Massimo Breccia, Corrado Girmenia, Roberto Latagliata, Giuseppina Loglisci, Michelina Santopietro, Vincenzo Federico, Luigi Petrucci, Alessandra Serrao, Adriano Salaroli and Giuliana Alimena
Published: May 16, 2011
Received: April 18, 2011
Accepted: May 9, 2011
Mediterr J Hematol Infect Dis 2011, 3: e2011021, DOI 10.4084/MJHID.2011.021
This article is available from: http://www.mjhid.org/article/view/8428
Abstract
Material and methods: From January 2001 to September 2006 we treated with imatinib 250 patients in first line (early CP) or after interferon failure (late CP), out of clinical trials and recorded all the bacterial and viral infections occurred.
Results: We recorded a similar incidence of bacterial and viral infections both in first line and late CP patients (respectively, 16% and 13%) during 3.5 years of follow-up. Analysis of presenting features predisposing to infections revealed differences only in late CP patients, with elevated percentage of high Sokal risk patients and a more longer median time from diagnosis to start of imatinib.
Conclusions: Opportunistic infections and reactivation of Herpes Zoster are observed during imatinib therapy at very low incidence.
Targeted
inhibition of BCR-ABL with imatinib mesylate has become the standard
therapy
for patients with chronic myeloid leukaemia (CML) where it induces a
complete
cytogenetic response in more than 80% of newly diagnosed patients.[1] A
recent 8-year follow-up of IRIS study showed an overall survival (OS)
of 85%
and event-free survival (EFS) of 81%.[2] Several years
after the
introduction of imatinib in clinical practice no significant major
incidence of
infections was reported. However, different evidences that imatinib can
impair
several cellular functions involved in the immune response have been
observed,
in particular in cell-mediated immunity.[3-5] We refer
here on the
incidence of infectious episodes observed during imatinib treatment in
our
large series of early and late chronic phase CML patients.
Patients
and methods
All
consecutive patients with Ph+ CML in chronic phase out of clinical
trials who
started with imatinib therapy during the period from January
2001
to September 2006 were included in the study. The observation was
interrupted on December 2009 and patients lost to follow-up were
considered until last visit. Overall 250 patients were
considered: 150 patients had received prior therapy with Interferon
(IFN) for a median of 24 months (range 5-68) and were considered in
late chronic phase (CP) and, whereas 100 patients received imatinib
soon after diagnosis (early CP). No differences were observed regarding
Sokal risk: in early CP patients at baseline, 45% were low risk, 24%
intermediate and 3% were high risk. In late CP group, Sokal risk at the
time of imatinib start revealed 43% of patients as low risk, 50%
intermediate and 6.6% as high risk. Before starting imatinib, all
patients had baseline evaluation including physical examination,
complete blood cell count, renal and hepatic function tests, serum
protein electrophoresis, serum levels of IgG, IgA and IgM, bone marrow
(BM) aspirate with cytogenetic and molecular analyses, chest X ray and
cardiac evaluation. Physical examination and complete blood cell
count were performed weekly for the first month, then monthly. During
treatment, all side effects possibly related to drug and all infective
episodes, were evaluated and collected by medical staff. Only
clinically and microbiologically documented infections were included.
Febrile episodes of unknown origin in non-neutropenic patients (ANC
> 500/cmm), upper respiratory tract syndromes possibly related to a
viral infection and localized Herpes simplex were not considered in the
analysis.
Results
Overall, 36
clinically or microbiologically documented infections that required
antibacterial or antiviral therapy and discontinuation of imatinib were
recorded in 35 patients (incidence 14%). The characteristics of the
