Review Articles
E.M. Rego and Gil C. De Santis
Published: October 24, 2011
Received: August 30, 2011
Accepted: September 19, 2011
Mediterr J Hematol Infect Dis 2011, 3(1): e2011048, DOI 10.4084/MJHID.2011.048
This article is available from: http://www.mjhid.org/article/view/9101
Abstract
Differentiation syndrome (DS)
represents a life-threatening
complication in patients with acute promyelocytic leukemia (APL)
undergoing induction therapy with all-trans retinoic acid (ATRA) or
arsenic trioxide (ATO). It affected about 20-25% of all patients and so
far there are no definitive diagnostic criteria. Clinically, DS is
characterized by weight gain, fever not attributable to infection,
respiratory distress, cardiac involvement, hypotension, and/or acute
renal failure. At the histological point of view, there is an extensive
interstitial and intra-alveolar pulmonary infiltration by maturing
myeloid cells, endothelial cell damage, intra-alveolar edema,
inter-alveolar hemorrhage, and fibrinous exsudates. DS pathogenesis is
not completely understood, but it is believed that an excessive
inflammatory response is the main phenomenon involved, which results in
increased production of chemokines and expression of adhesion molecules
on APL cells. Due to the high morbidity and mortality associated with
DS, its recognition and the prompt initiation of the treatment is of
utmost importance. Dexamethasone is considered the mainstay of
treatment of DS, and the recommended dose is 10 mg twice daily by
intravenous route until resolution of DS. In severe cases (respiratory
or acute renal failure) it is recommended the discontinuation of ATRA
or ATO until recovery.
Introduction
Contemporary
treatment of acute promyelocytic leukemia (APL) consists of a
combination of all-trans retinoic acid (ATRA) with
anthracycline-containing chemotherapy, or arsenic trioxide (ATO). These
modalities of treatment lead to complete remission rates greater than
90% and cure rates of approximately 80%, in contrast with results
reported before introduction of ATRA, in which disease free survival
after 3 years were inferior to 20%.[1] The
introduction of ATRA, which
belongs to a class of chemical compounds related to vitamin A known as
retinoids, to treat APL in the 1980s revolutionized the concept of
treatment of cancer, of which, besides the aim to destroy by
chemotherapy the pathologic cells, there is the objective to enforce
its maturation.[2] This latter process culminates with
cell death. ATRA,
when administered in pharmacological doses, triggers APL cells to
differentiate into mature granulocytes. The molecular process is not
fully known but the main aspect involves the degradation of the complex
PML-RARα, a phenomenon that unleashes the mechanisms required for
terminal maturation.
Besides ATRA, arsenic trioxide (ATO) is an important agent that is
being used for APL treatment since the early 1990s. ATO, or white
arsenic, is one of the three forms in which arsenic exists.[3]
ATO is one
of the oldest drugs known to man.[4] Despite its
reputation of being a
poison and carcinogenic agent, ATO is usually well tolerated. This drug
degrades PML-RARα by targeting its PML moiety (it also degrades normal
PML). ATO provokes apoptosis when used at high concentration (1-2 x
10-6 M), or partial maturation of APL cells when used at low
concentration (0.25-0.5 x 10-6 M) and for a longer period of time.
Through these actions it improves the clinical outcome of refractory,
relapsed or newly diagnosed APL.[5,6] Both ATRA and
ATO, alone or in
combination, can trigger differentiation syndrome (DS), a relatively
common complication of APL treatment previously named retinoic acid
syndrome.[7]
Clinically, DS is characterized by weight gain, fever not attributable
to infection, respiratory distress, cardiac involvement, hypotension,
and acute renal failure.[7] DS is
potentially fatal and its recognition
and treatment is of utmost importance. Histological analysis of
patients who died of DS revealed extensive interstitial and
intra-alveolar pulmonary infiltration by maturing myeloid cells,
endothelial cell damage intra-alveolar edema, inter-alveolar
hemorrhage, and fibrinous exsudates.[7,8]
Regarding DS incidence, most studies reported that approximately one
fourth of APL patients receiving ATRA as induction therapy,[9]
but
depending on the criteria employed; this incidence was lower. For
instance, Mandelli et al, from GIMEMA, considered DS when at least five
