Review Articles
C. Quadrelli, P. Barozzi, G.
Riva, D. Vallerini, E. Zanetti, L. Potenza, F. Forghieri and M.
Luppi
Published: October 24, 2011
Received: June 04, 2011
Accepted: September 20, 2011
Mediterr J Hematol Infect Dis 2011, 3(1): e2011043, DOI 10.4084/MJHID.2011.043
This article is available from: http://www.mjhid.org/article/view/8348
Abstract
Similarly to Epstein-Barr virus
(EBV), the human herpesvirus-8 (HHV-8)
is a γ-herpesvirus, recently recognized to be associated with the
occurrence of rare B cell lymphomas and atypical lymphoproliferations,
especially in the human immunodeficiency virus (HIV) infected subjects.
Moreover, the human herpesvirus-6 (HHV-6), a β-herpesvirus, has been
shown to be implicated in some non-malignant lymph node proliferations,
such as the Rosai Dorfman disease, and in a proportion of Hodgkin’s
lymphoma cases. HHV-6 has a wide cellular tropism and it might play a
role in the pathogenesis of a wide variety of human diseases, but given
its ubiquity, disease associations are difficult to prove and its role
in hematological malignancies is still controversial. The involvement
of another β-herpesvirus, the human cytomegalovirus (HCMV), has not yet
been proven in human cancer, even though recent findings have suggested
its potential role in the development of CD4+ large granular lymphocyte
(LGL) lymphocytosis. Here, we review the current knowledge on the
pathogenetic role of HHV-8 and human β-herpesviruses in human
lymphoproliferative disorders.
Introduction
Epstein-Barr virus (EBV) is a γ-herpesvirus well recognized to be
involved in the development of human B and NK/T cell lymphomas, either
in the general population or in the immunosuppressed individuals. EBV
is a lympho- and epitheliotropic γ-herpesvirus apparently carried as an
harmless passenger in the immunocompetent host. Alterations in the
delicate balance between the virus and the host immune control may
result in a wide range of EBV-associated diseases: the simplest
scenario is the outgrowth of EBV-transformed B-lymphoblasts, expressing
the full array of EBV latent gene (EBNA1, 2, 3A, 3B, 3C, LP, LMP1 and
LMP2) leading to the development of post-transplant lymphoproliferative
disease (PTLD) in immunodeficient subjects. EBV is also associated to
malignancies in immunocompetent hosts arising from either epithelial, T
cell or B cell origin in which is present with a limited pattern of
latency genes: latency II (expression of EBNA1, LMP2A) is typical of
Nasopharingeal Carcinoma, Gastric Carcinoma and Hodgkin’s Lymphoma;
latency I (expression of EBNA1) is associated to the Burkitt Lymphoma.[1]
In addition to EBV, another γ-herpesvirus, Kaposi’s sarcoma-associated
herpesvirus (KSHV or HHV-8) is oncogenic. Among β-herpesviruses,
several investigators have suggested that human herpesvirus-6 (HHV-6)
also may be an oncogenic virus. Here, we review the current knowledge
on the pathogenetic role of human β-herpesviruses and HHV-8 in human
lymphoproliferative disorders.
b-HERPESVIRUSES:
HHV-6. Epidemiology and biology.
HHV-6 was first isolated in 1986 and later two viral variants have been
identified, namely HHV-6A and HHV-6B, showing an overall nucleotide
sequence identity of 90%. HHV-6 is ubiquitous in human throughout the
world, with seroconversion occurring early in life.[2,3]
Salivary contact is likely to be the vehicle for transmission, but
intrauterine passage is also possible. HHV-6 can be transmitted by
blood products and with bone marrow and solid organ transplantation.
Through its cellular receptor CD46, an ubiquitary complement regulatory
glycoprotein,[4] HHV-6 can primarily infect either
early self-renewing bone marrow precursors or mature blood cells, as
well as oropharinx/salivary glands, epithelial mucosa of female genital
tract and brain tissue. Following primary infection, HHV-6 can persist
lifelong mainly in monocytes and other peripheral blood mononuclear
cells.[3] Only rare cells remain latently infected in
healthy individuals, as shown by PCR testing. Of note, HHV6, unique
among all the herpesviruses, exhibits a particular form of persistence
in the infected cell, consisting in the integration of the whole viral
genome into host chromosomes. The prevalence of the ‘chromosomal
integration of HHV-6’ (CIHHV-6) ranges from 0.2% to 3% among different
geographical areas.[5,6] It has been observed that the
main route of acquisition of CIHHV-6 is the vertical transmission,
which implies that at least one copy of viral DNA is present in all the
nucleated cells of the host. The HHV-6 genome shows human telomere-like
repeat sequences at both its ends and this may foster the viral
integration in some preferred chromosomal regions (mainly 17p13.3,
22q13, 1q44), which are close to or within the telomeres.[7-10]
HHV-6 has been demonstrated to efficiently replicate in vitro and cause
a cytopathic effect either in CD4+ T lymphocytes or in thymocytes,
inducing a suppression of T-cell functions. Cells transfected with
HHV-6 can cause tumors in nude mice.[11] However, the
evidence linking HHV-6 to human hematological malignancies is
circumstantial, and far from definitive.[12] HHV-6
DNA can transform human epidermal keratinocytes and NIH 3T3 cells in
vitro.[13-14]
HHV-6 has a number of unique genes that are plausible causes of
oncogenesis. Its ORF-1 gene encodes a protein that is capable of
transforming NIH 3T3 cells in vitro, and cells expressing ORF-1 protein
produce fibrosarcomas when injected into nude mice.[15]
The ORF-1 protein appears to maintain the transformed state of tumor
cells by binding p53 and thereby inhibiting its tumor suppressor
properties.[16] HHV-6 also has a unique immediate
early gene called U95 that has binding sites for nuclear factor-kappa B
(NF-kB).[17]
Dysregulation of NF-kB has been postulated to contribute to cancer,
through its effects on both the proliferative and apoptotic pathways.[18] (Table 1)
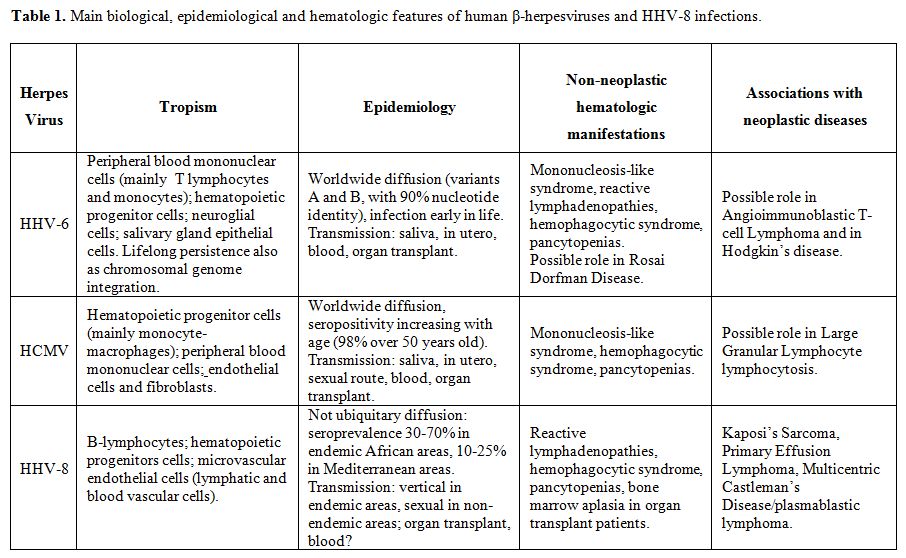
Table 1. Main biological, epidemiological and hematologic features of human β-herpesviruses and HHV-8 infections.
Hodgkin’s disease. Reports differ as to the possible role of HHV-6 in Hodgkin’s disease (HD). Torelli et al.[19] reported finding HHV-6 sequences by PCR in 3 of 25 cases of HD, all nodular sclerosis type, and in none of 41 cases of non-Hodgkin’s lymphoma. Krueger et al.[20] performed immunohistochemical studies of tumors from 103 patients with HD, and found tissue sections to be infected frequently by both EBV and HHV-6; lymphocytes and histiocytes were infected preferentially. Lacroix et al.[21] found HHV-6 DNA more frequently in the nodular sclerosis form of HD: of 73 patients with nodular sclerosis, 39 (49%) had both HHV-6 and EBV DNA, 25 (34%) had only HHV-6, and 8 (11%) had only EBV. In contrast, of 10 cases of the mixed cellularity form of HD, 4 (40%) had both viruses, 1 had HHV-6 only, 4 had EBV only, and 1 had neither. HHV-6+/EBV- patients were younger than the EBV+/HHV-6- patients and 92% of the HHV-6+ lymph nodes contained variant B. However, Luppi et al.[22] examined a large series of patients with HD in which HHV-6 DNA was found by both PCR and Southern blot analysis, did not detect either latent or lytic HHV-6 antigens in neoplastic cells, and detected only limited expression in Reed–Sternberg cells. Thus, the role of HHV-6 in any form of HD remains unclear. Recently, Lacroix et al.[23] showed the transforming, transactivating and oncogenic properties of HHV-6B and localized the transforming activity into DR7 gene. Cells expressing viral DR7 protein revealed tumorigenic properties when injected into nude mice. The expression of DR7B protein in Reed-Sternberg cells from HD patients causes molecular alterations into the cells typical of the lymphoproliferative disorder. In particular, the oncoprotein protects infected cells from apoptosis by retaining human p53 within the cytoplasm and by increasing NF-kB cellular transcription factor. The action on NF-kB is mainly exerted through two mechanisms: the transactivation of the expression of its subunities p65 and p50-p105 and the direct interaction of DR7B with the assembled protein. Lastly, DR7B promotes the overexpression of Id2, inhibitor of E2A transcription factor, that negatively regulates cell differentiation.[23] Further studies are needed to confirm a plausible pathogenetic role of HHV-6 infection in HD.
Non-Hodgkin’s lymphomas.
Luppi et al.[24]
reported a higher frequency of HHV-6 DNA in a well-characterized series
of patients with angioimmunoblastic T-cell lymphoma (AITL), a subtype
of T-cell non-Hodgkin’s lymphoma (NHL), compared with other lymphoma
subtypes and controls. These findings have been confirmed by Zhou et
al.[25] showing a clear association between
histological progression of AITL and the detectable copy number of both
EBV and HHV-6B in the AITL lesional tissue. While this increased viral
load could reflect a role for HHV-6 in the pathogenesis and progression
of AITL, it could also be the consequence of increasing dysfunction of
the immune system during lymphoma progression. Immunohistochemical
studies have so far failed to demonstrate HHV-6 antigens in the CD4+ T
cells (the likely proliferating elements) within AITL lesions.
Leukemias.
