Original
Articles
Acute
Promyelocytic Leukemia: an Experience on 95 Greek Patients Treated in
the All-Trans-Retinoic Acid EraMaria Pagoni1, Maria Garofalaki1, Fotios Panitsas1, Kalliopi Manola2, Katerina Psarra3, Panagiotis Economopoulos1, Aggeliki Vourtsi1, Marios Antoniades4, Kostas Gkirkas5, Evangelia Tzouvara6, Fotis Katis7, Chrystalla Prokopiou8, Irene Tziotziou1, Artemis Balta1, Eleni Lemissiou4, Panagiotis Tsirigotis5, Panagiotis Repoussis7 and Nicolas Harhalakis1
1Hematology-Lymphoma
Department - BMT Unit, Evangelismos Hospital, Athens, Greece
2Department of Cytogenetics, Laboratory of Health Physics and Environmental Hygiene, National Center for Scientific Research (NCSR) “Demokritos”, Athens, Greece
3Immunology and Histocompatibility Department, Evangelismos Hospital, Athens, Greece
4Haematology Clinic, Nicosia General Hospital, Cyprus
5Attikon Hospital Athens, Greece,
6Hematology Department, University Hospital of Patras, Greece
7Metaxa Anticancer Hospital, Peiraias, Greece
8Haematology Clinic, Limassol General Hospital, Cyprus
2Department of Cytogenetics, Laboratory of Health Physics and Environmental Hygiene, National Center for Scientific Research (NCSR) “Demokritos”, Athens, Greece
3Immunology and Histocompatibility Department, Evangelismos Hospital, Athens, Greece
4Haematology Clinic, Nicosia General Hospital, Cyprus
5Attikon Hospital Athens, Greece,
6Hematology Department, University Hospital of Patras, Greece
7Metaxa Anticancer Hospital, Peiraias, Greece
8Haematology Clinic, Limassol General Hospital, Cyprus
Correspondence
to:
Maria Pagoni, MD, Hematology-Lymphoma Department-BMT Unit, Evangelismos
Hospital, 45-47 Ipsilantou str., 10676 Athens, Greece, Tel.:
+302107201733, Fax: +302107248313, +302107203256, e-mail: marianpagoni@yahoo.com
Published: November 28, 2011
Received: September 18, 2011
Accepted: October 4, 2011
Mediterr J Hematol Infect Dis 2011, 3(1): e2011053, DOI 10.4084/MJHID.2011.053
This article is available from: http://www.mjhid.org/article/view/9197
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract.
Acute promyelocytic leukemia (APL) is highly curable with the combination of all-trans-retinoic acid (ATRA) and anthracycline based chemotherapy, but the percentage of early deaths remains high. In the present study, we report the clinical, immunophenotypic, cytogenetic and molecular characteristics and outcome of APL patients diagnosed and treated in various Hospitals of Greece and Cyprus.
We describe the data of ninety-five APL patients who were diagnosed during the last 15 years. Seven (7.4%) newly diagnosed APL patients died due to intracranial hemorrhage within 72 hours of presentation. All but two patients were induced with ATRA alone or ATRA plus chemotherapy. The early death rate was 14.9%. After induction all 80 evaluable patients achieved complete hematologic remission. The cumulative incidence of relapse was 18.3%. Eight of the ten relapsed patients were successfully salvaged, while both patients with molecularly resistant disease died during salvage treatment. Overall survival (OS) at 5 years was 78.4% and disease free survival (DFS) 73.6%. In multivariate analysis of OS age over 60 years, DIC at diagnosis and marginally major hemorrhage at presentation were identified as adverse prognostic factors. In the subgroup of patients with available data on FLT3 mutation status (49 out of 94), ITD positivity also remained as an independent prognostic factor in the final model of OS, together with major hemorrhage and marginally high Sanz score. We found a close correlation between the CD2 expression and the development of the differentiation syndrome (DS). In conclusion, the main problem in managing patients with APL is still the high early death rate.
Introduction
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myelogenous leukemia (AML) with special biological, morphological, cytogenetic, and molecular characteristics and also clinical features. Morphologically APL is identified by the FAB classification as M3 subtype (hypergranular) and its microgranular variant (M3v).[1-5] Cytogenetically is characterized by the t(15;17)(q22;q21) which is considered to be a favorable cytogenetic aberration.[6-9]
In rare cases other translocations as t(11;17), t(5;17) can be detected.[10] Additional chromosome aberrations to t(15;17) have been observed in 23%-43% of APL cases without changing the favorable prognosis.[11-15]
At the molecular level, the result of the t(15;17) is the formation of two functional fusion genes, PML-RARα and RARα-PML on the derivative chromosomes 15 and 17 respectively.[5,8,9,16] A variety of PML-RARα transcripts [bcr1 or Long (L), bcr3 or Short (S), bcr2 or Variable (V)] are produced due to different breakpoints in PML gene and alternative splicing in chromosome [15.17,18] The FLT3 gene aberrations, including internal tandem duplications (ITDs) and D835 (tyrosine kinase domain, TKD) mutations occur in 30%-50% in APL. However, the significance of FLT3 mutations as a prognostic factor is not firmly established.[19-22]
With current treatment strategies with all-trans-retinoic acid (ATRA) in combination with anthracycline based chemotherapy, approximately 70%-80% of patients with newly diagnosed APL carrying PML/RARα achieve long-term remission and are probably cured. However, relapses occur in 10%-30% of patients. The role of cytosine arabinoside and other agents remains controversial. For patients in whom chemotherapy is contraindicated, for elderly patients and for some relapsing patients Arsenic Trioxide (ATO) is a suitable alternative, as a single agent or in combination with ATRA. There is not agreement on the most appropriate consolidation therapy but generally two to three cycles of anthracycline-based chemotherapy are considered mandatory. Finally, there is an open debate about the need for maintenance treatment.[23-31]
Besides the excellent results, serious problems still remain: a high early death rate (up to 10% for patients who enrolled in clinical trials and higher in real life), cardiotoxicity, secondary myelodysplastic syndromes, and relapse rising up to 20%-30% in high risk patients.[30,32,33-37]
The APL published data in Greek patients are very limited, usually restricted to case reports or small series.[38-41]
In the present study, we report on 95 patients from Greece and Cyprus in order to clarify the clinical, cytogenetic and molecular features as well as their outcome.
Design and methods
Between January 1996 and June 2011 a total of 95 patients with APL were diagnosed and treated in 6 tertiary hospitals in Athens, Patras, Nicosia and Limassol. The diagnosis was confirmed with cytogenetics for the presence of t(15;17) and/or molecular studies for the presence of the PML/RARα fusion gene. Informed consent was obtained from all patients according the Declaration of Helsinki and all the protocols were approved by the Research Ethics Committee of the participating hospitals.