symptoms were present, and as a result DS incidence was of only 2.5%
(6/240).[10]
As mentioned previously, ATO is also associated with DS with a similar
frequency.[11] However, the combination of ATRA and
ATO was reported to
have induced DS in only 16% (13/82) of patients.[12]
This finding is not
a surprise, as it was demonstrated that the combination ATRA/ATO was
associated with fewer complications than ATRA or ATO alone.[13]
Clinical Picture
Table 1 lists the main symptoms of DS and their respective
frequency. De Botton et al reported in a large series of patients who
developed DS revealed that respiratory signs/symptoms and fever were
the most frequent clinical presentation of DS. These authors described
413 patients with newly diagnosed APL, 64 (15%) of which developed DS
(median at day 7), and 9 (14%) patients died of it.[14]
Respiratory
involvement was reviewed recently.[15] Its most
common manifestations are
pulmonary infiltrate, pleural effusion and respiratory distress.
Cardiac involvement is more commonly characterized by pericardial
effusion, but it can also present as chest pain typical of coronary
obstruction.[16] Rarer clinical presentations such as
musculoskeletal
symptoms were also reported.[17] There is no
pathognomonic clinical sign
or laboratory test to diagnose DS. For this reason, sometimes DS can be
misdiagnosed or confounded with other concurrent medical condition,
such as infection and heart failure. It was suggested to consider DS
when at least three of the following signs or symptoms are present:
fever, weight gain, respiratory distress, pulmonary infiltrates, pleura
or pericardial effusions, hypotension, and renal failure.[9]
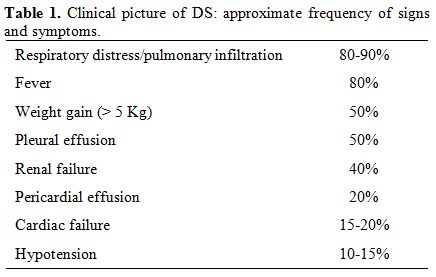
Table 1. Clinical picture of DS: approximate frequency of signs and symptoms.
Among
the conditions that clinically resemble DS are: acute respiratory
distress syndrome (ARDS),[18]
hyperkeukocytosis/leukostasis in AML[19] and
engraftment syndrome after hematopoietc stem cell transplantation.[20] In
all these conditions, there is an organ infiltration, especially the
lungs, by granulocytes or leukemic cells activated by inflammatory
cytokines, growth factors or differentiation agents. Curiously, and
certainly not fortuitously, some of them can be successfully treated
with corticosteroids.
Pathogenesis
The molecular mechanisms leading to DS development are not fully known,
but it is believed that an excessive inflammatory response is the main
phenomenon involved. This inflammatory response is provoked by leukemic
cells in the process of differentiation, which results in increased
production of chemokines and expression of adhesion molecules on APL
cells. The inflammation would result in organ infiltration by blast
cells, especially the lungs, capillary-leak syndrome and organ failure.
The mechanism must be similar to what occur with normal granulocytes
recruited to sites with inflammation, in which the circulating
leukocytes are captured by the endothelial cells (EC) on which occurs
the process called rolling (mediated by selectins), followed by their
firm attachment to EC (mediated by integrins), and finally, their
transendothelial migration into the tissue.[21]
Leukocyte transmigration
requires secretion of proteases (metalloproteases and neutrophil
elastase) that disrupts the endothelial barrier. As a consequence there
is extravasation of fluids into alveolar space.[22,23]
A large array of chemokines has their production greatly increased by
treatment of APL cells with ATRA.[24,25] The
migration of blast cells to
lungs is triggered by increased specific chemokines production by
alveolar epithelial cells. APL cells are the attracted to lungs where
they transmigrate into tissue and alveolar space. APL cells also
contribute to chemokine production, which increase further blast
recruitment. Administration of corticosteroids suppresses chemokine
production by alveolar cells and then abrogates lung infiltration.
Besides corticosteroids, neutralizing antibodies was able to reduce in
vitro transmigration of leukemic cells (NB4) towards alveolar
epithelial cells (A549).[26,27]
In addition to chemokine increased production, it was demonstrated by
our group and others that treatment with ATRA up-regulated the
expression of adhesion molecules on blast cells and on endothelial
cells, and that dexamethasone counteracted the effects of ATRA.[28-30]