infective episodes are summarized in table
1.
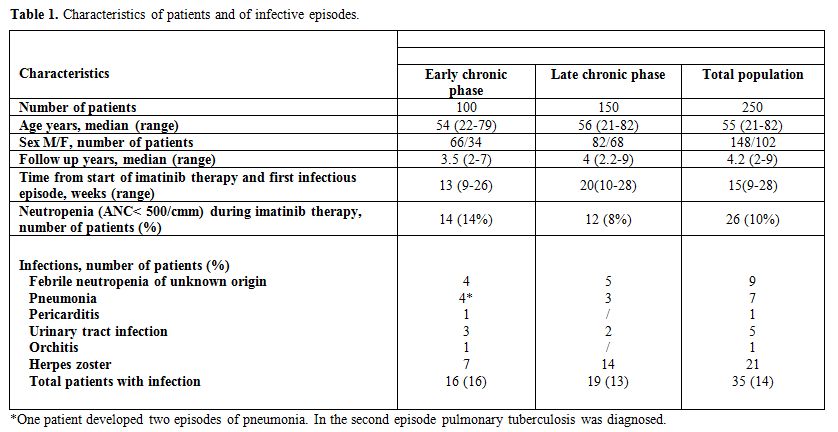
Table 1. Characteristics of patients and of infective episodes.
In the series
of 100 CP patients who received imatinib as first line
therapy, with a median follow-up of 3.5 years, we recorded 17
infectious episodes in 16 patients (incidence 16%). They accounted for
0.02 infectious episodes per 1000 patient days. All the infective
episodes occurred at a median time of 13 weeks (range 9-26) from the
onset of imatinib treatment. No episode was associated to deep
neutropenia (absolute neutrophyl count < 500/cmm). Median
gammaglobulin dosage was 0.82 g/dl (range 0.7-2), with only 1 patient
presented with a slightly inferior dosage. Herpes zoster and pneumonia
represented the two more frequently observed infections occurring in 7%
and 4% of patients, respectively. In all patients who developed Herpes
zoster a reduction of lymphocyte count (median lymphocytes count 0.6 x
109/l, range 0.2-1.1, compared to 0.9 x 109/l of patients who did not
developed viral infections) was evidenced at the time of viral
infection (median time of development 11 weeks), in the absence of
induced leukopenia and serum Ig level (0.9 g/dl). One out of the 4
subjects who developed pneumonia presented two additional episodes of
fever with radiological evidence of pulmonary infiltrate diagnosed as
tuberculosis. This second infection occurred soon after underlying
hematologic disease progression to accelerated phase.
In the series of 150 patients treated with imatinib after
resistance/intolerance to interferon, observed for a median
follow-up of 4 years, we recorded 19 infective episodes (13%). They
accounted for 0.003 infectious episodes per 1000 patient days. Three
episodes of pneumonia and 2 urinary tract infections occurred during
neutropenia phase. Median gammaglobulin dosage was 0.93 g/dl (range
0.6-2.1), with only 2 patients presented with a slightly inferior
dosage. Fourteen late CP patients developed a Herpes zoster infection
during treatment with imatinib (median time of development 14 weeks):
these patients, as observed in the cohort of early CP patients, had a
significant reduction of lymphocyte count at the time of viral
infection (median lymphocytes count 0.8 x 109/l, range 0.2-1.2,
compared to 1.1 x 109/l of patients who did not developed viral
infections), in the absence of leukopenia and serum Ig level (0.8
g/dl). The infective episodes developed at a median time of 20 weeks
(range 10-28) from the onset of imatinib treatment and all occurred
during neutropenia induced by the drug, differently from what observed
in early CP patients. No infection-related death occurred and all
infections resolved in both groups of patients. Presenting features of
patients who developed infections and of those who did not were
compared (Table 2). In the
group of subjects in early CP there were no
statistically significant differences between patients who developed
infections and patients who did not. In patients in late CP a longer
interval of time between diagnosis and the start of imatinib (17 vs 9
months, p=0.02) and a prevalence of high Sokal risk (p=0.03) was
observed in patients who developed an infectious complication.
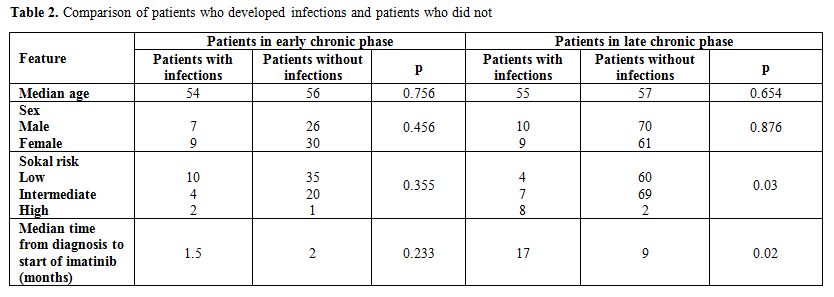
Table 2. Comparison of patients who developed infections and patients who did not
Discussion
Clinical and
laboratory findings seem to show that imatinib can impair several
cellular functions involved in the immune response. Two different
studies referred on a reduction of the immunoglobulin levels during
imatinib treatment for CML and for gastrointestinal stromal tumors
(GIST).[6,7] In the report of Steegmann et al6 only a
slight decrease of Ig levels in imatinib treated patients who had
became resistant or intolerant to interferon-alpha was documented: the
authors cannot rule out the possibility of an influence by the prior
therapy and suggested an impairment of B-lymphocytes or a mediated