Laboratory monitoring at diagnosis consisted of complete blood count, coagulation studies, and biochemical profile. Bone marrow aspirates were collected for morphology, immunophenotyping, molecular studies and cytogenetics. A comprehensive cardiac assessment was performed. The disease status was assessed with morphology and molecular studies in bone marrow aspirates in all patients before each cycle of chemotherapy, every three months during the first year after the treatment was completed, every four months during the second year, and subsequently every six months up to five years. In case of a PCR positive result after the third consolidation course or during subsequent follow-up a new sample, collected at least 2 weeks but no more than a month apart, was obtained. In case of repeated PCR positivity the patient was defined as having molecular relapse.[27,28,31] The Sanz risk score was used in order to decide the treatment intensity in some patients and for analyzing the results.[42]
Treatment protocols: During the first period (up to 2004) most patients followed the AIDA0493 protocol, as it has been described previously.[43] In brief, induction: idarubicin+ATRA, 1st consolidation: idarubicin+cytarabine, 2nd consolidation: mitoxantrone+etoposide, 3rd consolidation: idarubicin+cytarabine+6-thioguanine. From 2004 onwards most patients were treated according to the PETHEMA protocols (LPA1999 and LPA2005), as they have been described previously.[35,44,45] In brief, induction: idarubicin+ATRA, 1st consolidation: idarubicin, 2nd consolidation: mitoxantrone, 3rd consolidation: idarubicin. ATRA was added in each consolidation cycle in the intermediate and high risk patients (protocol LPA1999). In protocol LPA2005 ATRA was added in consolidation cycles of all patients, irrespective of their risk group. In addition, cytarabine was added in the 1st and the 3rd cycle of consolidation in the high risk patients. A few patients were treated according to the European APL93 and APL2000 protocols.[30,46-48] In brief, induction: ATRA+daunorubicin+cytarabine, 1st and 2nd consolidation: daunorubicin+cytarabine. In most patients, regardless the treatment protocol, maintenance treatment with ATRA+mercaptopourine+methotrexate (ATRA+6-MP+MTX) or ATRA alone was given for two years.[28,49-51] No CNS prophylaxis was given.[27,52]
Supportive care: When coagulopathy was present fresh-frozen plasma and platelet transfusions were given with a platelet target of 30x109/L. Heparin or tranexamic acid was not given. After resolution of the coagulopathy fresh-frozen plasma was not given and the platelet transfusions were given when hemorrhagic manifestations and/or infection were present.[27,28] Prophylaxis for the retinoic acid syndrome was not given, as the effectiveness of this procedure is not established according to previous reports.[47,53-57]
The prophylaxis during neutropenia and the management of neutropenic fever or infection followed the protocols of the participating hospitals.
Immunophenotypic studies: Immunophenotypic studies were performed on erythrocyte lysed whole bone marrow samples with directly conjugated monoclonal antibodies. The expression of HLA-DR, CD34, CD15, CD13, CD33, MPO as well as the side scatter (SS) of the abnormal cell population was assessed. Various lymphoid markers (including CD2 and CD56) were also used in order to assess their aberrant expression.[27,58-61]
Cytogenetic studies: Chromosome studies were performed on unstimulated bone marrow cells, cultured for 24 and 48 hours at the time of diagnosis, after the end of treatment and when any suspicion for the development of myelodysplastic syndrome was raised.[27] Cytogenetic analyses were performed on trypsin G-banded chromosome preparations and imaging and karyotyping were performed via microscopy and computer imaging techniques. Karyotypes were described according to the International System for Human Cytogenetic Nomenclature (ISCN) 2009. Whenever possible at least 20 metaphases were analyzed in each case.
Molecular studies: Conventional nested reverse transcription PCR (RT-PCR) was used for the detection of the various isoforms of the PML-RARα hybrid gene.[5,17,27,62] The same method was used for minimal residual disease (MRD) monitoring until early 2004. Subsequently, for a more accurate MRD assessment we optimized the RQ-PCR protocols of the three PML-RARα isoforms for use with the Lightcycler®/Roche and we established a standard approach of fluorescence data acquisition. MRD assessment was always performed in bone marrow samples.[63-68] DNA or RNA was used in PCR or RT-PCR respectively for the detection of the internal tandem duplication (ITD) or the point mutation D835 (TKD) of the FLT3 gene.[19,69]
Outcome definitions: Complete hematologic remission (CHR) and relapse were defined according to the National Cancer Institute criteria.[70,71] Molecular remission was defined upon a negative PCR for the PML-RARα hybrid gene, as described above. Early death was defined as death occurring during induction therapy or during the aplasia that follows induction. Death before treatment was considered “early death” but was also reported separately. Molecular relapse was met when there were two positive PCR results for the PML-RARα hybrid gene, at least two weeks apart each other, in a patient being in molecular remission. The differentiation syndrome was defined as “definitely present” or “probable” according to Frankel et al.[27,72,73]
Statistical methods: Nominal variables were summarized with frequencies and percentages, while continuous variables with median value and range. Comparisons between groups were done using the Mann-Whitney or Kruskal-Wallis test for continuous variables, and Pearson’s chi square or Fisher’s exact test for categorical data.
OS was calculated from the first day of induction treatment, while DFS was calculated from the date of complete hematologic response. Molecular and/or hematologic relapse, PML/RARα PCR positivity at the end of consolidation treatment, diagnosis of MDS and death irrespective of cause were defined as DFS events. OS and DFS were estimated by the Kaplan-Meier method.
Response and relapse cumulative incidences were calculated considering death as competing risk. Cumulative incidence of relapse (CIR) was calculated from the date of complete hematologic remission. Molecular or hematologic relapse and PCR positivity at the end of consolidation were defined as relapse events.
Effects on survival outcomes were tested by the log-rank test (OS and DFS) and Cox proportional hazards model. Cause specific hazard analysis was done in the presence of competing risks. Significant (p≤0.1) predictors from univariate analysis were further tested in multivariate models. The final model in multivariate analysis was reached by stepwise backward selection.
Logistic regression was employed for analysis of early death rate and differentiation syndrome risk.
All analyses were performed with STATA 11 software (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
Results
Demographic and baseline characteristics of the patients are shown in Table 1.
In nine patients the disease was therapy related (t-APL). Six patients had been treated with chemotherapy or irradiation because of some malignant disorder. In addition, there were three patients with multiple sclerosis that had been treated with mitoxantrone. Details for these patients are shown in Table 2.[74-78]
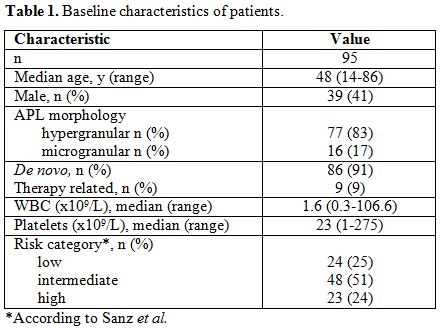
Table 1. Baseline characteristics of patients.
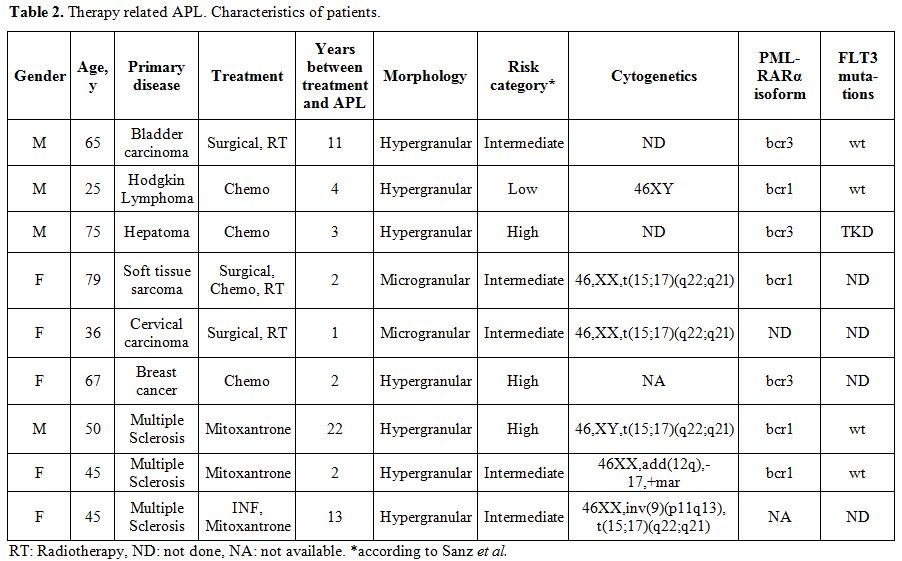
Table 2. Therapy related APL. Characteristics of patients.