Our group has demonstrated that NB4 cells (an APL cell lineage) and
primary APL cells treated with ATRA enhanced their expression of CD54
and CD18. Moreover, mice injected with APL cells previously exposed to
ATRA had the lungs infiltrated by blasts, demonstrated by increased
myeloperoxidase (MPO) activity. The infiltration however could be
blocked by dexamethasone and neutralizing antibodies against adhesion
molecules CD54 and CD18. Furthermore, knockout mice for CD54 had not
increased MPO activity in lungs after injection of treated APL cells.
These findings demonstrated the main role certain adhesion molecules
have in DS development and unveil part of the mechanisms of action of
corticosteroids in treatment of this syndrome.[30]
Risk Factors
Risk factors for developing DS are controversial. Vahdat et al have
shown that peripheral blood leukocyte count peak at the onset of DS
symptoms.[31] In agreement of this finding, Tallman
and colleagues
reported that in 44 of 167 (26%) patients with APL receiving ATRA, the
median white blood cell (WBC) count at diagnosis was 1,450/µL, and
31,000/µL at the time the syndrome was diagnosed. However, neither the
initial leukocyte count nor the rate of rise in leukocyte counts on
days preceding DS correlated with its incidence. The European trial
showed that patients with WBC > 5 x 109/L at presentation and DS
tended to require mechanical ventilation more frequently than patients
with lower WBC.[14]
Regarding the expression of myeloid associated markers, in the study of
Vahdat et al. the basal expression of CD13 (amino-peptidase N) was
highly associated with both development of DS as well as with elevated
leukocyte count. However, others and our group did not find correlation
between basal WBC count or immune-phenotype characteristics (expression
of CD33, CD13 and CD117) with incidence of DS.[32]
Recently, Montesinos et al reported the outcome of 739 APL patients
treated with ATRA and idarubicin and found that variables predictive of
severe DS included WBC > 5 x 109/L, abnormal serum creatinine
levels, FTL3-ITD mutation, the microgranular subtype, the short
PML-RARA isoform, and male sex.[33] However, in a
multivariate analysis,
only WBC counts and serum creatinine levels were significant. Moreover,
with respect to APL morphological subtype, the microgranular variant
(M3V) was found by others to protect against DS by the US Intergroup
study.[8] However, recently, Tallman and colleagues,
in a large joint
study of the North American Intergroup and the PETHEMA Group, found
that the incidence of DS was 26% in M3V and 25% for the classical APL
(P= 0.66).[34]
Another factor that seems to influence the incidence of DS is the
timing of initiation of chemotherapy. De Botton et al have shown that
early onset of chemotherapy can reduce the incidence of DS in newly
diagnosed APL with low WBC count at presentation (< 5 x 109/L). The
incidence of DS in the ATRA with concurrent chemotherapy arm was lower
than in the arm in which chemotherapy was not started concurrently
(9.2% and 18% respectively, P= 0.035).[35]
Dore et al reported an association between development of DS and the AA
genotype at Codon 469 of ICAM-1, which suggests that susceptibility to
DS in APL patients may be influenced by genetic variation in adhesion
molecule loci.[36] Finally, it was recently
demonstrated that high body
mass index is an independent predictor of DS.[37]
Management and Outcome
As DS can have a subtle clinical picture at presentation but progress
rapidly, it is of utmost importance to be aware of this complication
and initiate therapy as soon as it was suspected. Initial measures
involves ventilatory and blood pressure support. Dexamethasone is
considered the mainstay of treatment of DS, and should be administered
at the first sign or symptom of this syndrome. The dose recommended is
10 mg twice daily by intravenous route until resolution of DS, after
which the dose can be progressively reduced in the next few days or
weeks. There is no need to discontinue ATRA if DS is not severe.
However, in severe cases (respiratory or acute renal failure) it seems
reasonable to discontinue the drug until clinical recovery, when ATRA
could then be restarted (Table 2).
In the study by Tallman et al. ATRA was discontinued in 36 of the 44
patients (82%) that developed DS and resumed in 19 of the 36 patients
(53%). However, DS recurred in 3 (16%) of those 19 patients.[8]

