registration.
Treatment-related risk factor analyses have primarily focused on irradiation, alkylating agents, and topoisomerase II inhibitors (anthracyclines, epipodophyllotoxins) that induce DNA damage, whereas less attention has been paid to mechanisms that interfere with DNA control, including drugs that may modify DNA repair.[5] Previously, children with higher risk ALL had the highest risk of SMN due to their more intensive chemotherapy and radiotherapy and the use of hematopoietic stem cell transplantation. However, studies from the Nordic Society for Paediatric Haematology and Oncology (NOPHO) and from St Jude Children’s Research Hospital have indicated that even the less intensive 6-mercaptopurine (6MP)/methotrexate (MTX) maintenance therapy may enhance the risk of SMN not least for patients with thiopurine methyltransferase (TPMT) low-activity polymorphisms.[11,12] Since TPMT methylates 6MP and some of its metabolites and thus reduces the intracellular amounts of cytotoxic 6-thioguanine nucleotides (6TGN) available for DNA incorporation, the 5-10% of patients who have low TPMT activity will have higher intracellular 6TGN levels for DNA incorporation and DNA damage.
Based on these findings, the predominance of therapy-related acute myeloid leukemia and myelodysplasia (t-AML/MDS) in the two most recent Nordic ALL protocols (Figure 1), and since 75% of the SMNs occurred among standard risk patients, we analyzed in depth the occurrence of SMN in the NOPHO ALL92 by taking advantage of an extensive registration of individual data on maintenance therapy during the first years of the protocol.
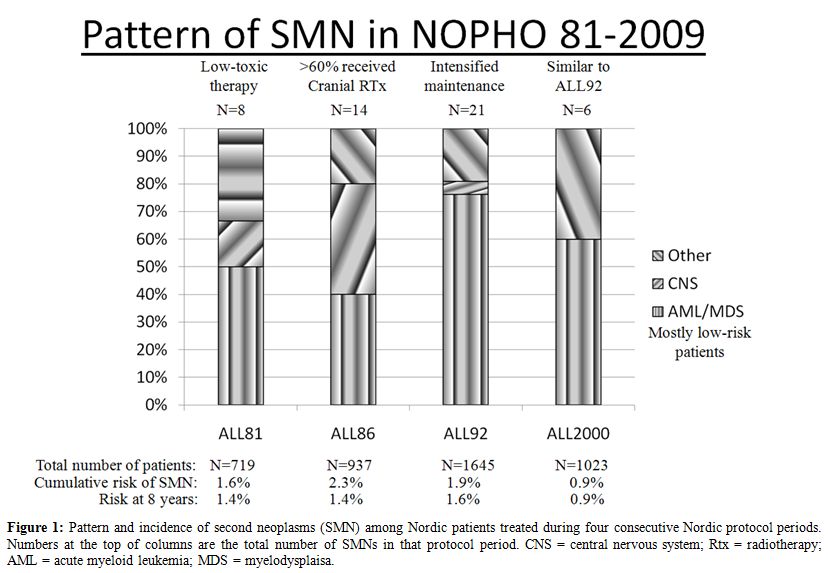
Figure 1: Pattern and incidence of second neoplasms (SMN) among Nordic patients treated during four consecutive Nordic protocol periods. Numbers at the top of columns are the total number of SMNs in that protocol period. CNS = central nervous system; Rtx = radiotherapy; AML = acute myeloid leukemia; MDS = myelodysplaisa.
Patients and Methods
Patients: From January 1992 until October 2001, 1614 children 1.0-14.9 years of age were diagnosed with B-cell precursor or T-cell ALL in the Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden) and were treated according to the NOPHO ALL92 protocol. [8]
Risk grouping: The risk group criteria are given in table 1.13 The patients who had higher risk features at diagnosis and were assigned to the very high risk (VHR) treatment arm received prophylactic or therapeutic central nervous system (CNS) irradiation as well as LSA2L2 instead of MTX/6MP maintenance therapy.[14]
Induction therapy and consolidation therapy have previously been published in detail.[13]
CNS irradiation and stem cell transplantation: Due to CNS disease at diagnosis or allocation to the very high risk protocol arm 123 non-transplanted patients (7.6% of the total study population) received cranial irradiation in 1st remission (CR1). 31 boys and 21 girls with higher-risk criteria received a hematopoitetic stem cell transplantation in CR1.[15]