Immunophenotyping: Most patients studied showed an “abnormal” cell population with intermediate to high side scatter in the CD45/SS dot plot. All patients but one (59/60, 98.3%) showed the characteristic absence to low percentage of HLA-DR. Actually this particular patient showed the variant translocation t(11;17). Only 3/58 (5.2%) were CD34 positive. CD15 positivity was not so rare, to be found in 12/62 patients (19.3%). The sharp strong expression of CD33 was a constant feature in all patients and it was accompanied by variable CD13 expression. MPO, where studied, was found positive in all patients. CD11b was found positive in a low percentage of patients (3/43, 7.0%) and CD117 expression was variable. Aberrant expression of lymphoid markers was found in 8/49 (16.3%) patients. CD2 expression was the more frequent finding (7/44, 15.9%), whereas CD7 was found in only one patient (1/60, 1.7%) and CD56 in two patients (2/49, 4.1%). Aberrant expression of lymphoid markers was not associated with bad prognosis (OS and DFS); as we observed only one molecular relapse event and no deaths among these eight patients. There was a close correlation between the expression of lymphoid markers and the development of the differentiation syndrome: 7/8 cases expressing lymphoid markers developed DS. No other correlation was found between immunophenotype and other features or outcomes of the patients, but it has to be stressed that the numbers were rather small.[58-61,79]
Chromosomal abnormalities: Comprehensive karyotypic results were available in 68 patients. The detailed karyotypic results are shown in Table 3. Normal karyotype was found in 9 patients (13.2%).
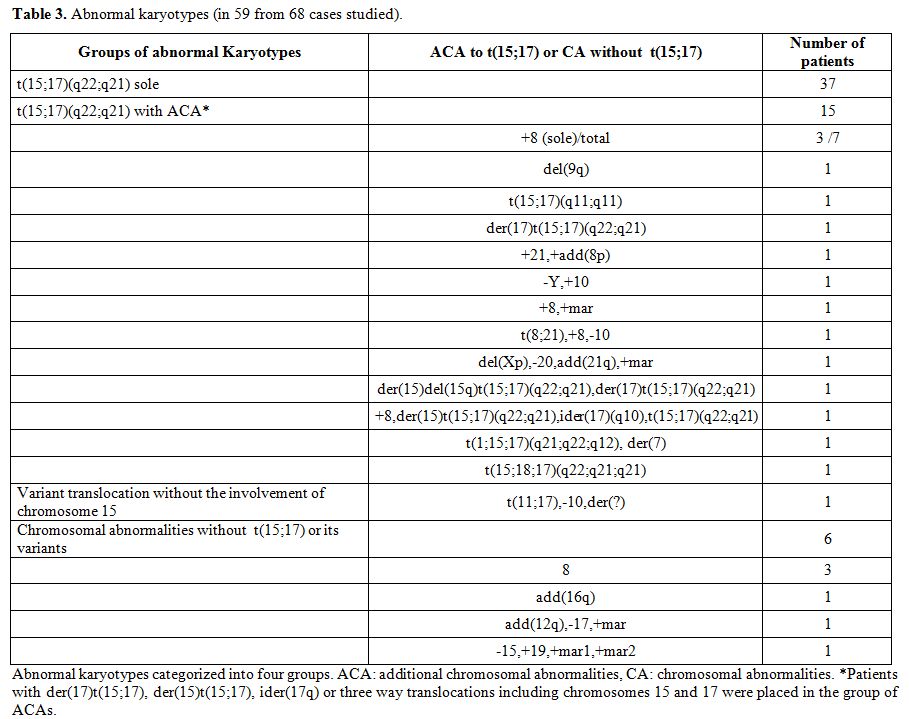
Table 3. Abnormal karyotypes (in 59 from 68 cases studied).
Among the 59 cases with abnormal karyotype there were 42 cases with a single abnormality, seven cases with two abnormalities, and 10 cases with 3 abnormalities (complex karyotype). The t(15;17) was found in 52 cases (76.5%) and it was the sole abnormality in 37 cases. In 15 cases (28.8%) additional chromosomal abnormalities (ACAs) were present. Among the ACAs trisomy 8 was by far the most frequent;[12-15] it was present in 7/15 cases. One patient showed t(8;21) in addition to t(15;17). He was treated with a hybrid protocol (AIDA and a conventional AML protocol) but he never achieved molecular CR.[80,81] There was not any difference between the patients with ACAs and those without ACAs concerning the clinical features (data not shown) or the response to treatment.[11,15]
Molecular findings: The presence of PML-RARα was tested in all but two patients and it was detected in 92/93 cases (98.9%). It was absent in the case with t(11;17), as expected. In 75 patients the PML-RARα isoforms were determined: L isoform (bcr1) in 38 cases (51%), V isoform (bcr2) in 5 cases (7%), and S isoform (bcr3) in 32 cases (42%).[5,17,18] There was a striking difference in the frequency of the isoforms between the patients from Greece and those from Cyprus. In the Greek origin patients the L isoform was present in 36/64 cases (56.25%) and the S isoform in 24/64 (37.5%) while the corresponding figures for the Cyprus origin patients was 2/11 (18.2%) and 8/11 (72.7%) (p=0.036).
Fifty one bone marrow samples were analyzed for FLT3 mutations at presentation. Mutations were found in 20 cases (39%): ITD in 11 cases and TKD in 9 cases.[19,20,82] The presence of FLT3 mutations was significantly associated with intermediate/high risk score (p=0.035).[82] The microgranular variant was rather more frequent in FLT3 mutated cases (p=0.09). Coagulopathy was significantly more prevalent among FLT3 mutated cases (p=0.037). FLT3 ITD was an adverse prognostic factor for early death and OS (p=0.017) but not for response rate or relapse incidence.[19,20,82] In one patient we observed a constant positivity for FLT3 TKD for 4 years while he remained in molecular remission.[19,21]
Clinical course: At presentation laboratory findings of disseminated intravascular coagulation were present in 54/95 patients (57%). In 26 cases major hemorrhagic events were noticed. There were 9 patients with intracranial bleeding. Hemorrhage was the main cause of death in 9 patients: 3 within the first 24 hours, 3 at day two, and the others at day 8, 10, and 14. Two of these patients died before specific treatment was given. Twenty six patients presented with infection.[46,47,83]
Induction therapy: Induction treatment was given in 88 patients: AIDA protocol (n=69),[43] ATRA alone (n=4),[84-86] ATRA+idarubicin+cytarabine (n=13).[30] Two patients misdiagnosed as AML-M2 were treated with “3+7”.[87] When the diagnosis was confirmed they followed the AIDA protocol. One patient discontinued treatment because cytogenetics showed t(11;17) and her age in conjunction with poor performance status prohibited further treatment.[88,89] She died a few weeks later. She was excluded from further analysis. Twenty seven patients (31%) developed differentiation syndrome.[73] In 5 cases (6%) this was definite. In all the patients developing DS, dexamethasone and furosemide were administered. ATRA was temporarily discontinued in 9 cases and its dose reduced in one case. In 3 cases the DS was the main or a contributory cause of death during induction. In all the other cases it resolved within a few days.[27,72] In 4 patients (5%) pseudotumor cerebri developed. All these patients were female and their age was 19 (n=2), 22 and 67 years old. Temporal discontinuation or dose reduction of ATRA in conjunction with dexamethasone and diuretic administration was followed by resolution of the symptoms.[27,43] Infection experienced 62 patients during induction with neutropenic fever being the most common manifestation (n=42). In one case, pneumonia was the direct cause of death and in two cases a contributing cause. Eight patients died during induction. Median time to death was 14 days (range, 8-55). All but one of those patients died before being evaluated for response. The patient that died on day 55 was in CHR. Causes of death: intracranial hemorrhage (n=3), pneumonia and differentiation syndrome (n=2), pneumonia (n=1) septic shock (n=1), and respiratory failure because of differentiation syndrome (n=1). All 80 patients that completed induction and survived the early period achieved CHR at a median of 35 days (range, 19-99). These patients were candidates for consolidation.