Table 2. Measures at suspicion of DS.
Prophylactic administration of steroids is controversial. There is no evidence of its benefit in reducing DS incidence or severity (Sanz et al, 2009). For this reason, this approach cannot be recommended at moment, despite the report that the prophylactic use of corticosteroids from the start of ATRA reduced the incidence of severe DS, but not its mortality.[33]
Some patients have DS that are refractory to corticosteroids. There are yet no widely accepted alternative to it. It seems reasonable to employ in future agents that block migration, adhesion or transmigration of APL cells. A few years ago, Kawasaki and colleagues administered sivelestat, a small molecule that inhibits neutrophil elastase, and that has been shown to be effective in animal models of ARDS/ALI, reported its successful use in two patients with DS.[38]
The frequency of death due to DS varied from 7.8% to 33% in clinical trials.[7,39] In the study by De La Serna et al DS was responsible for approximately one fifth of induction deaths, which occurred at a median of 171-26 days of starting induction.[40] Taken together these results suggest that there was a decrease in DS mortality in recent years, probably due to a more prompt recognition of its symptoms and signals and early introduction of therapeutic measures.
Conclusions
DS is an unpredictable complication of treatment of APL with ATRA or
ATO, and occurs usually after a few days or weeks of initiation of
induction therapy. It is extremely uncommon during maintenance
treatment. There are no laboratory tests or clinical exams specific for
DS. Considered alone, demonstration of diffuse opacity of lungs on Rx
suggestive of edema is perhaps the most suggestive exam for DS.
Treatment consists of ventilation support and administration of
steroids by intravenous route for a few days or weeks after clinical
resolution. In severe cases (respiratory or acute renal failure) it
seems reasonable to discontinue ATRA until clinical recovery, when it
could then be restarted. Concurrent chemotherapy can be useful to
reduce incidence and severity of DS, despite its potential dangerous
effect on blood hemostasis. Despite the fact that, in the last years,
concurrent chemotherapy has reduced the incidence and severity of DS,
the most important action to reduce DS morbidity and mortality remains
the early recognition of its symptoms, and institution of supportive
measures and treatment with dexamethasone.
References
- Fenaux
P, Chomienne C, Degos L. All-trans retinoic acid and chemotherapy in
the treatment of acute promyelocytic leukemia. Semin Hematol 2001
Jan;38(1):13-25. http://dx.doi.org/10.1016/S0037-1963(01)90002-2