Oral 6MP/MTX maintenance therapy was initiated at treatment week 13 (standard risk, SR), 32 (intermediate risk, IR), or 63 (high risk, HR) and scheduled to continue until 2 years (IR and HR) or 2½ years (SR) after diagnosis. Between 1992 and 1996, 538 patients with SR-, IR-, or HR-ALL were included in the randomised ALL-92 maintenance therapy trial.16 As part of that study we registered all data on MTX and 6MP doses as well as all blood counts (28.000 data sets in total), and furthermore added data on MTX and 6MP dosing and blood counts during maintenance therapy for the patients with SMN that were not included in the randomised study. The genotype (low activity polymorphisms G460A and A719G) and/or erythrocyte TPMT activity was available for 609 patients.[17] Erythrocyte TPMT activity was measured during maintenance therapy and at least 8 weeks from the last red blood cell transfusion. For 484 of these patients only the erythrocyte TPMT activity was available. The antimode of the TPMT activity distribution was 14 IU/ml, and patients with TPMT below that level, and for whom no TPMT genotype was available, were classified as TPMT presumed heterozygous.
Results and Discussion
With a median follow-up of 10.4 years for patients who remain in first remission, a total of 20 patients developed an SMN at a median of 3.4 years from the diagnosis of ALL (risk at 12 years: 1.6%) (Table 1). The 12-year cumulative risk of developing an SMN (pSMN12y) was 1.6 +/- 0.4%. The projected risk of SMN among the non-transplanted patients was significantly related to the risk group and was 2.4 +/- 0.7% for SR-ALL, 1.2 +/- 0.7% for IR-ALL, and 0.3 +/- 0.3% for higher risk ALL patients (p=0.007) (Table 1). The cumulative risk of developing SMN was 1.7 +/- 0.4% for the 1316 patients who were in CR1 when they initiated MTX/6MP maintenance therapy vs 0% for the 169 patients in CR1 at the start of LSA2L2 maintenance therapy. Twelve of the 20 SMN patients died within 21 months from diagnosis of their SMN with a median time to death of 8.8 months and a projected survival of 39% +/- 11%.
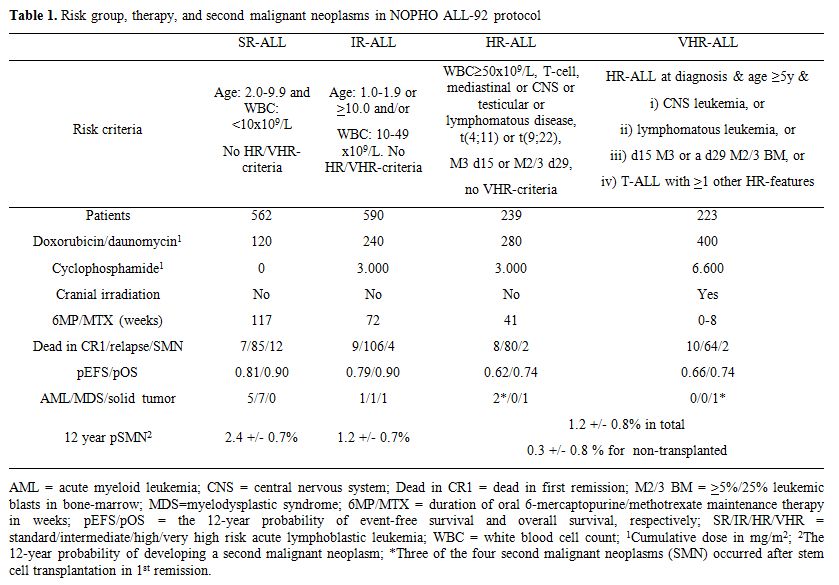
Table 1. Risk group, therapy, and second malignant neoplasms in NOPHO ALL-92 protocol
Sixteen of the twenty SMNs were t-AML/MDS, and 9 of these had either monosomy 7 (n=7) or 7q deletions (n=2). Eight of the nine -7/7q- t-AML/MDS cases occurred among patients with high-hyperdiploidy (n=4), t(12;21)(p13;q22) (n=1), or a normal/missing (n=4) karyotype at diagnosis of ALL, which indicates a biological propensity for non-disjunction, although the results did not reach statistical significance. In Cox multivariate analysis, longer duration of oral MTX/6MP maintenance therapy (p=0.02; being longest for standard risk patients) and presence of high-hyperdiploidy (p=0.07) were related to increased risk of SMN. Among all 562 SR-ALL patients, the 161 patients with a high-hyperdiploid karyotype had a 4.8 +/- 1.8% risk of developing SMN compared to a 1.5 +/- 0.6% risk for the remaining 401 SR patients (p=0.02).