Persistent IL-2-regulated HHV-6 infection of adult T-cell leukemia
cells causes T-cell leukemia to progress more rapidly,[26]
but in vivo studies have not yet confirmed a pathogenetic role for
HHV-6 in this disease. Few other studies aiming to investigate the
association of HHV-6 with acute leukemia have been reported. The
largest study showed significant higher titres of HHV-6 antibodies in
patients with acute myeloid leukemia, but not with acute lymphoblastic
leukemia.[27] Salonen et al.[28]
found that 40% of children with leukemia had IgM antibodies to HHV-6
compared to 7.7% of age- and sex-matched children with various
neurological diseases. However, molecular studies have so far failed to
show a higher rate of HHV-6 DNA in peripheral blasts from children with
acute lymphoblastic leukemia compared with healthy subjects.[29]
A recent report found higher rates of seropositivity to human
cytomegalovirus (HCMV) among patients with B-cell chronic lymphocytic
leukemia than among healthy control subjects, although restricted only
to some geographical areas, but the same was not true for
seropositivity to HHV-6 (or EBV and HHV-7).[30] In
conclusion, with the possible exception of adult T-cell leukemia,
available data do not lend support to a role for HHV-6 in human acute
leukemias.
Non-malignant lymphatic
tissue proliferation.
Of interest, HHV-6 late antigens have so far been detected only in
non-malignant lymph node proliferations, namely in cases of reactive
lymphadenitis,[31,32] in which HHV-6 antigens appear
to be restricted to CD4+T cells. HHV-6 late antigens have also been
identified in cases of Rosai Dorfman disease, otherwise known as sinus
histiocytosis with massive lymphadenopathy, a benign chronic disease,
mainly affecting children and young adults and with no progression to
lymphoma. HHV-6 infection appears to be restricted to follicular
dendritic cells and, more significantly, to the abnormal histiocytes
that represent the proliferating elements and the hallmark of this
disease.[33] (Table 1)
HCMV.
Epidemiology and
biology.
HCMV was simultaneously isolated from salivary glands by Rowe and Smith
in 1956. This virus was designated ‘cytomegalovirus’ and the associated
clinical syndrome was referred to as ‘cytomegalovirus inclusion
disease’ because viral cytopathic effects typically result in cell
swelling and intranuclear inclusions.
HCMV infection is widespread in the entire human population, with
prevalence increasing with age. In Western Countries, seropositivity
rates range 40-70%, while in developing countries are much higher.[34]
Using PCR, CMV viremia has been detected in about 98% of healthy
individuals over 50 years of age. HCMV can be transmitted orally,
sexually, and parenterally; primary infection may be subclinical in
healthy subjects. Even asymptomatic carriers may at times shed HCMV in
urine and saliva.[35-36]
HCMV productive infection (lytic cycle) is restricted to endothelial
cells and fibroblasts, typically causing cell death and tissue damage
in lung, liver, colon, brain and retina. Similarly to other
herpesviruses, HCMV can establish lifelong latent infection in the
host, mainly in macrophages and hematopoietic stem cells/progenitors
(then passively transmitted to the mature myeloid progenies), and is a
recognized cause of mononucleosis-like syndromes.[36]
(Table 1)
Large granular
lymphocyte proliferation. In contrast to HHV-6, HCMV has not
been proven to be involved in human cancer. However,
Rodriguez-Caballero and colleagues[37]
suggested a role of HCMV in the pathogenesis of a specific subtype of
large granular lymphocyte (LGL) proliferation involving CD4+/CD8+/-dim
T cells. In particular, they used microarray gene expression profile
(GEP) to show that CD4+ T cells in patients with CD4+ LGL expansions,
differ significantly from HCMV-specific memory CD4+ lymphocytes derived
from healthy control individuals. The chronic antigenic stimulation of
T cells by HCMV can lead to persistent monoclonal expansion of
vβ13.1/CD4+ NKa+ CD8dim+ lymphocytes presenting a deregulation of genes
involved in cell cycle progression, resistance to apoptosis and genetic
instability. The observed deregulation of key genes allows these cells
to accumulate in excess to what is required to control HCMV infection
and to abnormally proliferate. (Table 1)
γ-HERPESVIRUSES:
HHV-8. Epidemiology and biology.
Human herpesvirus-8 (HHV-8) was identified by Moore & Chang in
1994, from the Kaposi’s sarcoma (KS) tissues of patients with AIDS.
HHV-8 is not ubiquitous in the general population: the infectious rates
are low in the United Kingdom, United States and Asia, intermediate in
Mediterranean countries and high in Central Africa. The seroprevalence
of HHV-8 among blood donors ranges from 0.2% in Japan, to up to 10% in
the United States, and to more than 50% in Africa,[38]
with rates in Italy and other Mediterranean countries falling between
these percentages.[39,40]
HHV-8 is mainly spread by sexual route in non-endemic areas, while
non-sexual transmission may be important in endemic areas where
infection is usually acquired early in the childhood.
HHV-8 is classified as a γ-herpesvirus, related to EBV and Herpesvirus
Saimiri. Like other herpesviruses, HHV-8 is a large, double-stranded
DNA virus that replicates in the nucleus as a closer circular episome
during latency, but linearizes during virion packaging and replication.
The HHV-8 genome typically contains genes that are homologous to
cellular genes involved in the control of cell cycle and apoptosis.
Similarly to other herpesviruses, HHV-8 has evolved to persist within
the lymphoid system and has shown an oncogenic potential. HHV-8
infection has been described in association with rare
lymphoproliferative disorders, including primary effusion lymphoma
(PEL), multicentric Castleman’s disease (MCD), and MCD-associated
plasmablastic lymphoma, often occurring in AIDS patients. A subset of
viral proteins is expressed in HHV-8-associated lymphoproliferative
disorders and are involved in the viral lymphomagenesis. The viral
proteins expressed in most PEL cells are the following:
latency-associated nuclear antigen 1 (LANA -1), v-Cyclin, v-FLICE
inhibitory protein (v-FLIP), v-interferon regulatory factor/LANA-2,
Kaposin, v-Interleukein-6 (v-IL-6). LANA-1, v-Cyclin, v-FLIP and v-IL-6
are also expressed in most of the MCD cases. Two additional proteins,
namely the K1 and the v-G-protein-coupled receptor (v-GPCR) are
expressed in few cases of PEL and MCD.[41] (Table 1)
Primary Effusion
Lymphoma. PEL
has been included in the WHO classification as a distinct entity among
AIDS-associated NHLs, representing about 3-4% of all AIDS-NHLs.[42-44] The lymphoma grows predominantly in serous
effusions, without solid tumor masses in the affected body cavity,[45-47] while involvement of lymph nodes,[48]
bone marrow[49,50] or other tissues[51]
is occasionally seen. A number of continuous cell lines has been
established from such lymphomatous effusions and peripheral blood of
PEL patients.[52] The PEL cells can include features
of large cell immunoblastic and anaplastic lymphoma,[53]
and also sometimes express a more plasmacytoid cytology.[42]
PEL cells generally lack immunophenotypical expression of
differentiated B- or T- cell antigens, but for MUM1 and CD138,
reflecting their post-germinal centre B-cell origin. Consistent with
this, the gene expression profile analysis suggests a plasmablastic
derivation of PEL cells.[54,55] They express cell
activation associated markers, including HLA-DR, CD23, CD25, CD30 and
CD38, and the epithelial membrane antigen whereas adhesion markers are
variably expressed.[43,52] The B
cell lineage derivation of PEL cells is established on the basis of
clonal rearrangements of the heavy immunoglobulin (Ig) genes,[45,52]
and the PCR-based findings of a preferential expression of certain
lambda light chain genes in AIDS-related PELs, suggesting clonal
proliferation by an antigen selection process.[56] A
few cases of AIDS-related PEL did not demonstrate Ig gene
rearrangements, consistent with a polyclonal pattern of
lymphoproliferation.[47] In contrast to other non
Hodgkin’s B-cell lymphoma types, neither c-MYC nor other proto-oncogene
rearrangements were detected in PELs.[54] Likewise, a
wild type of the tumor suppressor p53 gene is expressed by PELs, while
mutations of the BCL-6 5’ non-coding regions have been documented in
most of the cases.[52] PELs show complex karyotypes,
the most frequent chromosomal abnormalities being trisomy 7, 12 and
aberrations of chromosomal bands 1q21-q25.52 Virtually all reported
cases of PEL have a relatively high number of HHV-8 DNA copies (40-150)
per cell, most cells being latently infected and relatively few
permissive for lytic infection as obtained in cultured PEL lines.
Analysis of HHV-8 terminal repeats (TR) by pulsed-field gel
electrophoresis has shown monoclonal or oligoclonal fused TR fragments
in all examined cases of PEL, suggesting HHV-8 infection of clonogenic
cells, supporting an etiologic role of the virus in these
lymphoproliferations.[57] EBV co-infection is
detected in many cases of PEL, also with a monoclonal infection pattern
and with a restricted antigen expression pattern of latency. Human