- Huang ME, Ye YC, Chen SR, Chai JR, Lu JX,
Zhoa L, et al. Use of all-trans retinoic acid in the treatment of acute
promyelocytic leukemia. Blood1988 Aug;72(2):567-72. PMid:3165295

- Wang ZY, Chen Z. Acute promyelocytic
leukemia: from highly fatal to highly curable. Blood2008 Mar
1;111(5):2505-15. http://dx.doi.org/10.1182/blood-2007-07-102798
PMid:18299451

- Zhu J, Chen Z, Lallemand-Breitenbach V, de
The H. How acute promyelocytic leukaemia revived arsenic. Nat Rev
Cancer2002 Sep;2(9):705-13. http://dx.doi.org/10.1038/nrc887 PMid:12209159

- de The H, Chen Z. Acute promyelocytic
leukaemia: novel insights into the mechanisms of cure. Nat Rev
Cancer2010 Nov;10(11):775-83. http://dx.doi.org/10.1038/nrc2943
PMid:20966922

- Grimwade D, Mistry AR, Solomon E, Guidez F.
Acute promyelocytic leukemia: a paradigm for differentiation therapy.
Cancer Treat Res2010;145:219-35. http://dx.doi.org/10.1007/978-0-387-69259-3_13
PMid:20306254

- Frankel SR, Eardley A, Lauwers G, Weiss M,
Warrell RP, Jr. The "retinoic acid syndrome" in acute promyelocytic
leukemia. Ann Intern Med1992 Aug 15;117(4):292-6. PMid:1637024

- Tallman MS, Andersen JW, Schiffer CA,
Appelbaum FR, Feusner JH, Ogden A, et al. Clinical description of 44
patients with acute promyelocytic leukemia who developed the retinoic
acid syndrome. Blood2000 Jan 1;95(1):90-5. PMid:10607690

- Larson RS, Tallman MS. Retinoic acid
syndrome: manifestations, pathogenesis, and treatment. Best Pract Res
Clin Haematol2003 Sep;16(3):453-61. http://dx.doi.org/10.1016/S1521-6926(03)00043-4

- Mandelli F, Diverio D, Avvisati G, Luciano
A, Barbui T, Bernasconi C, et al. Molecular remission in PML/RAR
alpha-positive acute promyelocytic leukemia by combined all-trans
retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie
Ematologiche Maligne dell'Adulto and Associazione Italiana di
Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood1997 Aug
1;90(3):1014-21. PMid:9242531

- Soignet SL, Frankel SR, Douer D, Tallman
MS, Kantarjian H, Calleja E, et al. United States multicenter study of
arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin
Oncol2001 Sep 15;19(18):3852-60. PMid:11559723

- Ravandi F, Estey E, Jones D, Faderl S,
O'Brien S, Fiorentino J, et al. Effective treatment of acute
promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide,
and gemtuzumab ozogamicin. J Clin Oncol2009 Feb 1;27(4):504-10. http://dx.doi.org/10.1200/JCO.2008.18.6130
PMid:19075265

- Wang G, Li W, Cui J, Gao S, Yao C, Jiang
Z, et al. An efficient therapeutic approach to patients with acute
promyelocytic leukemia using a combination of arsenic trioxide with
low-dose all-trans retinoic acid. Hematol Oncol2004 Jun;22(2):63-71. http://dx.doi.org/10.1002/hon.728
PMid:15468344

- De Botton S, Dombret H, Sanz M, Miguel JS,
Caillot D, Zittoun R, et al. Incidence, clinical features, and outcome
of all trans-retinoic acid syndrome in 413 cases of newly diagnosed
acute promyelocytic leukemia. The European APL Group. Blood1998 Oct
15;92(8):2712-8. PMid:9763554

- Luesink M, Jansen JH. Advances in
understanding the pulmonary infiltration in acute promyelocytic
leukaemia. Br J Haematol2010 Nov;151(3):209-20. http://dx.doi.org/10.1111/j.1365-2141.2010.08325.x
PMid:20735400

- De Santis GC, Madeira MI, de Oliveira LC,
Falcao RP, Rego EM. Cardiac stunning as a manifestation of ATRA
differentiation syndrome in acute promyelocytic leukemia. Med Oncol
2011
Jan 26.