The patients who developed an SMN had significantly lower TPMT activity than the remaining patients (Median: 12.1 vs 18.1 IU/ml; p=0.02), and they received 10-15% higher 6MP doses than those that did not develop an SMN, and this was the case both among patients classified as TPMT low activity (Median: 55.5 (N=3) vs 50.3 mg/m2 (N=57); p=0.31) and those classified as TPMT wild type (Median: 69.7 (N=9) vs 60.4 mg/m2 (N=418); p=0.03). The positive relation between 6MP dosage and risk of SMN is likely to reflect that TPMT-mediated thiopurine methylation, not least through 6-methyl-thioinosine 5'-monophosphate, will inhibit purine de novo synthesis,18 and since 6-methyl-thioinosine 5'-monophosphate levels preferentially increase with upward dose adjustments of 6MP,19 this may cause enhanced DNA-6TGN incorporation. Such a relationship is supported by the recent demonstration of a positive correlation between erythrocyte levels of methylated 6MP metabolites versus the index between DNA-6-thioguanine nucleotide levels in nucleated cells and the erythrocyte levels of 6-thioguanine nucleotides.[20]
Several drugs have been suggested to modify the risk of SMN by promoting the growth of a malignant clone or by interference with DNA-repair. Such agents include granulocyte colony stimulating factor ,[21] L-asparaginase,[22] and thiopurines.[11,23,24] The present population-based, retrospective study strongly indicates that maintenance therapy, and especially 6MP pharmacotherapy, influenced the development of SMNs, since 6MP can cause DNA damage,[25-27] and the risk of t-AML/MDS was related to i) longer duration of 6MP/MTX maintenance therapy, ii) low TPMT activity, and iii) significantly higher doses of 6MP for TPMT wild type patients. During oral 6MP therapy 1:103-1:104 DNA nucleotides in leukocytes have been shown to be substituted with 6TGN (DNA-6TGN),[20] but it is unknown, if the risk of t-AML/MDS is related to the individual leukocyte DNA-6TGN levels.
Canclusion
The relation between TPMT activity was not found in a recent large German BFM-study,[28] but this may reflect both significant differences between that study and the present Nordic study with respect to the MTX/6MP dosages in the two protocols, and furthermore by the fact that only genotyping was used in the BFM study, whereas most patients in the Nordic study was classified by TPMT phenotyping. Thus, it remains to be explored whether TPMT genotyping or phenotyping is superior in predicting the risk of SMN. Since 2001, determination of TPMT geno-/phenotype has been mandatory in NOPHO protocols, and during the initial maintenance therapy 6MP dose is reduced to 50 mg/m2 for TPMT heterozygous patients. With longer follow-up it will be explored, if this approach has reduced the risk of SMN for such patients without compromising their low relapse rate.[17]
To further increase the biological understanding of the development of SMN, an international study on SMN chaired by Maria Grazia Valsecchi (AIEOP) and Kjeld Schmiegelow (NOPHO) on behalf of the Ponte di Legno group has been initiated. This stuy will collect data on more than 700 SMNs occuring as the first event in children treated for ALL by 17 major childhood ALL groups in U.S., Europe and Asia.
Acknowledgement