interleukin-6 (IL-6) and -10 (IL-10), v-IL-6 and vascular endothelial
growth factor (VEGF) are the major growth factors released and used by
PEL cells for autocrine growth stimulation.[52,58-60]
The occurrence of PEL in a non-AIDS setting appears to be very rare and
has been reported in very few cases of solid organ transplant patients,[50,61,62] and a few cases have also
been described in HIV negative elderly men, most of them originating
from HHV-8 endemic areas.[63,64]
The clinical outcome of AIDS-related and post-transplant PEL is very
poor, with a median survival from 2 to 6 months, despite chemotherapy.[50,52,57]
Decreasing CD4+ cell counts seem to be the most important indicator of
progression of AIDS-related PEL.65 In HIV negative patients, PEL may
have a more indolent clinical course without specific therapy and may
initially respond to drainage procedures.[63]
Recently, it has been reported that azidothymidine and interferon-g induce apoptosis in PEL cells
either in vitro or in vivo,[66,67] and PEL remission
was observed in a patient on anti-retroviral therapy.[68]
We have demonstrated that cidofovir at high doses induces in vitro
apoptosis in PEL cell lines and PEL remission in four HIV negative,
elderly Italian men treated with intrapleural/intraperitoneal
injections of cidofovir, who had recurrent effusions not responding
either to pleural/peritoneal drainages or to chemotherapy.[69,70]
Recent in vitro data have shown that glycyrrhizic acid, contained in
the licorice root, induces apoptosis of PEL cells, by down-regulating
the synthesis of the HHV-8 LANA-1.[71] Other
approaches have recently been considered for the treatment of PEL,
based on the targeting of viral gene products,[72,73]
providing the basis for new therapeutic options for PEL patients who
are generally poor candidates for aggressive chemotherapy.
Multicentric
Castleman’s Disease (MCD) and MCD-associated plasmablastic lymphoma. MCD
of plasma cell type is an atypical lymphoproliferative disorder, which
is histologically characterized by abundance and prominent alterations
of the germinal centers, marked plasmacytic infiltration, and vascular
hyperplasia.[74] Two types of malignancies, lymphoma
and KS, have been reported to occur during the course of MCD in 18% and
13% of cases respectively.[74] HHV-8 DNA sequences
have been detected in most of MCD cases occurring in HIV positive
patients, but only in few HIV negative cases.[75-78]
HHV-8 infection is also found in most MCD patients with associated
POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, skin
changes) syndrome.[79] One case of HHV-8 positive MCD
has also been reported in a renal transplant patient with KS.[80]
HHV-8 positive MCD cells, expressing LANA-1, morphologically resemble
plasmablasts and are localized in the mantle zone of the follicles.[81] These plasmablasts show g
light-chain restriction and coalesce to form microscopic lymphomas in
some MCD cases, which could herald the development of frank HHV-8
positive plasmablastic lymphoma.[82-84] A role in the
pathogenesis of MCD for an over-expression of IL-6, a cytokine which
promotes B cell survival and proliferation, has been proposed.[74] The expression of v-IL-6 in a proportion of HHV-8
infected MCD cells[85-88]
thus appears to support such a pathogenic mechanism. This is consistent
with findings that exacerbations of systemic symptoms in MCD correlate
with an increase in HHV-8 viral load together with IL-6 and IL-10,
which thus represent markers of disease activity.[89,90]
Recent studies suggest that HHV-8 positive MCD cases have a more
aggressive clinical course and a poorer prognosis.[82]
With regard to therapy, single agent chemotherapy with vinblastine is
the most effective therapeutic option and may prolong survival.[91] A patient with MCD has successfully been treated
with retinoic acid and prednisone.[92]
Ganciclovir has also been effective in attenuating the constitutional
symptoms in some cases of HIV-associated HHV-8 positive MCD cases.[93]
Recently, treatment of MCD with humanized anti-IL-6 receptor antibody
has been reported to be safe and to alleviate chronic inflammatory
symptoms and wasting in a series of 28 patients, followed-up for 60
weeks.[94]
Other diseases.
The pathogenetic association between HHV-8 infection and the
development of multiple myeloma, proposed by Rettig and colleagues,[95] has not been confirmed.[96-98]
HHV-8 infection is certainly rare in lymphoproliferative diseases other
than PEL or MCD, both in HIV positive and HIV negative subjects.
Moreover, the occurrence of HHV-8 positive solid lymphomas, usually
extranodal and extracavitary, but with pathobiological features
mimicking those of PEL, has been described in AIDS as well as in HIV
negative patients.[99] HHV-8 infection was documented
in association with hepatitis C virus infection in one case of plasma
cell leukemia,[100]
and three HIV negative cases of a germinotropic lymphoproliferative
disorder characterized by plasmablasts coinfected by HHV-8 and EBV have
also been described.[101] HHV-8 DNA and LANA-1
antigens have been detected in liver, lung and bone marrow tissues from
patients affected with common variable immunodeficiency and
granulomatous/lymphocytic interstitial lung disease, suggesting a
pathogenetic viral role in this disorder.[102] HHV-8
DNA was also found in a single case of primary cerebral lymphoma, in a
woman who had received long-term steroid therapy for uveitis,
suggesting that HHV-8 infection may be occasionally involved in a
lymphoproliferation process associated with iatrogenic
immunesuppression.[103] Consistent with this, the
occurrence of an EBV negative, HHV-8 positive, monoclonal,
lymphoproliferative disease of polymorphic type has recently been
reported in a HHV-8 seronegative Jewish man, nine months after
receiving a kidney from his HHV-8 seropositive father.[104]
Non malignant plasmacytic proliferations have also been reported in two
solid organ transplant patients[105] as well as in a
few Italian cases of HIV negative angioimmunoblastic lymphadenopathy.[106]
Interestingly, a few cases of benign lymphadenopathy with germinal
center hyperplasia and increased vascularity in which HHV-8 DNA
sequences were detected, have been reported in HIV negative[75,106] and HIV positive[106,107]
young adults. The only one case of well documented HHV-8 primary
infection in HIV positive subjects has been reported to be associated
with the development of fever, splenomegaly and a cervical
lymphadenopathy, characterized by angiolymphoid hyperplasia.[108]
Thus, the above mentioned histologic features of florid follicular
hyperplasia and increased vascularity, which are observed also in MCD,
are likely to represent the distinct histologic pattern of lymphoid
response induced by HHV-8. Interestingly, a lymphoproliferative disease
characterized by persistent angiofollicular lymphadenopathy is induced
in simian immunodeficiency-virus infected Rhesus Macaques, following
infection with the simian homologue of HHV-8.[109]
It is also likely that, as with other human herpesviruses, a HHV-8
primary infection or reactivation, is manifested by non neoplastic
pathological changes. Thus, HHV-8 DNA has been detected in the
pathologic lung tissue of HIV negative and positive patients with
interstitial pneumonitis.[110,111] Fever, cutaneous
rash and hepatitis have also been reported in an Italian patient with
NHL, who received autologous peripheral blood stem cell (PBSC)
transplantation and showed HHV-8 reactivation.[112]
Recently, we had also the possibility to study primary HHV-8 infection
in two patients four months after kidney transplantation from the same
HHV-8-seropositive cadaveric donor. Seroconversion and viremia
coincided with development of a disseminated KS in one patient and with
an acute syndrome of fever, splenomegaly, cytopenia, and marrow failure
with plasmacytosis in the other patient.[113] We
also reported a further case of HHV-8 reactivation associated with
fever and marrow aplasia with plasmacytosis in a patient with NHL,
after autologous PBSC transplantation. HHV-8 transcripts and latency
associated nuclear antigen were expressed in the immature myeloid
progenitors of the aplastic marrow of these patients.[113]
In recent studies we and others have observed that HHV-8 may also exert
a myelosuppressive effect in vitro,[114,115]
suggesting that HHV-8 could also be implicated in the complex
pathophysiology of cytopenias often occurring in HIV infected patients.[116] (Table 1)
Acknowledgments
This study was supported by the Associazione Italiana per la Ricerca
sul Cancro (AIRC), Milan, Italy; the European Commission’s FP6
Life-Science-Health Programme (INCA project; LSHC-CT-2005-018704); the
Associazione Italiana Lotta alle Leucemie, Linfoma e Mieloma
(AIL)-Modena ONLUS; and the Programma di ricerca Regione-Università PRU
2007-2009 (M.L.).
References
- Comoli P and Locatelli F. T-cell therapy for the treatment of Epstein Barr virus-associated malignancies. Immunotheapy Insights. 2009;1 3-14 (available from http://www.la-press.com).
- Caserta MT, Mock DJ, Dewhurst S. Human
Herpes Virus 6. Clin Inf Dis. 2001; 33:829-833. http://dx.doi.org/10.1086/322691
PMid:11512088