- Yu W, Burns CM. All-trans retinoic
acid-induced focal myositis, synovitis, and mononeuritis. J Clin
Rheumatol2009 Oct;15(7):358-60. http://dx.doi.org/10.1097/RHU.0b013e31818866d8
PMid:20009973

- Kollef MH, Schuster DP. The acute
respiratory distress syndrome. N Engl J Med1995 Jan 5;332(1):27-37. http://dx.doi.org/10.1056/NEJM199501053320106
PMid:7646623

- Oliveira LC, Romano LG, Prado-Junior BP,
Covas DT, Rego EM, De Santis GC. Outcome of acute myeloid leukemia
patients with hyperleukocytosis in Brazil. Med Oncol2010
Dec;27(4):1254-9. http://dx.doi.org/10.1007/s12032-009-9367-9
PMid:19937404

- Spitzer TR. Engraftment syndrome following
hematopoietic stem cell transplantation. Bone Marrow Transplant2001
May;27(9):893-8. http://dx.doi.org/10.1038/sj.bmt.1703015 PMid:11436099

- Zarbock A, Ley K. Neutrophil adhesion and
activation under flow. Microcirculation2009 Jan;16(1):31-42. http://dx.doi.org/10.1080/10739680802350104
PMid:19037827 PMCid:2851240

- Moraes TJ, Chow CW, Downey GP. Proteases
and lung injury. Crit Care Med2003 Apr;31(4 Suppl):S189-94. http://dx.doi.org/10.1097/01.CCM.0000057842.90746.1E
PMid:12682439

- Kawabata K, Hagio T, Matsuoka S. The role
of neutrophil elastase in acute lung injury. Eur J Pharmacol2002 Sep
6;451(1):1-10. http://dx.doi.org/10.1016/S0014-2999(02)02182-9

- Shibakura M, Niiya K, Niiya M, Asaumi N,
Yoshida C, Nakata Y, et al. Induction of CXC and CC chemokines by
all-trans retinoic acid in acute promyelocytic leukemia cells. Leuk
Res2005 Jul;29(7):755-9. http://dx.doi.org/10.1016/j.leukres.2005.01.005
PMid:15927671

- Luesink M, Pennings JL, Wissink WM,
Linssen PC, Muus P, Pfundt R, et al. Chemokine induction by all-trans
retinoic acid and arsenic trioxide in acute promyelocytic leukemia:
triggering the differentiation syndrome. Blood2009 Dec
24;114(27):5512-21. http://dx.doi.org/10.1182/blood-2009-02-204834
PMid:19828696

- Tsai WH, Hsu HC, Lin CC, Ho CK, Kou YR.
Role of interleukin-8 and growth-regulated oncogene-alpha in the
chemotactic migration of all-trans retinoic acid-treated promyelocytic
leukemic cells toward alveolar epithelial cells. Crit Care Med2007
Mar;35(3):879-85. http://dx.doi.org/10.1097/01.CCM.0000256844.38259.27
PMid:17235257

- Tsai WH, Shih CH, Lin CC, Ho CK, Hsu FC,
Hsu HC. Monocyte chemotactic protein-1 in the migration of
differentiated leukaemic cells toward alveolar epithelial cells. Eur
Respir J2008 May;31(5):957-62. http://dx.doi.org/10.1183/09031936.00135707
PMid:18216048

- Larson RS, Brown DC, Sklar LA. Retinoic
acid induces aggregation of the acute promyelocytic leukemia cell line
NB-4 by utilization of LFA-1 and ICAM-2. Blood1997 Oct 1;90(7):2747-56.
PMid:9326242

- Ninomiya M, Kiyoi H, Ito M, Hirose Y, Naoe
T. Retinoic acid syndrome in NOD/scid mice induced by injecting an
acute promyelocytic leukemia cell line. Leukemia2004 Mar;18(3):442-8. http://dx.doi.org/10.1038/sj.leu.2403284
PMid:14749706

- Cunha De Santis G, Tamarozzi MB, Sousa RB,
Moreno SE, Secco D, Garcia AB, et al. Adhesion molecules and
Differentiation Syndrome: phenotypic and functional analysis of the
effect of ATRA, As2O3, phenylbutyrate, and G-CSF in acute promyelocytic
leukemia. Haematologica2007 Dec;92(12):1615-22. http://dx.doi.org/10.3324/haematol.10607
PMid:18055984