This meeting review was written with support from The Danish Childhood Cancer Foundation; The Danish Cancer Society; the Home Secretary Research Grant for Individualised Therapy; and the Danish Research Council for Health and Disease. Kjeld Schmiegelow holds the Danish Childhood Cancer Foundation Research Professorship in Pediatric Oncology.
One of the most devastating complications to the therapy of childhood acute lymphoblastic leukemia (ALL) is the development of second cancers (SMN), a complication that in general has a poor prognosis.[1-5] Although on average it occurs in 2% of all patients depending on the therapy given, the published cumulative incidences vary from less than 1% to almost 10%.[6-8] Furthermore, since the overall survival of childhood ALL now approaches 85%, SMN now encompass 15-20% of all deaths after childhood ALL therapy.
The incidence of SMN will be influenced not only by the treatment modalities applied but also by the duration of follow-up, since SMN may emerge several decades from the initial ALL treatment. Furthermore, some centres actively screen for subclinical cancers,
e.g. meningeomas [9] and thyroid cancers.[10] Finally, the strategies or reporting of SMNs differ widely between collaborative groups ranging from no-news-are-good-news to follow-up by regular questionnaires or systematic exchange of data with national or regional cancer registries, which in the Nordic countries are population-based and enforced by legislation and by exchange of data with the National hospital discharge registration.
Treatment-related risk factor analyses have primarily focused on irradiation, alkylating agents, and topoisomerase II inhibitors (anthracyclines, epipodophyllotoxins) that induce DNA damage, whereas less attention has been paid to mechanisms that interfere with DNA control, including drugs that may modify DNA repair.[5] Previously, children with higher risk ALL had the highest risk of SMN due to their more intensive chemotherapy and radiotherapy and the use of hematopoietic stem cell transplantation. However, studies from the Nordic Society for Paediatric Haematology and Oncology (NOPHO) and from St Jude Children’s Research Hospital have indicated that even the less intensive 6-mercaptopurine (6MP)/methotrexate (MTX) maintenance therapy may enhance the risk of SMN not least for patients with thiopurine methyltransferase (TPMT) low-activity polymorphisms.[11,12] Since TPMT methylates 6MP and some of its metabolites and thus reduces the intracellular amounts of cytotoxic 6-thioguanine nucleotides (6TGN) available for DNA incorporation, the 5-10% of patients who have low TPMT activity will have higher intracellular 6TGN levels for DNA incorporation and DNA damage.
Based on these findings, the predominance of therapy-related acute myeloid leukemia and myelodysplasia (t-AML/MDS) in the two most recent Nordic ALL protocols (Figure 1), and since 75% of the SMNs occurred among standard risk patients, we analyzed in depth the occurrence of SMN in the NOPHO ALL92 by taking advantage of an extensive registration of individual data on maintenance therapy during the first years of the protocol.