Consolidation therapy: From the 80 patients that achieved complete hematologic remission after induction, 76 proceeded to consolidation. One patient died because of lung infection while in CHR. One patient 84 years old was induced with ATRA alone and achieved molecular remission.[84-86] Because of her poor performance status she was offered no further treatment. She had a molecular relapse after 39 months and a hematologic relapse after 30 further months. She then received AIDA induction 43 but she died in aplasia. The other two patients decided by themselves to discontinue further treatment. The first consolidation cycle varied according to the protocol that the patient followed: AIDA (n=39),43 PETHEMA (n=24),[35,44,45] European APL (n=7),[30,46-48] other (n=6). There were two deaths because of infection and one patient experienced severe organ toxicity after the 1st consolidation cycle and, therefore, 73 patients received the 2nd consolidation cycle. The patients followed the same protocols. One patient died after the 2nd consolidation because of infection, one developed severe organ toxicity, one relapsed, one developed myelodysplasia and two were considered unfit for any further chemotherapy. Two other patients were scheduled to receive the 3rd consolidation when the charts were reviewed. Therefore the 3rd consolidation cycle was given to 65 patients. No deaths or severe toxicity was noticed after the 3rd consolidation cycle.
Maintenance: A total of 64 patients received maintenance at a median time of 187 days (range, 89-303) from the start of the induction therapy.[24,28,49-51] Thirty five patients received ATRA alone (median, 8 cycles), 28 received ATRA+MTX+6-MP (median, 8 cycles) and one patient received only MTX+6-MP because her pseudotumor cerebri symptoms reappeared when ATRA was given. No other grade 3 hematologic or non-hematologic toxicity was noticed during maintenance. Fifteen patients are still on maintenance. Four patients discontinued maintenance because of relapse.
Outcomes
Early death: The early death rate was 14.9%. (Table 4) Significant predictors of increased risk of early death on univariate analysis were older age, leykocytosis, thrombocytopenia, higher Sanz score, DIC, major hemorrhage, infection at diagnosis and FLT3 ITD mutation (Table 5). No association of early death with morphologic subtype, secondary APL, transcript breakpoint, FLT3 TKD, karyotypic group or gender could be detected. In multivariate logistic regression, baseline characteristics that remained in the final model as independent predictors of early death were DIC, major bleeding and higher age (Table 5).[32,33,71]
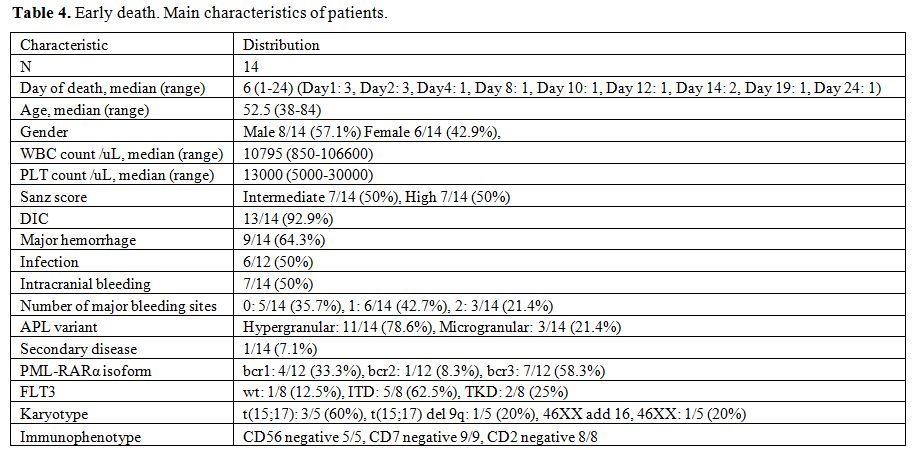
Table 4. Early death. Main characteristics of patients.
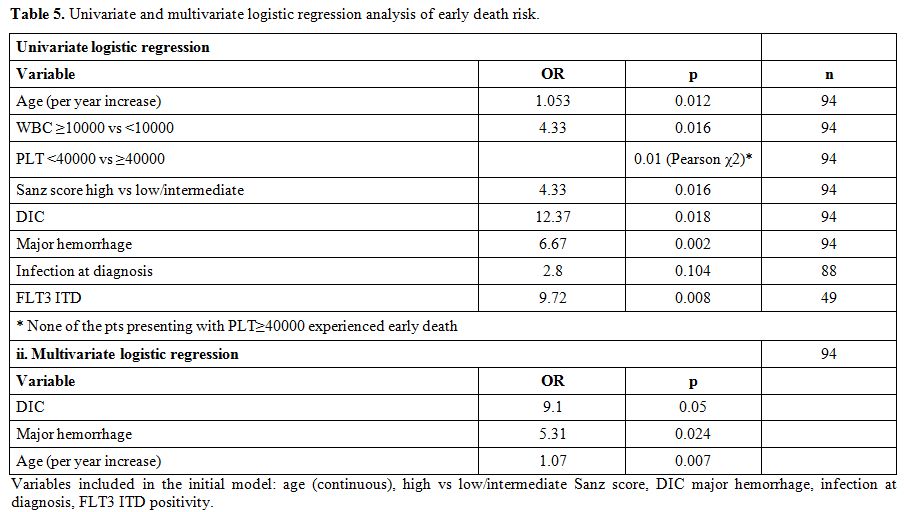
Table 5. Univariate and multivariate logistic regression analysis of early death risk.
Response to treatment: As mentioned above, all 80 patients that completed induction therapy and were evaluated for response achieved CHR. Seven patients died before induction therapy was introduced and eight died early after induction. The cumulative incidence of CHR from the beginning of the induction therapy was
85.1% (95% CI, 76.1-90.9).(Figure 1) At the end of the consolidation therapy 73 of the 75 evaluable patients achieved molecular remission. Only two patients never achieved molecular remission. Both died after aggressive chemotherapy or allogeneic stem cell transplantation.[83,90] With a median follow-up of the surviving patients of 55 months (range, 1.3-182) the OS at 5 years was 78.4% (95% CI, 68.5-85.5). The OS for the patients of the low/intermediate risk group was 83.1% and that of the high risk group 62.9%. (Table 6, Figure 2) If we consider only the patients that received full induction therapy (i.e. until day 8, n=87), the OS at 5 years was 84.7% (95% CI, 75.1-90.8).