- Clark DA. Human herpes virus 6. Rev Med
Virol. 2000; 10(3): 155-73. http://dx.doi.org/10.1002/(SICI)1099-1654(200005/06)10:3<155::AID-RMV277>3.0.CO;2-6

- Santoro F, Kennedy PE, Locatelli G, Malnati
MS, Berger EA, Lusso P. CD46 is a cellular receptor for human
herpesvirus 6. Cell. 1999;99(7):817-27. http://dx.doi.org/10.1016/S0092-8674(00)81678-5

- Ward KN, Leong HN, Nacheva EP, Howard J,
Atkinson CE, Davies NW, Griffiths PD, Clark DA. Human herpesvirus 6
chromosomal integration in immunocompetent patients results in high
levels of viral DNA in blood, sera, and hair follicles. J Clin
Microbiol. 2006; 44: 1571-1574. http://dx.doi.org/10.1128/JCM.44.4.1571-1574.2006
PMid:16597897 PMCid:1448653

- Potenza L, Barozzi P, Masetti M; Pecorari
M, Bresciani P, Gautheret-Dejean A, Riva G, Vallerini D, Tagliazucchi
S, Codeluppi M, Di Benedetto F, Gerunda GE, Narni F, Torelli G, Luppi
M. Prevalence of human herpesvirus-6 chromosomal integration (CIHHV-6)
in Italian solid organ and allogeneic stem cell transplant patients. Am
J Transplant. 2009; 9:1-8. http://dx.doi.org/10.1111/j.1600-6143.2009.02685.x
PMid:19519818

- Torelli G, Barozzi P, Marasca R,
Cocconcelli P, Merelli E, Ceccherini-Nelli L, Ferrari S, Luppi M.
Targeted integration of human herpesvirus 6 in the p arm of chromosome
17 of human peripheral blood mononuclear cells in vivo. J Med Virol.
1995;46(3): 178-88. http://dx.doi.org/10.1002/jmv.1890460303
PMid:7561787

- Morris C, Luppi M, McDonald M, Barozzi P,
Torelli G. Fine mapping of an apparently targeted latent human
herpesvirus type 6 integration site in chromosome band 17p13.3. J Med
Virol. 1999;58(1):69-75. http://dx.doi.org/10.1002/(SICI)1096-9071(199905)58:1<69::AID-JMV11>3.0.CO;2-3

- Daibata Taguchi T, Taguchi H, Miyoshi I.
Integration of human herpesvirus 6 in a Burkitt's lymphoma cell line.
Br J Haematol. 1998;102(5):1307-13. PMid:9753061

- Daibata M, Taguchi T, Nemoto Y, Taguchi H,
Miyoshi I. Inheritance of chromosomally integrated human herpesvirus 6
DNA. Blood. 1999; 1; 94(5): 1545-9.

- Puri RK, Leland P, Razzaque A.
Antigen(s)-specific tumour infiltrating lymphocytes from tumour induced
by fhuman herpesvirus-6 (HHV-6) DNA transfected NIH 3T3 transformants.
Clin Exp Immunol.199; 83:96–101.

- OgataM, Satou T, Kawano R, Takakura S,
Goto K, Ikewaki J,Kohno K, Ikebe T, Ando T, Miyazaki Y, Ohtsuka E,
Saburi Y, Saikawa T, Kadota J. Correlations of HHV-6 viral load and
plasma IL-6 concentration with HHV-6 encephalitis in allogeneic stem
cell transplant recipients. Bone Marrow Transplant. 2009; 45:129–136. http://dx.doi.org/10.1038/bmt.2009.116
PMid:19465942

- Razzaque A. Oncogenic potential of human
herpesvirus-6 DNA. Oncogene. 1990; 5:1365–1370. PMid:2170897

- Razzaque A, Williams O, Wang J, Rhim JS.
Neoplastic transformation of immortalized human epidermal keratinocytes
by two HHV-6 DNA clones. Virology. 1993; 195:113–120. http://dx.doi.org/10.1006/viro.1993.1351
PMid:8391179

- Kashanchi F, Araujo J, Doniger J,
Muralidhar S, Hoch R, Khleif S,Mendelson E, Thompson J, Azumi N, Brady
JN, Luppi M, Torelli G, Rosenthal LJ. 1997. Human herpesvirus 6 (HHV-6)
ORF-1 transactivating gene exhibits malignant transforming activity and
its protein binds to p53. Oncogene 14:359–367. http://dx.doi.org/10.1038/sj.onc.1200840
PMid:9018122

- Doniger J, Muralidhar S, Rosenthal LJ.
Human cytomegalovirus and human herpesvirus 6 genes that transform and
transactivate. Clin Microbiol Rev. 1999; 12:367–382. PMid:10398670
PMCid:100243

- Takemoto M, Shimamoto T, Isegawa Y,
Yamanishi K. The R3 region, one of three major repetitive regions of
human herpesvirus 6, is a strong enhancer of immediate-early gene U95.
J Virol. 2001; 75:10149–10160. http://dx.doi.org/10.1128/JVI.75.21.10149-10160.2001
PMid:11581383 PMCid:114589

- Campbell KJ, Perkins ND. Regulation of
NF-kappaB function. Biochem Soc Symp. 2006; 73:165–180.
PMid:16626297