Figure 1: Pattern and incidence of second neoplasms (SMN) among Nordic patients treated during four consecutive Nordic protocol periods. Numbers at the top of columns are the total number of SMNs in that protocol period. CNS = central nervous system; Rtx = radiotherapy; AML = acute myeloid leukemia; MDS = myelodysplaisa.
Patients and Methods
Patients: From January 1992 until October 2001, 1614 children 1.0-14.9 years of age were diagnosed with B-cell precursor or T-cell ALL in the Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden) and were treated according to the NOPHO ALL92 protocol. [8]
Risk grouping: The risk group criteria are given in table 1.13 The patients who had higher risk features at diagnosis and were assigned to the very high risk (VHR) treatment arm received prophylactic or therapeutic central nervous system (CNS) irradiation as well as LSA2L2 instead of MTX/6MP maintenance therapy.[14]
Induction therapy and consolidation therapy have previously been published in detail.[13]
CNS irradiation and stem cell transplantation: Due to CNS disease at diagnosis or allocation to the very high risk protocol arm 123 non-transplanted patients (7.6% of the total study population) received cranial irradiation in 1st remission (CR1). 31 boys and 21 girls with higher-risk criteria received a hematopoitetic stem cell transplantation in CR1.[15]
Oral 6MP/MTX maintenance therapy was initiated at treatment week 13 (standard risk, SR), 32 (intermediate risk, IR), or 63 (high risk, HR) and scheduled to continue until 2 years (IR and HR) or 2½ years (SR) after diagnosis. Between 1992 and 1996, 538 patients with SR-, IR-, or HR-ALL were included in the randomised ALL-92 maintenance therapy trial.16 As part of that study we registered all data on MTX and 6MP doses as well as all blood counts (28.000 data sets in total), and furthermore added data on MTX and 6MP dosing and blood counts during maintenance therapy for the patients with SMN that were not included in the randomised study. The genotype (low activity polymorphisms G460A and A719G) and/or erythrocyte TPMT activity was available for 609 patients.[17] Erythrocyte TPMT activity was measured during maintenance therapy and at least 8 weeks from the last red blood cell transfusion. For 484 of these patients only the erythrocyte TPMT activity was available. The antimode of the TPMT activity distribution was 14 IU/ml, and patients with TPMT below that level, and for whom no TPMT genotype was available, were classified as TPMT presumed heterozygous.
Results and Discussion
With a median follow-up of 10.4 years for patients who remain in first remission, a total of 20 patients developed an SMN at a median of 3.4 years from the diagnosis of ALL (risk at 12 years: 1.6%) (Table 1). The 12-year cumulative risk of developing an SMN (pSMN12y) was 1.6 +/- 0.4%. The projected risk of SMN among the non-transplanted patients was significantly related to the risk group and was 2.4 +/- 0.7% for SR-ALL, 1.2 +/- 0.7% for IR-ALL, and 0.3 +/- 0.3% for higher risk ALL patients (p=0.007) (Table 1). The cumulative risk of developing SMN was 1.7 +/- 0.4% for the 1316 patients who were in CR1 when they initiated MTX/6MP maintenance therapy vs 0% for the 169 patients in CR1 at the start of LSA2L2 maintenance therapy. Twelve of the 20 SMN patients died within 21 months from diagnosis of their SMN with a median time to death of 8.8 months and a projected survival of 39% +/- 11%.