effect through the inhibition of ABL kinase. Santachiara et al [7]
reported on 87 late CP patients treated with imatinib who presented a
reduction of Ig levels; these patients were treated with imatinib after
IFN for CML or were affected by GIST. The authors did exclude that this
effect had to be ascribed to interferon therapy and suggested that it
had to be considered the consequence of the imatinib treatment. CML
patients in CP are at low infectious risk compared to patients in more
advanced disease phases, however the epidemiological impact of
infectious complications in the imatinib era is unknown as infections
have not been enclosed in the safety analysis of large imatinib studies.[2,8]
Few reports on non-viral infections have been published during imatinib
therapy and anecdotic cases of pulmonary nocardiosis, pulmonary
tuberculosis, fungal pneumonia and listeria meningitis have been
reported.[9-12] In two of these observations, the
infections were concomitant to lymphopenia and monocytopenia. On the
contrary, viral infections, particularly those caused by herpes
viruses, seem to have a higher epidemiological impact.[13-15]
Mattiuzzi et al [13]
reported on a frequency of 2% of VZV reactivation in CML patients
treated with imatinib: baseline features of these subjects did not
differ significantly from those of patients who did not develop VZV
infection, except for the time from diagnosis to imatinib treatment and
the number of prior therapies. Few case reports reported on the
occurrence of other viral infections during imatinib therapy.[14-16]
In particular, the case of a fatal HHV8 infection in a CML patient in
complete molecular remission after imatinib was recently reported.[15]
In our experience, bacterial infection had a very low epidemiological
and clinical impact but VZV disease occurred more frequently than
previously reported.[13] Contrary to the study by
Mattiuzzi et al,[13]
all patients enclosed in our series were out of clinical trials,
probably with less favourable clinical characteristics. In fact, all
patients who developed the viral infection were aged over 50 years and
had comorbidities, such as diabetes and/or cardiac disease, conditions
which contraindicated the inclusion in clinical trials. We did not find
differences in the VZV incidence between early and late CP patients (7%
vs 9%). In any case, VZV infections accounted for only 0.04 infectious
episodes per 1000 patient days in the overall population, therefore
prophylaxis of VZV infection may not be recommended in this setting,
considering also the limited extent of the disease and the prompt
response to therapy. As to bacterial infections in late CP group, we
found that a longer interval of time between diagnosis and the start of
imatinib and a prevalence of high Sokal risk were prognostic adverse
events compared to early CP group: a possible explanation is that
previous treatment and high Sokal risk may influence the rate of
infections due to the possible scarce reserve of Ph negative cells.
Infectious episodes appeared concentrated in the first period of
treatment: this is in line with data from trials,[2]
in which toxicity was observed in the first year of treatment and new
events did not occur lately, again probably related to the initial
massive reduction of leukemic burden.
Conclusion
We observed
that opportunistic infections are an unusual complication also in a
“real life” population of CP - CML patients under imatinib therapy. No
life threatening and easy to treat Herpes zoster reactivations
represents the most frequently observed infectious complications.
Acknowledgements
No
grants were received from any of the authors for this paper.
Authorship: MB wrote and created the paper; RL, LC, GL, MS, AS, LP, VF
followed the patients; CG done microbacterial analysis; GA revised the
final version of the paper.
References
- Hochhaus A. First-Line management of CML: a
state of the art review. J Natl Compr Canc Netw Suppl 2008; 2: S1-S10