- Torelli G, Marasca R, Luppi M, Selleri L,
Ferrari S, Narni F, Mariano MT, Federico M, Ceccherini-Nelli L,
Bendinelli M, Montagnani G, Montorsi M, Artusi T. Human herpesvirus-6
in human lymphomas: Identification of specific sequences in Hodgkin’s
lymphomas by polymerase chain reaction. Blood. 1991; 77:2251–2258.
PMid:1674220

- Krueger GR, Huetter ML, Rojo J, Romero M,
Cruz-Ortiz H. Human herpesviruses HHV-4 (EBV) and HHV-6 in Hodgkin’s
and Kikuchi’s diseases and their relation to proliferation and
apoptosis. Anticancer Res. 2001; 21:2155–2161 PMid:11501840

- Lacroix A, Jaccard A, Rouzioux C, Piguet
C, Petit B, Bordessoule D, Ranger-Rogez S. HHV-6 and EBV DNA
quantitation in lymph nodes of 86 patients with Hodgkin’s lymphoma. J
Med Virol. 2007; 79:1349–1356. http://dx.doi.org/10.1002/jmv.20868
PMid:17607791

- Luppi M, Barozzi P, Garber R, Maiorana A,
Bonacorsi G, Artusi T,Trovato R, Marasca R, Torelli G. Expression of
human herpesvirus-6 antigens in benign and malignant
lymphoproliferative diseases. Am J Pathol. 1998; 153:815–823. http://dx.doi.org/10.1016/S0002-9440(10)65623-4

- Lacroix A, Collot-teixeira S, Mardivirin
L, Jaccard A, Petit B, Piguet C, Sturtz F, Preux PM, Bordeussoule D,
Ranger-Rogez S. Involvement of Human Herpesvirus-6 Variant B in classic
Hodgking’s Lymphoma via DR7 oncoprotein. Clin Cancer Res. 2010;
16:4711-4721. http://dx.doi.org/10.1158/1078-0432.CCR-10-0470
PMid:20858841

- Luppi M, Marasca R, Barozzi P, Artusi T,
Torelli G. Frequent detection of human herpesvirus-6 sequences by
polymerase chain reaction in paraffin-embedded lymph nodes from
patients with angioimmunoblastic lymphadenopathy and angioimmunoblastic
lymphadenopathy-like lymphoma. Leuk Res. 1993;.17:1003–1011.

- Zhou Y, Attygalle AD, Chuang SS, Diss T,
Ye H, Liu H, Hamoudi RA, Munson P, Bacon CM, DoganA, Du MQ.
Angioimmunoblastic T-cell lymphoma: Histological progression associates
withEBVand HHV6B viral load. Br J Haematol. 2007; 138:44–53. http://dx.doi.org/10.1111/j.1365-2141.2007.06620.x
PMid:17555446

- Ojima T, Abe K, Ohyashiki JH, Shirakata M,
Yamamoto K. IL-2-regulated persistent human herpesvirus-6 B infection
facilitates growth of adult T cell leukemia cells. J Med Dent Sci.
2005; 52:135–141. PMid:16187619

- Levine PH, Ablashi DV, Saxinger WC,
Connelly RR. Antibodies to human herpes virus-6 in patients with acute
lymphocytic leukemia. Leukemia. 1992a 6:1229–1231. PMid:1331626

- Salonen MJ, Siimes MA, Salonen EM, Vaheri
A, Koskiniemi M. Antibody status to HHV-6 in children with leukaemia.
Leukemia. 2002;16:716–719. http://dx.doi.org/10.1038/sj.leu.2402437
PMid:11960354

- Barozzi P, Luppi M, Marasca R, Trovato R,
Ceccherini-Nelli L, Torelli G. Human herpesvirus-6 genome in acute
lymphoblastic leukemia: Evidence against an etiologic relationship.
Acta Haematol. 1995; 94:169–172 http://dx.doi.org/10.1159/000204004
PMid:7502638

- Steininger C, Rassenti LZ, Vanura K,
Eigenberger K, Jager U, Kipps TJ, Mannhalter C, Stilgenbauer
S,Popow-Kraupp T.Relative seroprevalence of human herpes viruses in
patients with chronic lymphocytic leukaemia. Eur J Clin Invest. 2009;
39:497–506. http://dx.doi.org/10.1111/j.1365-2362.2009.02131.x
PMid:19490058

- Akashi K, Eizuru Y, Sumiyoshi Y, Minematsu
T, Hara S, Harada M, Kikuchi M, Niho Y, Minamishima Y. Brief report:
Severe infectious mononucleosis-like syndrome and primary human
herpesvirus 6 infection in an adult. N Engl J Med. 1993; 329:168–171. http://dx.doi.org/10.1056/NEJM199307153290304
PMid:8390615

- Maric I, Bryant R, Abu-Asab M, Cohen JI,
Vivero A, Jaffe ES, Raffeld M, Tsokos M, Banks PM, Pittaluga S. Human
herpesvirus-6- associated acute lymphadenitis in immunocompetent
adults. Mod Pathol. 2004;17:1427–1433 http://dx.doi.org/10.1038/modpathol.3800179
PMid:15494709 PMCid:2288737

- Levine PH, Jahan N, Murari P, ManakM,
Jaffe ES. Detection of human herpesvirus 6 in tissues involved by sinus
histiocytosis with massive lymphadenopathy (Rosai–Dorfman disease). J
Infect Dis. 1992;166:291–295. http://dx.doi.org/10.1093/infdis/166.2.291
PMid:1321861

- Arbeitskreis Blut, Untergruppe “Bewertung
Blutassoziierter Krankheitserreger”. Human Cytomegalovirus (HCMV).
Transfus Med Hemother. 2010; 37: 365-375. http://dx.doi.org/10.1159/000322141
PMid:21483467 PMCid:3048947

- Boeckh M and Geballe AP. Cytomegalovirus:
pathogen, paradigm, and puzzle. J Clin Invest. 2011. 121(5). 1673-80. http://dx.doi.org/10.1172/JCI45449
PMid:21659716

- Sinzger C, Digel M, Jahn G.Cytomegalovirus
cell tropism. Curr Top Microbiol Immunol. 2008;325:63-83. http://dx.doi.org/10.1007/978-3-540-77349-8_4

- Rodríguez-Caballero A, García-Montero AC,
Bárcena P, Almeida J, Ruiz-Cabello F, Tabernero MD, Garrido P,
Muñoz-Criado S, Sandberg Y, Langerak AW, González M, Balanzategui A,
Orfao A. Expanded cells in monoclonal TCR alphabeta+/ CD4+/ NKa+/
CD8-/+dim T-LGL lymphocytosis recognize hCMV antigens. Blood.
2008;112(12):4609-16. http://dx.doi.org/10.1182/blood-2008-03-146241
PMid:18768393

- Lennette ET, Blackbourn DJ, Levy JA.
Antibodies to human herpesvirus type 8 in the general population and in
Kaposi’s sarcoma patients. Lancet 1996;348:858-61. http://dx.doi.org/10.1016/S0140-6736(96)03240-0

- Whitby D, Luppi M, Barozzi P, Boshoff C,
Weiss RA, Torelli G. Human herpesvirus 8 seroprevalence in blood donors
and lymphoma patients from different regions of Italy. J Natl Cancer
Inst 1998;90:395-7. http://dx.doi.org/10.1093/jnci/90.5.395

- Simpson GR, Schulz TF, Whitby D, et al.
Prevalence of Kaposi’s sarcoma associated herpesvirus infection
measured by antibodies to recombinant capsid protein and latent
immunofluorescence antigen. Lancet 1996; 348:1133-8 http://dx.doi.org/10.1016/S0140-6736(96)07560-5

- Damania B. Oncogenic γ-herpesviruses:
comparison of viral proteins involved in tumorigenesis. Nat Rev
Microbiol. 2004; 2: 656-667. http://dx.doi.org/10.1038/nrmicro958
PMid:15263900

- Carbone A and Gaidano G. HHV-8 positive
body-cavity-based lymphoma: a novel lymphoma entity Br J Haematol.
1997; 97: 515-22. http://dx.doi.org/10.1046/j.1365-2141.1997.00064.x
PMid:9207392

- Harris NL, Jaffe ES, Diebold J, Flandrin
G, Muller-Hermelink K, Vardiman J, Lister TA, Bloomfield CD. World
Health Organization Classification of neoplastic diseases of the
hematopoietic and lymphoid tissues: report of the clinical advisory
committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol.
1999; 17: 3835-49. PMid:10577857