Table 1. Risk group, therapy, and second malignant neoplasms in NOPHO ALL-92 protocol
Sixteen of the twenty SMNs were t-AML/MDS, and 9 of these had either monosomy 7 (n=7) or 7q deletions (n=2). Eight of the nine -7/7q- t-AML/MDS cases occurred among patients with high-hyperdiploidy (n=4), t(12;21)(p13;q22) (n=1), or a normal/missing (n=4) karyotype at diagnosis of ALL, which indicates a biological propensity for non-disjunction, although the results did not reach statistical significance. In Cox multivariate analysis, longer duration of oral MTX/6MP maintenance therapy (p=0.02; being longest for standard risk patients) and presence of high-hyperdiploidy (p=0.07) were related to increased risk of SMN. Among all 562 SR-ALL patients, the 161 patients with a high-hyperdiploid karyotype had a 4.8 +/- 1.8% risk of developing SMN compared to a 1.5 +/- 0.6% risk for the remaining 401 SR patients (p=0.02).
The patients who developed an SMN had significantly lower TPMT activity than the remaining patients (Median: 12.1 vs 18.1 IU/ml; p=0.02), and they received 10-15% higher 6MP doses than those that did not develop an SMN, and this was the case both among patients classified as TPMT low activity (Median: 55.5 (N=3) vs 50.3 mg/m2 (N=57); p=0.31) and those classified as TPMT wild type (Median: 69.7 (N=9) vs 60.4 mg/m2 (N=418); p=0.03). The positive relation between 6MP dosage and risk of SMN is likely to reflect that TPMT-mediated thiopurine methylation, not least through 6-methyl-thioinosine 5'-monophosphate, will inhibit purine de novo synthesis,18 and since 6-methyl-thioinosine 5'-monophosphate levels preferentially increase with upward dose adjustments of 6MP,19 this may cause enhanced DNA-6TGN incorporation. Such a relationship is supported by the recent demonstration of a positive correlation between erythrocyte levels of methylated 6MP metabolites versus the index between DNA-6-thioguanine nucleotide levels in nucleated cells and the erythrocyte levels of 6-thioguanine nucleotides.[20]
Several drugs have been suggested to modify the risk of SMN by promoting the growth of a malignant clone or by interference with DNA-repair. Such agents include granulocyte colony stimulating factor ,[21] L-asparaginase,[22] and thiopurines.[11,23,24] The present population-based, retrospective study strongly indicates that maintenance therapy, and especially 6MP pharmacotherapy, influenced the development of SMNs, since 6MP can cause DNA damage,[25-27] and the risk of t-AML/MDS was related to i) longer duration of 6MP/MTX maintenance therapy, ii) low TPMT activity, and iii) significantly higher doses of 6MP for TPMT wild type patients. During oral 6MP therapy 1:103-1:104 DNA nucleotides in leukocytes have been shown to be substituted with 6TGN (DNA-6TGN),[20] but it is unknown, if the risk of t-AML/MDS is related to the individual leukocyte DNA-6TGN levels.
Canclusion
The relation between TPMT activity was not found in a recent large German BFM-study,[28] but this may reflect both significant differences between that study and the present Nordic study with respect to the MTX/6MP dosages in the two protocols, and furthermore by the fact that only genotyping was used in the BFM study, whereas most patients in the Nordic study was classified by TPMT phenotyping. Thus, it remains to be explored whether TPMT genotyping or phenotyping is superior in predicting the risk of SMN. Since 2001, determination of TPMT geno-/phenotype has been mandatory in NOPHO protocols, and during the initial maintenance therapy 6MP dose is reduced to 50 mg/m2 for TPMT heterozygous patients. With longer follow-up it will be explored, if this approach has reduced the risk of SMN for such patients without compromising their low relapse rate.[17]
To further increase the biological understanding of the development of SMN, an international study on SMN chaired by Maria Grazia Valsecchi (AIEOP) and Kjeld Schmiegelow (NOPHO) on behalf of the Ponte di Legno group has been initiated. This stuy will collect data on more than 700 SMNs occuring as the first event in children treated for ALL by 17 major childhood ALL groups in U.S., Europe and Asia.
Acknowledgement
This meeting review was written with support from The Danish Childhood Cancer Foundation; The Danish Cancer Society; the Home Secretary Research Grant for Individualised Therapy; and the Danish Research Council for Health and Disease. Kjeld Schmiegelow holds the Danish Childhood Cancer Foundation Research Professorship in Pediatric Oncology.
References
- Hijiya N, Hudson MM, Lensing S, Zacher M,
Onciu M, Behm FG, et al. Cumulative incidence of secondary neoplasms as
a first event after childhood acute lymphoblastic leukemia. JAMA 2007
Mar 21;297:1207-15. doi:10.1001/jama.297.11.1207
PMid:17374815