- Deininger MW, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571(IRIS) 8-year follow-up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood 2009; 114: 1126
- Cwynarski K, Laylor R, Macchiarulo E,
Goldman J, Lombardi G, Melo JV, Dazzi F. Imatinib inhibits the
activation and proliferation of normal T lymphocytes in vitro. Leukemia
2004; 18: 1332-1339 doi:10.1038/sj.leu.2403401
PMid:15190258

- Sinai P, Berg RE, Haynie JM, Egorin MJ,
Ilaria RL Jr, Forman J. Imatinib mesylate inhibits antigen-specific
memory CD8 T cell responses in vivo. J Immunol 2007; 178:
2028-2037PMid:17277106

- Chen CI, Maecker HT, Lee PP. Development
and dynamics of robust T-cell responses to CML under imatinib
treatment. Blood 2008; 111: 5342-5349 doi:10.1182/blood-2007-12-128397
PMid:18326818 PMCid:2396727

- Steegmann JL, Moreno G, AlŠez C, Osorio S,
Granda A, de la Camara R, Arranza E, Reino FG, Salvanes FR,
Fernandez-Ranada JM, Munoz C. Chronic myeloid leukemia patients
resistant to or intolerant of interferon alpha and subsequently treated
with imatinib show reduced immunoglobulin levels and
hypogammaglobulinemia. Haematologica 2003; 88: 762-768PMid:12857554

- Santachiara R, Maffei R, Martinelli S,
Arcari A, Piacentini F, Trabacchi E, Alfieri P, Ferrari A, Leonardi G,
Luppi G, Longo G, Vallisa D, Marasca R, Torelli G. Development of
hypogammaglobulinemia in patients treated with imatinib for chronic
myeloid leukemia or gastrointestinal stromal tumor. Haematologica 2008;
93: 1252-1255doi:10.3324/haematol.12642
PMid:18519520

- de Lavallade H, Apperley JF, Khorashad JS,
Milojkovic D, Reid AG, Bua M, Szydlo R, Olavarria E, Kaeda J, Goldman
JM, Marin D. Imatinib for newly diagnosed patients with chronic myeloid
leukemia: incidence of sustained responses in an intention-to-treat
analysis. J Clin Oncol. 2008 Jul 10;26:3358-63. Epub 2008 Jun 2.doi:10.1200/JCO.2007.15.8154
PMid:18519952

- Daniels JM, Vonk-Noordegraaf A, Janssen JJ,
Postmus PE, van Altena R. Tuberculosis complicating imatinib treatment
for chronic myeloid leukaemia. Eur Respir J 2009; 33: 670-672 doi:10.1183/09031936.00025408
PMid:19251803

- Speletas M, Vyzantiadis TA, Kalala F,
Plastiras D, Kokoviadou K, Antoniadis A, Korantzis I. Pneumonia caused
by Candida krusei and Candida glabrata in a patient with chronic
myeloid leukemia receiving imatinib mesylate treatment. Med Mycol 2008;
46:259-263 doi:10.1080/13693780701558969
PMid:17885950

- Lin JT, Lee MY, Hsiao LT, Yang MH, Chao
TC, Chen PM, Chiou TJ. Pulmonary nocardiosis in a patient with CML
relapse undergoing imatinib therapy after bone marrow transplantation.
Ann Hematol 2004; 83: 444-446 doi:10.1007/s00277-003-0813-zPMid:14689232

- Ferrand H, Tamburini J, Mouly S, Bouscary
D, Bergmann JF. Listeria monocytogenes meningitis following imatinib
mesylate-induced monocytopenia in a patient with chronic myeloid
leukemia. Clin Infect Dis 2005; 41:1684-1685 doi:10.1086/498031
PMid:16267747

- Mattiuzzi GN, Cortes JE, Talpaz M, Reuben
J, Rios MB, Shan J, Kontoyiannis D, Giles FJ, Raad I, Verstovsek S,
Ferrajoli A, Kantarjian HM. Development of Varicella-Zoster virus
infection in patients with chronic myelogenous leukemia treated with
imatinib mesylate. Clin Cancer Res 2003; 9:976-980 PMid:12631595

- Ikeda K, Shiga Y, Takahashi A, Kai T,
Kimura H, Takeyama K, Noji H, Ogawa K, Nakamura A, Ohira H, Sato Y,
Maruyama Y. Fatal hepatitis B virus reactivation in a chronic myeloid
leukemia patient during imatinib mesylate treatment. Leuk Lymphoma
2006; 47: 155-157 doi:10.1080/14639230500236818

- Bekkenk MW, Vermeer MH, Meijer CJ, Jansen
PM, Middeldorp JM, Stevens SJ, Willemze R. EBV-positive cutaneous
B-cell lymphoproliferative disease after imatinib mesylate. Blood 2003;
102: 4243 doi:10.1182/blood-2003-07-2436
PMid:14623772

- Anthony N, Shanks J, Terebelo H.
Occurrences of opportunistic infections in chronic myelogenous leukemia
patients treated with imatinib mesylate. Leuk Res 2010; 34:1250-1251 doi:10.1016/j.leukres.2010.05.019
PMid:20646761