- Simonelli C, Spina M, Cinelli R, Talamini
R, Tedeschi R, Gloghini A, Vaccher E, Carbone A, Tirelli U. Clinical
features and outcome of primary effusion lymphoma in HIV-infected
patients: a single-institution study. J Clin Oncol. 2003; 21: 3948-54. http://dx.doi.org/10.1200/JCO.2003.06.013
PMid:14581418

- Cesarman E, Chang Y, Moore PS, Said JW,
Knowles DM. Kaposi’ sarcoma-associated herpesvirus-like DNA sequences
in AIDS-related body-cavity-based lymphomas. New Engl J Med. 1995; 332:
1186-91. http://dx.doi.org/10.1056/NEJM199505043321802
PMid:7700311

- Nador RG, Cesarman E, Chadburn A, Dawson
DB, Ansari MQ, Said J, Knowles DM. Primary effusion lymphoma: a
distinct clinico-pathologic entity associated with the Kaposi’s
sarcoma-associated herpesvirus. Blood. 1996; 88: 645-56. PMid:8695812

- Komanduri KVJ, Luce JA, McGrath MS,
Herndier BG, Ng VL. The natural history and molecular heterogeneity of
HIV-associated primary malignant lymphomatous effusions. J Acquir Immun
Defic. 1996; 13: 215-26. http://dx.doi.org/10.1097/00042560-199611010-00003
PMid:8898666

- Ariad S, Benharroch D, Lupu L, Davidovici
B, Dupin N, Boshoff C. Early peripheral lymph node involvement of human
herpesvirus 8-associated body cavity-based lymphoma in a human
immunodeficiency virus-negative patient. Arch Pathol Lab Med. 2000;
124: 753-5. PMid:10782162

- Boshoff C, Gao S-J, Healy LE, Matthews S,
Thomas AJ, Coignet L, Warnke RA, Strauchen JA, Matutes E, Kamel OW,
Moore PS, Weiss RA, Chang Y. Establishing a KSHV+ cell line (BCP-1)
from peripheral blood and characterizing its growth in Nod/SCID mice.
Blood. 1998; 91: 1671-9. PMid:9473233

- Dotti, G., Fiocchi, R., Motta, T.,
Facchinetti, B., Chiodini, B., Boleri, G.M., Gavazzeni, G., Barbui, T.,
Rambaldi, S. Primary effusion lymphoma after heart transplantation: a
new entity associated with human herpesvirus-8. Leukemia. 1999; 13:
664-70. http://dx.doi.org/10.1038/sj.leu.2401390
PMid:10374868

- DePond W, Said JW, Tasaka T, de Vos S,
Kahn D, Cesarman E, Knowles DM, Koeffler HP. Kaposi’s
sarcoma-associated herpesvirus and human herpesvirus 8
(KSHV/HHV-8)-associated lymphoma of the bowel. Am J Surg Pathol. 1997;
21: 719-24. http://dx.doi.org/10.1097/00000478-199706000-00013
PMid:9199651

- Drexler HG, Huphoff CC, Gaidano G, Carbone
A. Lymphoma cell lines: in vitro models for the study of HHV-8 +
primary effusion lymphomas (body-cavity based lymphomas). Leukemia.
1998; 12: 1507-17. http://dx.doi.org/10.1038/sj.leu.2401160
PMid:9766492

- Jaffe E. Primary body cavity-based
AIDS-related lymphomas. Evolution of a new disease entity. Am J Clin
Pathol. 1996; 105: 141-3 PMid:8607435

- Carbone A. Emerging pathways in the
development of AIDS-related lymphomas. The Lancet Oncology. 2003; 4:
22-9. http://dx.doi.org/10.1016/S1470-2045(03)00957-4

- Klein U, Gloghini A, Gaidano G, Chadburn
A, Cesarman E, Dalla-Favera R, Carbone A. Gene expression profile
analysis of AIDS-related primary effusion lymphoma (PEL) suggests a
plasmablastic derivation and identifies PEL-specific transcripts.
Blood. 2003; 101: 4115-21. http://dx.doi.org/10.1182/blood-2002-10-3090
PMid:12531789

- Fais F, Gaidano G, Capello D, Gloghini A,
Ghiotto F, Roncella S, Carbone A, Chiorazzi N, Ferrarini M.
Immunoglobulin V region gene use and structure suggest antigen
selection in AIDS-related primary effusion lymphomas. Leukemia. 1999;
13: 1093-9. PMid:10400426

- Judde J-G, Lacoste V, Brière J,
Kassa-Kelembho E, Clyti E, Couppiè P, Buchrieser C, Tulliez M, Morvan
J, Gessain A. Monoclonality or oligoclonality of human herpesvirus 8
terminal repeat sequences in Kaposi’s sarcoma and other diseases. J
Natl Canc Inst. 2000; 22: 729-36. http://dx.doi.org/10.1093/jnci/92.9.729
PMid:10793109

- Jones KD, Aoki Y, Chang Y, Moore PS,
Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral
IL-6 in the spontaneous growth of Kaposi’s sarcoma
herpesvirus-associated infected primary effusion lymphoma cells. Blood.
1999; 94: 2871-79. PMid:10515891

- Aoki Y, Jaffe ES, Chang Y, Jones K,
Teruya-Feldstein J, Moore PS, Tosato G. Angiogenesis and hematopoiesis
induced by Kaposi’s sarcoma-associated herpesvirus-encoded
interleukin-6. Blood. 1999; 93: 4034-43. PMid:10361100

- Aoki Y, Tosato G. Role of vascular
endothelial growth factor/vascular permeability factor in the
pathogenesis of Kaposi sarcoma-associated herpesvirus-infected primary
effusion lymphomas. Blood. 1999; 94: 4247-54. PMid:10590069

- Jones D, Ballestas M.E, Kaye K.M, Gulizia
J.M, Winters G.L, Fletcher J, Scadden D.T, Aster J.C. Primary effusion
lymphoma and Kaposi’s sarcoma in a cardiac-transplant recipient. N Engl
J Med. 1998; 339: 444-9. http://dx.doi.org/10.1056/NEJM199808133390705
PMid:9700178

- Shaw RN, Waller EK, Offermann MK.
Induction of human herpesvirus 8 gene expression in a
post-transplantation primary effusion lymphoma cell line. Leuk
Lymphoma. 2002; 43: 631-4. http://dx.doi.org/10.1080/10428190290012173

- Strauchen JA, Hauser D, Burstein D,
Jimenez R, Moore PS, Chang Y. Body-cavity-based malignant lymphoma
containing Kaposi sarcoma-associated herpesvirus in an HIV-negative man
with previous Kaposi sarcoma. Ann Intern Med. 1996; 125: 822-5.
PMid:8928989

- Ascoli V, Lo Coco F, Torelli G, Vallisa D,
Cavanna L, Bergonzi C, Luppi M. Human herpesvirus 8-associated primayi
effusion lymphoma in HIV-patients: a clinico epidemiologic variant
resembling classic Kaposi’s sarcoma. Haematologica. 2002; 87: 339-43.
PMid:11940475

- Simonelli C, Tedeschi R, Gloghini A,
Bortolin MT, Spina M, Bidoli E, Cinelli R, De Paoli P, Carbone A,
Tirelli U. Characterization of immunologic and virological parameters
in HIV-infected patients with primary effusion lymphoma during
antiblastic therapy and highly active antiretroviral therapy. Clin
Infect Dis. 2005; 40: 1022-7. http://dx.doi.org/10.1086/428615
PMid:15824995

- Lee RK, Cai J-P, Deyev V, Gill PS, Cabral
L, Wood C, Agarwal RP, Xia W, Boise LH, Podack E, Harrington Jr WJ.
Azidothymidine and interferon-α induce apoptosis in
herpesvirus-associated lymphomas. Cancer Res. 1999; 59: 5514-20.
PMid:10554028

- Ghosh SK, Wood C, Boise LH, Mian AM, Deyev
VV, Feuer G, Toomey NL, Shank NC, Cabral L, Barber GN, Harrington WJ
Jr. Potentiation of TRAIL-induced apoptosis in primary effusion
lymphoma through azidothymidine-mediated inhibition of NF-kappa B.
Blood. 2003; 101: 2321-7. http://dx.doi.org/10.1182/blood-2002-08-2525
PMid:12406882

- Oksenhendler E, Clauvel JB, Jouveshomme S,
Davi F, Mansour G. Complete remission of a primary effusion lymphoma
with antiretroviral therapy. Am J Hematol. 1998; 57: 266 http://dx.doi.org/10.1002/(SICI)1096-
8652(199803)57:3<266::AID-AJH25>3.0.CO;2-7