- Vahdat L, Maslak P, Miller WH, Jr.,
Eardley A, Heller G, Scheinberg DA, et al. Early mortality and the
retinoic acid syndrome in acute promyelocytic leukemia: impact of
leukocytosis, low-dose chemotherapy, PMN/RAR-alpha isoform, and CD13
expression in patients treated with all-trans retinoic acid. Blood1994
Dec 1;84(11):3843-9. PMid:7949141

- Santos FL, Dore AI, Lima AS, Garcia AB,
Zago MA, Rizzatti EG, et al. [Hematological features and expression
profile of myeloid antigens of acute promyelocytic leukemia patients:
analysis of prognostic factors for development of the retinoic acid
syndrome]. Rev Assoc Med Bras2004 Jul-Sep;50(3):286-92. http://dx.doi.org/10.1590/S0104-42302004000300036

- Montesinos P, Bergua JM, Vellenga E, Rayon
C, Parody R, de la Serna J, et al. Differentiation syndrome in patients
with acute promyelocytic leukemia treated with all-trans retinoic acid
and anthracycline chemotherapy: characteristics, outcome, and
prognostic factors. Blood2009 Jan 22;113(4):775-83. http://dx.doi.org/10.1182/blood-2008-07-168617
PMid:18945964

- Tallman MS, Kim HT, Montesinos P,
Appelbaum FR, de la Serna J, Bennett JM, et al. Does microgranular
variant morphology of acute promyelocytic leukemia independently
predict a less favorable outcome compared with classical M3 APL? A
joint study of the North American Intergroup and the PETHEMA Group.
Blood2010 Dec 16;116(25):5650-9. http://dx.doi.org/10.1182/blood-2010-06-288613
PMid:20858857

- de Botton S, Chevret S, Coiteux V, Dombret
H, Sanz M, San Miguel J, et al. Early onset of chemotherapy can reduce
the incidence of ATRA syndrome in newly diagnosed acute promyelocytic
leukemia (APL) with low white blood cell counts: results from APL 93
trial. Leukemia2003 Feb;17(2):339-42. http://dx.doi.org/10.1038/sj.leu.2402807
PMid:12592333

- Dore AI, Santana-Lemos BA, Coser VM,
Santos FL, Dalmazzo LF, Lima AS, et al. The association of ICAM-1 Exon
6 (E469K) but not of ICAM-1 Exon 4 (G241R) and PECAM-1 Exon 3 (L125V)
polymorphisms with the development of differentiation syndrome in acute
promyelocytic leukemia. J Leukoc Biol2007 Nov;82(5):1340-3. http://dx.doi.org/10.1189/jlb.0207095
PMid:17704297

- Jeddi R, Ghedira H, Mnif S, Gouider E,
Fenaux P, Meddeb B. High body mass index is an independent predictor of
differentiation syndrome in patients with acute promyelocytic leukemia.
Leuk Res Apr;34(4):545-7.

- Kawasaki K, Akaike H, Miyauchi A, Ouchi K.
Sivelestat relieves respiratory distress refractory to dexamethasone in
all-trans retinoic acid syndrome: a report of two cases. Eur J
Haematol2006 Nov;77(5):448-52. http://dx.doi.org/10.1111/j.0902-4441.2006.t01-1-EJH2852.x
PMid:16930140 PMCid:1618807

- Fenaux P, Chastang C, Chevret S, Sanz M,
Dombret H, Archimbaud E, et al. A randomized comparison of all
transretinoic acid (ATRA) followed by chemotherapy and ATRA plus
chemotherapy and the role of maintenance therapy in newly diagnosed
acute promyelocytic leukemia. The European APL Group. Blood1999 Aug
15;94(4):1192-200. PMid:10438706

- de la Serna J, Montesinos P, Vellenga E,
Rayon C, Parody R, Leon A, et al. Causes and prognostic factors of
remission induction failure in patients with acute promyelocytic
leukemia treated with all-trans retinoic acid and idarubicin. Blood2008
Apr 1;111(7):3395-402. http://dx.doi.org/10.1182/blood-2007-07-100669
PMid:18195095