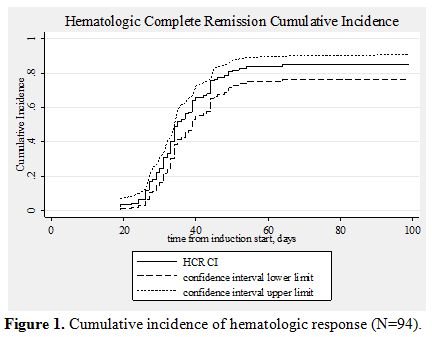
Figure 1. Cumulative incidence of hematologic response (N=94).
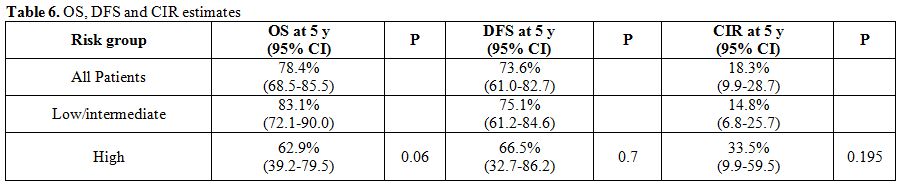
Table 6. OS, DFS and CIR estimates
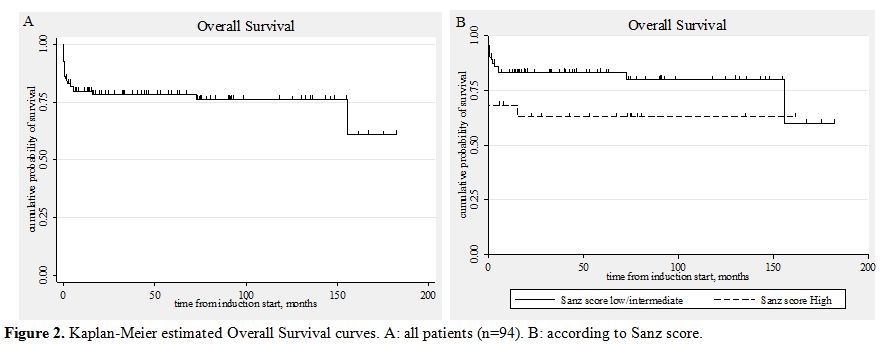
Figure 2. Kaplan-Meier estimated Overall Survival curves. A: all patients (n=94). B: according to Sanz score.
Ten patients relapsed. In seven patients the relapse was molecular and in three hematologic. There was no case of extramedullary relapse. Most of these patients were treated with ATO.[91,92] Two patients died during salvage therapy. Six achieved molecular remission and proceeded to autologous stem cell transplantation. In all these six cases the graft was PML-RARα negative.[91,93] Five of these six patients are alive and in molecular remission for 2-124 months after the transplantation (median, 36). Three relapsed patients did not achieve molecular remission and proceeded to allogeneic stem cell transplantation. All had an HLA identical sibling. They are alive and in molecular remission [62, 70], and 118 months after the transplantation. The cumulative incidence of relapse (calculated from the date of CHR) was 18.3% (95% CI, 9.9-28.7). It was 14.8% for the low/intermediate risk group and 33.5% for the high risk group (univariate, HR=2.21, p=0.195). (Table 6, Figure 3) Two patients developed a myelodysplastic syndrome 4 months and 3 years after the diagnosis of APL.[36,37,44,51,94] Both are alive and in complete molecular remission concerning PML/RARα seven months and eight years since the diagnosis of MDS, respectively.
The DFS for all 80 patients that achieved CHR was 73.6%. The DFS of the patients belonging to the low/intermediate risk group was 75.1%, and of those belonging to the high risk group 66.5%. (Table 6, Figure 4)
The results of univariate and multivariate analysis concerning overall survival are shown in tables 7 and 8. The same factors were studied with univariate analysis for the DFS and the CIR. None proved to be significant (data not shown).
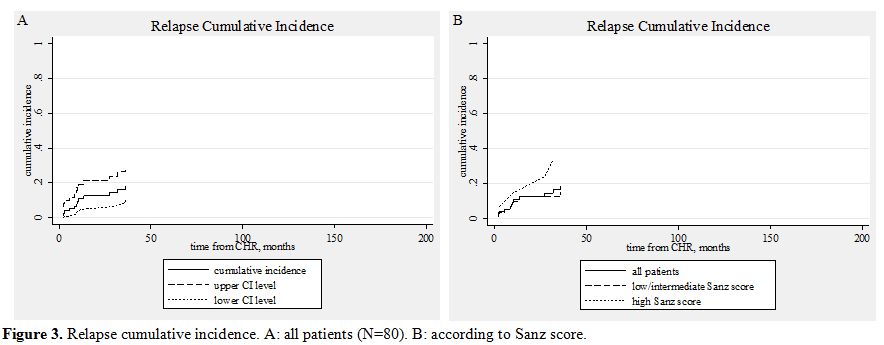
Figure 3. Relapse cumulative incidence. A: all patients (N=80). B: according to Sanz score.
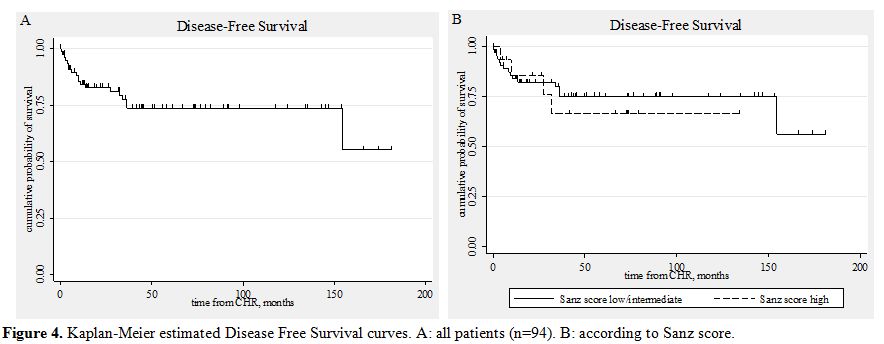
Figure 4. Kaplan-Meier estimated Disease Free Survival curves. A: all patients (n=94). B: according to Sanz score.
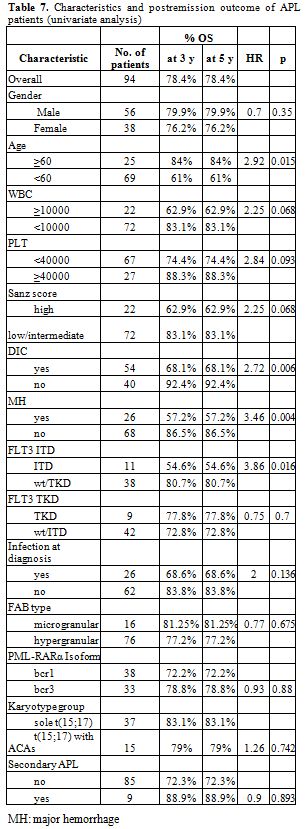
Table 7. Characteristics and postremission outcome of APL patients (univariate analysis).