- Luppi M, Trovato R, Barozzi P, Vallisa D,
Rossi G, Re A, Ravazzini L, Potenza L, Riva G, Morselli M, Longo G,
Cavanna L, Roncaglia R, Torelli G. Treatment of herpesvirus associated
primary effusion lymphoma with intracavity cidofovir. Leukemia. 2005;
19: 473-6. http://dx.doi.org/10.1038/sj.leu.2403646
PMid:15674353

- Halfdanarson TR, Markovic SN, Kalokhe U,
Luppi M. A non-chemotherapy treatment of a primary effusion lymphoma:
durable remission after intracavitary cidofovir in HIV negative PEL
refractory to chemotherapy. Ann Oncol. 2006;17(12):1849-50. http://dx.doi.org/10.1093/annonc/mdl139
PMid:16766593

- Curreli F, Friedman-Kien AE, Flore O.
Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus latency,
triggering p53-mediated apoptosis in transformed B lymphocytes. J Clin
Invest 2005; 115: 642-52. PMid:15765147 PMCid:1051998

- Cohen JI. Licking latency with licorice. J
Clin Invest. 2005; 115: 591-3. PMid:15765143 PMCid:1052015

- Klass CM and Offermann MK. Targeting human
herpesvirus-8 for the treatment of Kaposi’s sarcoma and primary
effusion lymphoma. Curr Opin Oncol. 2005; 17: 447-55. http://dx.doi.org/10.1097/01.cco.0000172823.01190.6c
PMid:16093794

- Peterson BA & Frizzera G. Multicentric
Castleman’s disease. Semin Oncol. 1993; 20: 636-47 PMid:8296200

- Soulier J, Grollet L, Oksenhendler E,
Cacoub P, Cazals-Hatem D, Babinet P, d’Agay M-F, Clauvel J-P, Raphael
M, Degos L, Sigaux F. Kaposi’s sarcoma-associated herpesvirus-like DNA
sequences in multicentric Castleman’s disease. Blood. 1995; 86:
1276-80. PMid:7632932

- Gessain A, Sudaka A, Brière J, Fouchard N,
Nicola M-A, Rio B, Arborio M, Troussard X, Audouin J, Diebold J, de Thè
G. Blood. 1996; 87: 414-6. PMid:8547672

- Corbellino M, Poirel L, Aubin JT, Paulli
M, Magrini U, Bestetti G, Galli M, Parravicini C. The role of human
herpesvirus 8 and Epstein-Barr virus in the pathogenesis of giant lymph
node hyperplasia (Castleman’s disease). Clin Infect Dis. 1996; 22:
1120-1. http://dx.doi.org/10.1093/clinids/22.6.1120
PMid:8783733

- Barozzi P, Luppi M, Masini L, Marasca R,
Savarino M, Morselli M, Ferrari MG, Bevini M, Bonacorsi G, Torelli G.
Lymphotropic herpes virus (EBV, HHV-6, HHV-8) DNA sequences in HIV
negative Castleman’s disease. J Clin Pathol Mol Pathol. 1996; 49:
M232-5. http://dx.doi.org/10.1136/mp.49.4.M232

- Bèlec L, Mohamed AS, Authier F-J, Hallouin
M-C, Soe AM, Cotigny S, Gaulard P, Gherardi RK. Human herpesvirus 8
infection in patients with POEMS syndrome-associated multicentric
Castleman’s disease. Blood. 1999; 93: 3643-3653. PMid:10339470

- Parravicini C, Olsen SJ, Capra M, Poli F,
Sirchia G, Gao SJ, Berti E, Nocera A, Rossi E, Bestetti G, Pizzuto M,
Galli M, Moroni M, Moore PS, Corbellino M. Risk of Kaposi’s
sarcoma-associated herpesvirus transmission from donor allografts among
Italian post-transplant Kaposi’s sarcoma patients. Blood. 1997; 90:
2826-9. PMid:9326251

- Dupin N, Fisher C, Kellam P, Ariad S,
Tulliez M, Franck N, Van Marck E, Salmon D, Gorin I, Escande J-P, Weiss
RA, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently
infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease,
and primary effusion lymphoma. Proc Natl Acad Sci. 1999; 96: 4546-51. http://dx.doi.org/10.1073/pnas.96.8.4546

- Dupin N, Diss TL, Kellam P, Tulliez M, Du
M-Q, Sicard D, Weiss RA, Isaacson PG, Boshoff C. HHV-8 is associated
with a plasmablastic variant of Castleman disease that is linked to
HHV-8-positive plasmablastic lymphoma. Blood. 2000; 95: 1406-12.
PMid:10666218

- Du MQ, Liu H, Diss TC, Ye H, Hamoudi RA,
Dupin N, Meignin V, Oksehendler E, Boshoff C, Isaacson PG. Kaposi
sarcoma-associated herpesvirus infects monotypic (IgM lambda) but
polyclonal naive B cells in Castleman disease and associated
lymphoproliferative disorders. Blood. 2001; 97: 2130-6. http://dx.doi.org/10.1182/blood.V97.7.2130
PMid:11264181

- Oksenhendler E, Boulanger E, Galicier L,
Du M-Q, Dupin N, Diss TC, Hamoudi R, Daniel M-T, Agbalika F, Boshoff C,
Clauvel J-P, Isaacson PG, Meignin V. High incidence of Kaposi
sarcoma-associated herpesvirus-related non–Hodgkin lymphoma in patients
with HIV infection and multicentric Castleman disease. Blood. 2002;
100: 3415-8. PMid:12384445

- Parravicini C, Corbellino M, Paulli M,
Magrini U, Lazzarino M, Moore PS, Chang Y. Expression of a
virus-derived cytokine, KSHV vIL-6 in HIV sieronegative Castleman’s
disease. Am J pathol. 1997; 6: 1517-22.

- Parravicini C, Chandran B, Corbellino M,
Berti E, Paulli M, Moore PS, Chang Y. Differential viral protein
expression in Kaposi’s sarcoma Am J Pathol. 2000; 156: 743-9. http://dx.doi.org/10.1016/S0002-9440(10)64940-1

- Staskus KA, Sun R, Miller G, Racz P,
Jaslowski A, Metroka C, Brett-Smith H, Haase AT. Cellular tropism and
viral interleukin-6 expression distinguish human herpesvirus 8
involvement in Kaposi’ sarcoma, primary effusion lymphoma, and
Castleman’s disease. J Virol. 1999; 73: 4181-7. PMid:10196314
PMCid:104197

- Luppi M, Barozzi P, Maiorana A, Trovato R,
Marasca R, Morselli M, Cagossi K, Torelli G. Expression of
cell-homologous genes of human herpesvirus-8 in human immunodeficiency
virus-negative lymphoproliferative diseases. Blood. 1999; 94: 2931-3.
PMid:10515899

- Grandadam MJ, Gorin I, Blum L, Kernbaum S,
Sicard D, Buisson Y, Agut H, Escande JB, Huraux JM. Exacerbations of
clinical symptoms in human immunodeficiency virus type-1 infected
patients with multicentric Castleman’s disease are associated with a
high increase in Kaposi’s sarcoma herpesvirus DNA load in peripheral
blood mononuclear cells. J Infect Dis.1997; 175: 1198-201. http://dx.doi.org/10.1086/593567
PMid:9129085

- Oksenhendler E, Carcelain G, Aoki Y,
Boulanger E, Maillard A, Clauvel J-P, Agbalika F. High levels of human
herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C
reactive protein correlate with exacerbation of multicentric Castleman
disease in HIV-infected patients. Blood. 2000; 96: 2069-73.
PMid:10979949

- Oksenhendler E, Cacoub P, Welker Y,
Cadranel J, Cazals-Hatem D, Autran B, Clauvel JP, Raphael M.
Multicentric Castleman disease in HIV infection: a clinical and
pathological study of 20 patients. AIDS. 1996; 10: 61-7. http://dx.doi.org/10.1097/00002030-199601000-00009
PMid:8924253

- Rieu P, Droz D, Gessain A, Grunfeld JB,
Hermine O. Retinoic acid for treatment of multicentric Castleman
disease. Lancet. 1999; 354: 1262-3. http://dx.doi.org/10.1016/S0140-6736(99)03957-4

- Casper C, Nicholas G, Huang M-L, Corey L,
Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman
disease with ganciclovir treatment. Blood. 2004; 103: 1632-4. http://dx.doi.org/10.1182/blood-2003-05-1721
PMid:14615380