- Garwicz S, Anderson H, Olsen JH, Dollner H,
Hertz H, Jonmundsson G, et al. Second malignant neoplasms after cancer
in childhood and adolescence: A population-based case-control study in
the 5 nordic countries. Int J Cancer 2000 Nov 15;88:672-8. doi:10.1002/1097-0215(20001115)88:4<672::AID-IJC24>3.0.CO;2-N

- Bhatia S, Sather HN, Pabustan OB, Trigg ME,
Gaynon PS, Robison LL. Low incidence of second neoplasms among children
diagnosed with acute lymphoblastic leukemia after 1983. Blood 2002 Jun
15;99:4257-64. doi:10.1182/blood.V99.12.4257
PMid:12036851

- Loning L, Zimmermann M, Reiter A, Kaatsch
P, Henze G, Riehm H, et al. Secondary neoplasms subsequent to
Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in
childhood: significantly lower risk without cranial radiotherapy. Blood
2000 May 1;95:2770-5. PMid:10779419

- Ng A, Taylor GM, Eden OB. Treatment-related
leukaemia - a clinical and scientific challenge. Cancer Treat Rev 2000
Oct;26:377-91. PMid:11006138

- Kamps WA, van der Pal-de Bruin KM, Veerman
AJ, Fiocco M, Bierings M, Pieters R. Long-term results of Dutch
Childhood Oncology Group studies for children with acute lymphoblastic
leukemia from 1984 to 2004. Leukemia 2010 Feb;24:309-19.doi:10.1038/leu.2009.258
PMid:20016528

- Pui CH, Pei D, Sandlund JT, Ribeiro RC,
Rubnitz JE, Raimondi SC, et al. Long-term results of St Jude Total
Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute
lymphoblastic leukemia. Leukemia 2010 Feb;24:371-82. doi:10.1038/leu.2009.252
PMid:20010620 PMCid:2820159

- Schmiegelow K, Forestier E, Hellebostad M,
Heyman M, Kristinsson J, Soderhall S, et al. Long-term results of NOPHO
ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia.
Leukemia 2010 Feb;24:345-54. doi:10.1038/leu.2009.251
PMid:20010622

- Banerjee J, Paakko E, Harila M, Herva R,
Tuominen J, Koivula A, et al. Radiation-induced meningiomas: a shadow
in the success story of childhood leukemia. Neuro Oncol 2009
Oct;11:543-9. doi:10.1215/15228517-2008-122
PMid:19179425 PMCid:2765343

- Lando A, Holm K, Nysom K, Krogh RA, Hoier
MM, Feldt-Rasmussen U, et al. Serum thyroglobulin as a marker of
thyroid neoplasms after childhood cancer. Acta Paediatr 2003
Nov;92:1284-90. PMid:14696848

- Thomsen JB, Schroder H, Kristinsson J,
Madsen B, Szumlanski C, Weinshilboum R, et al. Possible carcinogenic
effect of 6-mercaptopurine on bone marrow stem cells: relation to
thiopurine metabolism. Cancer 1999Sep15;86:1080-6.doi:10.1002/(SICI)1097-0142(19990915)86:6<1080::AID-CNCR26>3.0.CO;2-5

- Schmiegelow K, Al-Modhwahi I, Andersen MK,
Behrendtz M, Forestier E, Hasle H, et al. Methotrexate/6-mercaptopurine
maintenance therapy influences the risk of a second malignant neoplasm
after childhood acute lymphoblastic leukemia - results from the NOPHO
ALL-92 study. Blood 2009 Feb 17;113:6077-84. doi:10.1182/blood-2008-11-187880
PMid:19224761 PMCid:2699230

- Gustafsson G, Schmiegelow K, Forestier E,
Clausen N, Glomstein A, Jonmundsson G, et al. Improving outcome through
two decades in childhood ALL in the Nordic countries: the impact of
high-dose methotrexate in the reduction of CNS irradiation. Nordic
Society of Pediatric Haematology and Oncology (NOPHO). Leukemia 2000
Dec;14:2267-75.doi:10.1038/sj.leu.2401961
PMid:11187918

- Schmiegelow K, Heyman M, Kristinsson J,
Mogensen UB, Rosthoj S, Vettenranta K, et al. Oral
Methotrexate/6-Mercaptopurine may be superior to a multi-drug LSA2L2
maintenance therapy for higher risk childhood acute lymphoblastic
leukemia. J Pediatr Hematol Oncol. In press 2009.doi:10.1097/MPH.0b013e3181a6e171
PMid:19648786

- Saarinen-Pihkala UM, Heilmann C, Winiarski
J, Glomstein A, Abrahamsson J, Arvidson J, et al. Pathways through
relapses and deaths of children with acute lymphoblastic leukemia: role
of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol
2006 Dec 20;24:5750-62. doi:10.1200/JCO.2006.07.1225
PMid:17179109

- Schmiegelow K, Bjork O, Glomstein A,
Gustafsson G, Keiding N, Kristinsson J, et al. Intensification of
mercaptopurine/methotrexate maintenance chemotherapy may increase the
risk of relapse for some children with acute lymphoblastic leukemia. J
Clin Oncol 2003 Apr 1;21:1332-9.doi:10.1200/JCO.2003.04.039
PMid:12663723