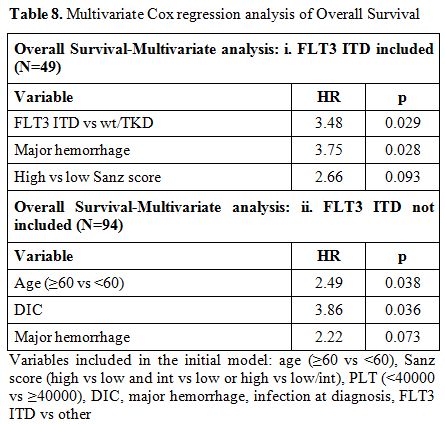
Table 8. Multivariate Cox regression analysis of Overall Survival .
Discussion
In this study we present the clinical, immunophenotypic, cytogenetic, and molecular findings as well as the clinical course of 95 Greek APL patients from 6 hospitals in Greece and Cyprus. Since there is not a national registry for this particular type of AML in Greece, the present study represents the largest series ever reported.
Demographics do not seem to differ substantially from previous literature reports.[43,45,51] Immunophenotyping is a useful tool for the correct diagnosis. Intermediate to high side scatter, strong CD33 expression and MPO positivity, CD34+/-, CD13++, CD15+ dim and lack of HLA-DR expression were characteristic findings in almost all cases studied. The only patient showing HLA-DR expression was found to be PML-RARα negative since her cytogenetic abnormality was t(11;17).[58-61] We noticed a strong correlation between the expression of lymphoid
markers and the development of differentiation syndrome since 7/8 cases expressing lymphoid markers developed the syndrome. By far the most frequent lymphoid marker expressed was CD2, a cell adhesion molecule. An explanation for such a correlation might be the adherence of the cells expressing such molecules to the capillary endothelium with consequent inflammation.[95] There were only two cases with CD56 expression, a rather low incidence and, therefore, no conclusions about its significance can be drawn.[ 96]
Taking into account that cytogenetically cryptic PML-RARα rearrangements are observed in 4%-6% of APL cases, we found a rather high number of cases with normal cytogenetics, 9/68 (13.2%). Chance and ‘technical reasons’ are the only explanations we can offer for this finding.[5,16,26] Additional chromosomal abnormalities were found in 15/59 cases (28.8%) and trisomy 8 was by far the most frequent abnormality. These findings are in accordance to other reports. The presence of additional chromosomal abnormalities did not influence the response to treatment. We therefore agree with the suggestion that ACAs is not a reason to intensify treatment.[11-15] The prognostic significance of ider(17q) and three way translocations of t(15;17) are currently unknown due to their low incidence.[41] In addition, the third chromosome that is involved in the three way translocation to the t(15;17) varies. One of the two variant translocations involving chromosomes 15, 17 and 18 was associated with a submicroscopic deletion of the 5’ part of the RARα gene, as evidenced by FISH.[97] Because only limited number of studies has examined such deletions associated with the PML-RARa fusion gene, their significance and their involvement in the pathogenesis of the disease if any, remain unclear. One of our patients manifested t(8;21) in addition to the t(15;17). Although the patient received induction therapy according to the AIDA protocol and subsequently two cycles of high dose cytarabine with idarubicin and ATRA he did not achieve molecular remission. The co-existence of t(15;17) and t(8;21) in a single leukemic clone is a very rare finding and the results of the few reported cases are conflicting. However, it has been proposed that PML-RARa and AML1/ETO fusion proteins may mutually affect their pathogenetic mechanism, rendering the cells resistant to ATRA which is in agreement with the resistance of our patient to ATRA based treatment.[38,80,81]
In the Greek origin patients the L isoform of the PML-RARa was the most frequent and this is in agreement with previous reports. In contrast, in the Cyprus origin patients we noticed a very high frequency of the S isoform.[5,17,18] We can offer no explanation for this finding and it definitely needs confirmation. The presence of FLT3 mutations was associated with various adverse features and generally with intermediate/high risk score, as previously reported. It seems therefore that these mutations are inherently connected with the biology of the disease. We observed one patient showing the FLT3 TKD permanently for 4 years while being in complete molecular remission. Such a finding supports the suggestion of not using FLT3 mutations as a marker for minimal residual disease in APL.[22,69,98,99]
One important finding of our study was the considerable early death rate. Actually there were 14/94 early deaths (14.9%), with half of these patients dying within the first few days before they could receive full induction treatment. Hemorrhage was the main cause of early death. (Table 4)[32,33,35,71] Such a figure is increased compared to the numbers reported in the clinical trials of APL (3%-10%). However, population based studies have confirmed that the early death rate reported in clinical trials does not correspond to real life circumstances and worryingly, such a finding has not appreciably changed in the ATRA era. Delays in referring the patients, delays in establishing diagnosis, and failure to promptly begin specific treatment on clinical suspicion and to provide aggressive supportive therapy are factors explaining such mortality and are the targets of the interventions urgently needed. Moreover, our data suggest that much of the prognostic effect of significant variables on OS outcome largely stems from the effect of these variables on the risk of early death. (Tables 5, 7, and 8) This is also supported by the observation that these variables had no discernible effect on disease-free survival or relapse incidence. As regards patients surviving induction treatment the response was excellent. All 80 evaluable patients achieved complete hematologic remission. At the end of the consolidation therapy 73/75 achieved molecular remission. These results are in accordance to the relevant reports of the protocols that our patients followed (AIDA0493, the LPA1999 and LPA2005 PETHEMA and the European APL1993 and APL2000). [30,35,43-51] Only two patients showed primary resistance, i.e. they never achieved molecular remission. Both died after being treated with aggressive chemotherapy or allogeneic stem cell transplantation. We observed 10 relapses (cumulative incidence of relapse 18.3%, 95% CI 9.9%-28.7%) (Figure 3). Because of the close monitoring most relapses were molecular and therefore the patients were salvaged rather early. The salvage treatment (for the majority ATO and autologous or allogeneic stem cell transplantation) was successful: five patients remain in molecular remission for 2-124 months (median, 36) after autologous stem cell transplantation and three patients for 62-118 months after allogeneic stem cell transplantation. These results further support the importance of the close molecular monitoring after the first line treatment is completed.[65,100-102] No CNS relapse was observed in the cohort of our patients in none of whom CNS prophylaxis was given.[27,52] There were two patients that developed myelodysplastic syndrome, an incidence not different from the one reported. [27,36,37,40,44,51,94] We were unable to confirm the value of various well established risk factors for the patients’ outcome. Most probable explanations are the rather small numbers in our study as well as the risk adapted therapy that was provided to most of our patients. In summary, our data confirm the excellent results of the current treatment of the disease, provided that the patient will survive the early period after the disease has been diagnosed.