- Nishimoto N, Kanakura Y, Aozasa K, Johkoh
T, Nakamura M, Nakano S, Nakano N, Ikeda Y, Sasaki T, Asaoku H, Kumagai
S, Kodama F, Nakahara H, Hagihara K, Yoshizaki K, Kishimoto T.
Humanized anti-interleukin receptor antibody treatment of multicentric
Castleman disease. Blood. 2005; 106: 2627-32. http://dx.doi.org/10.1182/blood-2004-12-4602
PMid:15998837

- Rettig MB, Ma HJ, Vescio RA, Pold M,
Schiller G, Belson D, Savage A, Nishikubo C, Wu C, Fraser J, Said JW,
Berenson JR. Kaposi’s sarcoma-associated herpesvirus infection of bone
marrow dendritic cells from multiple myeloma patients. Science. 1997;
276: 1851-4. http://dx.doi.org/10.1126/science.276.5320.1851
PMid:9188529

- Yi O, Ekman M, Anton D, Bergenbrant S,
Osterborg A, Goergii-Hemming P, Holm G, Nilsson K, Bibefeld P. Blood
dendritic cells from myeloma patients are not infected with Kaposi’s
sarcoma-associated herpesvirus (KSHV/HHV-8). Blood. 1998; 92: 402-4.
PMid:9657737

- Tarte K, Chang Y, Klein B. Kaposi’s
sarcoma-associated herpesvirus and multiple myeloma: lack of criteria
for causality. Blood. 1999; 93: 3159-63. PMid:10233868

- Ablashi DV, Chatlynne L, Thomas D,
Bourboulia D, Rettig MB, Vescio RA, Viza D, Gill P, Kyle RA, Berenson
JR, Whitman JE. Lack of serologic association of human herpesvrus-8
(KSHV) in patients with monoclonal gammopathy of undetermined
significance with and without progression to multiple myeloma. Blood.
2000; 96: 2304. PMid:10979981

- Carbone A, Gloghini A, Vaccher E, Cerri M,
Gaidano G, Dalla-Favera R, Tirelli U. Kaposi’s sarcoma-associated
herpesvirus/human herspesvirus 8-positive solid lymphomas. A
tissue-based variant of primary effusion lymphomas. J Mol Diagn. 2005;
7: 17-27. http://dx.doi.org/10.1016/S1525-1578(10)60004-9

- Hermouet S, Corre I, Gassin M,
Bigot-Corbel E, Sutton CA, Casey JW. Hepatitis C virus, human
herpesvirus 8, and the development of plasma cell leukemia. New Engl J
Med. 2003; 348: 178-9. http://dx.doi.org/10.1056/NEJM200301093480219
PMid:12519936

- Du MQ, Diss TC, Liu H, Ye H, Hamoudi RA,
Cabecadas J, Dong HY, Harris NL, Chan JK, Rees JW, Dogan A, Isaacson
PG. KSHV- and EBV-associated germinotropic lymphoproliferative
disorder. Blood. 2002; 100: 3415-8.
http://dx.doi.org/10.1182/blood-2002-02-0487 PMid:12384445

- Wheat WH, Cool CD, Morimoto Y, Rai PR,
Kirkpatrick CH, Lindenbaum BA, Bates CA, Ellison MC, Seris AE, Brown
KK, Routes JM. Possible role of human herpesvirus 8 in the
lymphoproliferative disorders in common variable immunodeficiency. J
Exp Med. 2005; 202: 479-84. http://dx.doi.org/10.1084/jem.20050381
PMid:16103407 PMCid:2212861

- Luppi M, Barozzi P, Marasca R, Savarino
M, Torelli G. HHV-8-associated primary cerebral B-cell lymphoma in
HIV-negative patient after long term steroids. The Lancet.1996; 347:
980. http://dx.doi.org/10.1016/S0140-6736(96)91473-7

- Kapelushnik, J., Ariad, S., Benharroch,
D., Landau, D., Moser, A., Delsol, G., Brousset, P. Post renal
transplantation human herpesvirus 8-associated lymphoproliferative
disorder and Kaposi’s sarcoma. Br J Haematol. 2001; 113: 425-8. http://dx.doi.org/10.1046/j.1365-2141.2001.02740.x
PMid:11380409

- Matsushima, A.Y., Strauchen, J.A., Lee,
G., Scigliano, E., Hale, E.E., Weisse, M.T., Burstein, D., Kamel, O.,
Moore, P.S., Chang, Y. Posttransplantation plasmacytic proliferations
related to Kaposi’s sarcoma-associated herpesvirus. Am J Surg Pathol.
1999; 23: 1393-1400. http://dx.doi.org/10.1097/00000478-199911000-00010
PMid:10555008

- Luppi, M., Barozzi, P., Maiorana, A.,
Artusi, T., Trovato, R., Marasca, R., Savarino, M., Ceccherini-Nelli,
L., Torelli, G. Human herpesvirus-8 DNA sequences in human
immunodeficiency virus-negative angioimmunoblastic lymphadenopathy and
benign lymphadenopathy with giant germinal center hyperplasia and
increased vascularity. Blood. 1996; 87: 3903-9. PMid:8611719

- Chang Y, Cesarman E, Pessin MS, Lee F,
Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like
DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994; 266:
1865-9. http://dx.doi.org/10.1126/science.7997879
PMid:7997879

- Oksenhendler, E., Cazals-Hatem, D.,
Schulz, T.F., Barateau, V., Grollet, L., Sheldon, J., Clauvel, J.P.,
Sigaux, F., Agbalika, F. Transient angiolymphoid hyperplasia and
Kaposi’s sarcoma after primary infection with human herpesvirus 8 in a
patient with human immunodeficiency virus infection. N Engl J Med.
1998; 338: 1585-90. http://dx.doi.org/10.1056/NEJM199805283382204
PMid:9603796

- Wong SW, Bergquam EP, Swanson RM, Lee FW,
Shiigi SM, Avery NA, Fanton JW, Axthelm MK. Induction of B cell
hyperplasia in simian immunodeficiency virus-infected Rhesus macaques
with the simian homologue of Kaposi’s sarcoma-associated herpesvirus. J
Exp Med.1999; 190: 827-40. http://dx.doi.org/10.1084/jem.190.6.827
PMid:10499921 PMCid:2195633

- Luppi M and Torelli G. Human herpesvirus
8 and interstitial pneumonitis in an HIV-negative patient. New Engl J
Med 1996; 335: 351-2. http://dx.doi.org/10.1056/NEJM199608013350514
PMid:8668220

- Muller A, Franzen C, Klussmann P, Wagner
M, Diehl V, Fatkenhenen G, Salzberger B, Ablashi DV, Krueger GR. Human
herpesvirus type 8 in HIV infected patients with interstitial
pneumonitis. J Infect Dis. 2000; 40: 242-7.

- Luppi M, Barozzi P, Schulz TF, Trovato R,
Donelli A, Narni F, Sheldon J, Marasca R, Torelli G. Non malignant
disease associated with human herpesvirus 8 reactivation in patients
who have undergone autologous peripheral blood stem cell
transplantation. Blood. 2000; 96: 2355-7. PMid:11001882

- Luppi M, Barozzi P, Schulz TF, Setti G,
Staskus K, Trovato R, Narni F, Donelli A, Maiorana A, Marasca R,
Sandrini S, Torelli G. Bone marrow failure associated with human
herpesvirus 8 infection after transplantation. New Engl J Med. 2000;
343: 1378-85. http://dx.doi.org/10.1056/NEJM200011093431905
PMid:11070102

- Luppi M, Trovato R, Barozzi P, Gibellini
F, Potenza L, Riva G, Torelli G. Suppressive effect of human
herpesvirus-8/Kaposi sarcoma-associated herpesvirus on in vitro colony
formation of hematopoietic progenitor cells. Leuk Res. 2005; 29: 251-3.
http://dx.doi.org/10.1016/j.leukres.2005.01.012
PMid:15978946

- Wu W, Vieira J, Fiore N, Banerjee P,
Sieburg M, Rochford R, Harrington Jr, Feuer G. KSHV/HHV-8 infection of
human hematopoietic progenitor (CD34+) cells: persistence of infection
during hematopoiesis in vitro and in vivo. Blood. 2006 Jul
1;108(1):141-51. http://dx.doi.org/10.1182/blood-2005-04-1697
PMid:16543476 PMCid:1895828

- Abrams DI, Chinn EK, Lewis BJ, Volberding
PA, Conant MA, Townsend RM. Hematologic manifestations in homosexual
men with Kaposi’s sarcoma Am J Clin Pathol. 1984; 81: 13-8.
PMid:6704205