- Schmiegelow K, Forestier E, Kristinsson J,
Soderhall S, Vettenranta K, Weinshilboum R, et al. Thiopurine
methyltransferase activity is related to the risk of relapse of
childhood acute lymphoblastic leukemia - results from the NOPHO ALL-92
study. Leukemia. In press 2008

- Wang L, Weinshilboum R. Thiopurine
S-methyltransferase pharmacogenetics: insights, challenges and future
directions. Oncogene 2006 Mar 13;25:1629-38. doi:10.1038/sj.onc.1209372
PMid:16550163

- Erb N, Harms DO, Janka-Schaub G.
Pharmacokinetics and metabolism of thiopurines in children with acute
lymphoblastic leukemia receiving 6-thioguanine versus 6-mercaptopurine.
Cancer Chemother Pharmacol 1998;42:266-72.doi:10.1007/s002800050816
PMid:9744770

- Hedeland RL, Hvidt K, Nersting J, Rosthoj
S, Dalhoff K, Lausen B, et al. DNA incorporation of 6-thioguanine
nucleotides during maintenance therapy of childhood acute lymphoblastic
leukaemia and non-Hodgkin lymphoma. Cancer Chemother Pharmacol 2010
Aug;66:485-91. doi:10.1007/s00280-009-1184-5
PMid:19956952

- Relling MV, Boyett JM, Blanco JG, Raimondi
S, Behm FG, Sandlund JT, et al. Granulocyte colony-stimulating factor
and the risk of secondary myeloid malignancy after etoposide treatment.
Blood 2003 May 15;101:3862-7. doi:10.1182/blood-2002-08-2405
PMid:12531808

- Pui CH, Relling MV, Behm FG, Hancock ML,
Boyett JM, Raimondi SC, et al. L-asparaginase may potentiate the
leukemogenic effect of the epipodophyllotoxins. Leukemia 1995
Oct;9:1680-4. PMid:7564509

- Relling MV, Rubnitz JE, Rivera GK, Boyett
JM, Hancock ML, Felix CA, et al. High incidence of secondary brain
tumours after radiotherapy and antimetabolites. Lancet 1999 Jul
3;354(9172):34-9. doi:10.1016/S0140-6736(98)11079-6

- Relling MV, Yanishevski Y, Nemec J, Evans
WE, Boyett JM, Behm FG, et al. Etoposide and antimetabolite
pharmacology in patients who develop secondary acute myeloid leukemia.
Leukemia 1998 Mar;12:346-52. doi:10.1038/sj.leu.2400928 PMid:9529129

- Waters TR, Swann PF. Cytotoxic mechanism
of 6-thioguanine: hMutSalpha, the human mismatch binding heterodimer,
binds to DNA containing S6-methylthioguanine. Biochemistry 1997 Mar
4;36:2501-6. doi:10.1021/bi9621573
PMid:9054555

- Swann PF, Waters TR, Moulton DC, Xu YZ,
Zheng Q, Edwards M, et al. Role of postreplicative DNA mismatch repair
in the cytotoxic action of thioguanine. Science 1996 Aug
23;273(5278):1109-11. doi:10.1126/science.273.5278.1109
PMid:8688098

- Karran P, Attard N. Thiopurines in current
medical practice: molecular mechanisms and contributions to
therapy-related cancer. Nat Rev Cancer 2008 Jan;8:24-36. doi:10.1038/nrc2292
PMid:18097462

- Stanulla M, Schaeffeler E, Moricke A,
Coulthard SA, Cario G, Schrauder A, et al. Thiopurine methyltransferase
genetics is not a major risk factor for secondary malignant neoplasms
after treatment of childhood acute lymphoblastic leukemia on
Berlin-Frankfurt-Munster protocols. Blood 2009 Aug 13;114:1314-8.doi:10.1182/blood-2008-12-193250 PMid:19535798