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myelogenous leukemia (AML) with special biological, morphological, cytogenetic, and molecular characteristics and also clinical features. Morphologically APL is identified by the FAB classification as M3 subtype (hypergranular) and its microgranular variant (M3v).[1-5] Cytogenetically is characterized by the t(15;17)(q22;q21) which is considered to be a favorable cytogenetic aberration.[6-9]
In rare cases other translocations as t(11;17), t(5;17) can be detected.[10] Additional chromosome aberrations to t(15;17) have been observed in 23%-43% of APL cases without changing the favorable prognosis.[11-15]
At the molecular level, the result of the t(15;17) is the formation of two functional fusion genes, PML-RARα and RARα-PML on the derivative chromosomes 15 and 17 respectively.[5,8,9,16] A variety of PML-RARα transcripts [bcr1 or Long (L), bcr3 or Short (S), bcr2 or Variable (V)] are produced due to different breakpoints in PML gene and alternative splicing in chromosome [15.17,18] The FLT3 gene aberrations, including internal tandem duplications (ITDs) and D835 (tyrosine kinase domain, TKD) mutations occur in 30%-50% in APL. However, the significance of FLT3 mutations as a prognostic factor is not firmly established.[19-22]
With current treatment strategies with all-trans-retinoic acid (ATRA) in combination with anthracycline based chemotherapy, approximately 70%-80% of patients with newly diagnosed APL carrying PML/RARα achieve long-term remission and are probably cured. However, relapses occur in 10%-30% of patients. The role of cytosine arabinoside and other agents remains controversial. For patients in whom chemotherapy is contraindicated, for elderly patients and for some relapsing patients Arsenic Trioxide (ATO) is a suitable alternative, as a single agent or in combination with ATRA. There is not agreement on the most appropriate consolidation therapy but generally two to three cycles of anthracycline-based chemotherapy are considered mandatory. Finally, there is an open debate about the need for maintenance treatment.[23-31]
Besides the excellent results, serious problems still remain: a high early death rate (up to 10% for patients who enrolled in clinical trials and higher in real life), cardiotoxicity, secondary myelodysplastic syndromes, and relapse rising up to 20%-30% in high risk patients.[30,32,33-37]
The APL published data in Greek patients are very limited, usually restricted to case reports or small series.[38-41]
In the present study, we report on 95 patients from Greece and Cyprus in order to clarify the clinical, cytogenetic and molecular features as well as their outcome.
Design and methods
Between January 1996 and June 2011 a total of 95 patients with APL were diagnosed and treated in 6 tertiary hospitals in Athens, Patras, Nicosia and Limassol. The diagnosis was confirmed with cytogenetics for the presence of t(15;17) and/or molecular studies for the presence of the PML/RARα fusion gene. Informed consent was obtained from all patients according the Declaration of Helsinki and all the protocols were approved by the Research Ethics Committee of the participating hospitals.
Laboratory monitoring at diagnosis consisted of complete blood count, coagulation studies, and biochemical profile. Bone marrow aspirates were collected for morphology, immunophenotyping, molecular studies and cytogenetics. A comprehensive cardiac assessment was performed. The disease status was assessed with morphology and molecular studies in bone marrow aspirates in all patients before each cycle of chemotherapy, every three months during the first year after the treatment was completed, every four months during the second year, and subsequently every six months up to five years. In case of a PCR positive result after the third consolidation course or during subsequent follow-up a new sample, collected at least 2 weeks but no more than a month apart, was obtained. In case of repeated PCR positivity the patient was defined as having molecular relapse.[27,28,31] The Sanz risk score was used in order to decide the treatment intensity in some patients and for analyzing the results.[42]
Treatment protocols: During the first period (up to 2004) most patients followed the AIDA0493 protocol, as it has been described previously.[43] In brief, induction: idarubicin+ATRA, 1st consolidation: idarubicin+cytarabine, 2nd consolidation: mitoxantrone+etoposide, 3rd consolidation: idarubicin+cytarabine+6-thioguanine. From 2004 onwards most patients were treated according to the PETHEMA protocols (LPA1999 and LPA2005), as they have been described previously.[35,44,45] In brief, induction: idarubicin+ATRA, 1st consolidation: idarubicin, 2nd consolidation: mitoxantrone, 3rd consolidation: idarubicin. ATRA was added in each consolidation cycle in the intermediate and high risk patients (protocol LPA1999). In protocol LPA2005 ATRA was added in consolidation cycles of all patients, irrespective of their risk group. In addition, cytarabine was added in the 1st and the 3rd cycle of consolidation in the high risk patients. A few patients were treated according to the European APL93 and APL2000 protocols.[30,46-48] In brief, induction: ATRA+daunorubicin+cytarabine, 1st and 2nd consolidation: daunorubicin+cytarabine. In most patients, regardless the treatment protocol, maintenance treatment with ATRA+mercaptopourine+methotrexate (ATRA+6-MP+MTX) or ATRA alone was given for two years.[28,49-51] No CNS prophylaxis was given.[27,52]
Supportive care: When coagulopathy was present fresh-frozen plasma and platelet transfusions were given with a platelet target of 30x109/L. Heparin or tranexamic acid was not given. After resolution of the coagulopathy fresh-frozen plasma was not given and the platelet transfusions were given when hemorrhagic manifestations and/or infection were present.[27,28] Prophylaxis for the retinoic acid syndrome was not given, as the effectiveness of this procedure is not established according to previous reports.[47,53-57]
The prophylaxis during neutropenia and the management of neutropenic fever or infection followed the protocols of the participating hospitals.
Immunophenotypic studies: Immunophenotypic studies were performed on erythrocyte lysed whole bone marrow samples with directly conjugated monoclonal antibodies. The expression of HLA-DR, CD34, CD15, CD13, CD33, MPO as well as the side scatter (SS) of the abnormal cell population was assessed. Various lymphoid markers (including CD2 and CD56) were also used in order to assess their aberrant expression.[27,58-61]
Cytogenetic studies: Chromosome studies were performed on unstimulated bone marrow cells, cultured for 24 and 48 hours at the time of diagnosis, after the end of treatment and when any suspicion for the development of myelodysplastic syndrome was raised.[27] Cytogenetic analyses were performed on trypsin G-banded chromosome preparations and imaging and karyotyping were performed via microscopy and computer imaging techniques. Karyotypes were described according to the International System for Human Cytogenetic Nomenclature (ISCN) 2009. Whenever possible at least 20 metaphases were analyzed in each case.
Molecular studies: Conventional nested reverse transcription PCR (RT-PCR) was used for the detection of the various isoforms of the PML-RARα hybrid gene.[5,17,27,62] The same method was used for minimal residual disease (MRD) monitoring until early 2004. Subsequently, for a more accurate MRD assessment we optimized the RQ-PCR protocols of the three PML-RARα isoforms for use with the Lightcycler®/Roche and we established a standard approach of fluorescence data acquisition. MRD assessment was always performed in bone marrow samples.[63-68] DNA or RNA was used in PCR or RT-PCR respectively for the detection of the internal tandem duplication (ITD) or the point mutation D835 (TKD) of the FLT3 gene.[19,69]
Outcome definitions: Complete hematologic remission (CHR) and relapse were defined according to the National Cancer Institute criteria.[70,71] Molecular remission was defined upon a negative PCR for the PML-RARα hybrid gene, as described above. Early death was defined as death occurring during induction therapy or during the aplasia that follows induction. Death before treatment was considered “early death” but was also reported separately. Molecular relapse was met when there were two positive PCR results for the PML-RARα hybrid gene, at least two weeks apart each other, in a patient being in molecular remission. The differentiation syndrome was defined as “definitely present” or “probable” according to Frankel et al.[27,72,73]
Statistical methods: Nominal variables were summarized with frequencies and percentages, while continuous variables with median value and range. Comparisons between groups were done using the Mann-Whitney or Kruskal-Wallis test for continuous variables, and Pearson’s chi square or Fisher’s exact test for categorical data.
OS was calculated from the first day of induction treatment, while DFS was calculated from the date of complete hematologic response. Molecular and/or hematologic relapse, PML/RARα PCR positivity at the end of consolidation treatment, diagnosis of MDS and death irrespective of cause were defined as DFS events. OS and DFS were estimated by the Kaplan-Meier method.
Response and relapse cumulative incidences were calculated considering death as competing risk. Cumulative incidence of relapse (CIR) was calculated from the date of complete hematologic remission. Molecular or hematologic relapse and PCR positivity at the end of consolidation were defined as relapse events.
Effects on survival outcomes were tested by the log-rank test (OS and DFS) and Cox proportional hazards model. Cause specific hazard analysis was done in the presence of competing risks. Significant (p≤0.1) predictors from univariate analysis were further tested in multivariate models. The final model in multivariate analysis was reached by stepwise backward selection.
Logistic regression was employed for analysis of early death rate and differentiation syndrome risk.
All analyses were performed with STATA 11 software (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
Results
Demographic and baseline characteristics of the patients are shown in Table 1.
In nine patients the disease was therapy related (t-APL). Six patients had been treated with chemotherapy or irradiation because of some malignant disorder. In addition, there were three patients with multiple sclerosis that had been treated with mitoxantrone. Details for these patients are shown in Table 2.[74-78]

Table 1. Baseline characteristics of patients.

Table 2. Therapy related APL. Characteristics of patients.
Immunophenotyping: Most patients studied showed an “abnormal” cell population with intermediate to high side scatter in the CD45/SS dot plot. All patients but one (59/60, 98.3%) showed the characteristic absence to low percentage of HLA-DR. Actually this particular patient showed the variant translocation t(11;17). Only 3/58 (5.2%) were CD34 positive. CD15 positivity was not so rare, to be found in 12/62 patients (19.3%). The sharp strong expression of CD33 was a constant feature in all patients and it was accompanied by variable CD13 expression. MPO, where studied, was found positive in all patients. CD11b was found positive in a low percentage of patients (3/43, 7.0%) and CD117 expression was variable. Aberrant expression of lymphoid markers was found in 8/49 (16.3%) patients. CD2 expression was the more frequent finding (7/44, 15.9%), whereas CD7 was found in only one patient (1/60, 1.7%) and CD56 in two patients (2/49, 4.1%). Aberrant expression of lymphoid markers was not associated with bad prognosis (OS and DFS); as we observed only one molecular relapse event and no deaths among these eight patients. There was a close correlation between the expression of lymphoid markers and the development of the differentiation syndrome: 7/8 cases expressing lymphoid markers developed DS. No other correlation was found between immunophenotype and other features or outcomes of the patients, but it has to be stressed that the numbers were rather small.[58-61,79]
Chromosomal abnormalities: Comprehensive karyotypic results were available in 68 patients. The detailed karyotypic results are shown in Table 3. Normal karyotype was found in 9 patients (13.2%).

Table 3. Abnormal karyotypes (in 59 from 68 cases studied).
Among the 59 cases with abnormal karyotype there were 42 cases with a single abnormality, seven cases with two abnormalities, and 10 cases with 3 abnormalities (complex karyotype). The t(15;17) was found in 52 cases (76.5%) and it was the sole abnormality in 37 cases. In 15 cases (28.8%) additional chromosomal abnormalities (ACAs) were present. Among the ACAs trisomy 8 was by far the most frequent;[12-15] it was present in 7/15 cases. One patient showed t(8;21) in addition to t(15;17). He was treated with a hybrid protocol (AIDA and a conventional AML protocol) but he never achieved molecular CR.[80,81] There was not any difference between the patients with ACAs and those without ACAs concerning the clinical features (data not shown) or the response to treatment.[11,15]
Molecular findings: The presence of PML-RARα was tested in all but two patients and it was detected in 92/93 cases (98.9%). It was absent in the case with t(11;17), as expected. In 75 patients the PML-RARα isoforms were determined: L isoform (bcr1) in 38 cases (51%), V isoform (bcr2) in 5 cases (7%), and S isoform (bcr3) in 32 cases (42%).[5,17,18] There was a striking difference in the frequency of the isoforms between the patients from Greece and those from Cyprus. In the Greek origin patients the L isoform was present in 36/64 cases (56.25%) and the S isoform in 24/64 (37.5%) while the corresponding figures for the Cyprus origin patients was 2/11 (18.2%) and 8/11 (72.7%) (p=0.036).
Fifty one bone marrow samples were analyzed for FLT3 mutations at presentation. Mutations were found in 20 cases (39%): ITD in 11 cases and TKD in 9 cases.[19,20,82] The presence of FLT3 mutations was significantly associated with intermediate/high risk score (p=0.035).[82] The microgranular variant was rather more frequent in FLT3 mutated cases (p=0.09). Coagulopathy was significantly more prevalent among FLT3 mutated cases (p=0.037). FLT3 ITD was an adverse prognostic factor for early death and OS (p=0.017) but not for response rate or relapse incidence.[19,20,82] In one patient we observed a constant positivity for FLT3 TKD for 4 years while he remained in molecular remission.[19,21]
Clinical course: At presentation laboratory findings of disseminated intravascular coagulation were present in 54/95 patients (57%). In 26 cases major hemorrhagic events were noticed. There were 9 patients with intracranial bleeding. Hemorrhage was the main cause of death in 9 patients: 3 within the first 24 hours, 3 at day two, and the others at day 8, 10, and 14. Two of these patients died before specific treatment was given. Twenty six patients presented with infection.[46,47,83]
Induction therapy: Induction treatment was given in 88 patients: AIDA protocol (n=69),[43] ATRA alone (n=4),[84-86] ATRA+idarubicin+cytarabine (n=13).[30] Two patients misdiagnosed as AML-M2 were treated with “3+7”.[87] When the diagnosis was confirmed they followed the AIDA protocol. One patient discontinued treatment because cytogenetics showed t(11;17) and her age in conjunction with poor performance status prohibited further treatment.[88,89] She died a few weeks later. She was excluded from further analysis. Twenty seven patients (31%) developed differentiation syndrome.[73] In 5 cases (6%) this was definite. In all the patients developing DS, dexamethasone and furosemide were administered. ATRA was temporarily discontinued in 9 cases and its dose reduced in one case. In 3 cases the DS was the main or a contributory cause of death during induction. In all the other cases it resolved within a few days.[27,72] In 4 patients (5%) pseudotumor cerebri developed. All these patients were female and their age was 19 (n=2), 22 and 67 years old. Temporal discontinuation or dose reduction of ATRA in conjunction with dexamethasone and diuretic administration was followed by resolution of the symptoms.[27,43] Infection experienced 62 patients during induction with neutropenic fever being the most common manifestation (n=42). In one case, pneumonia was the direct cause of death and in two cases a contributing cause. Eight patients died during induction. Median time to death was 14 days (range, 8-55). All but one of those patients died before being evaluated for response. The patient that died on day 55 was in CHR. Causes of death: intracranial hemorrhage (n=3), pneumonia and differentiation syndrome (n=2), pneumonia (n=1) septic shock (n=1), and respiratory failure because of differentiation syndrome (n=1). All 80 patients that completed induction and survived the early period achieved CHR at a median of 35 days (range, 19-99). These patients were candidates for consolidation.
Consolidation therapy: From the 80 patients that achieved complete hematologic remission after induction, 76 proceeded to consolidation. One patient died because of lung infection while in CHR. One patient 84 years old was induced with ATRA alone and achieved molecular remission.[84-86] Because of her poor performance status she was offered no further treatment. She had a molecular relapse after 39 months and a hematologic relapse after 30 further months. She then received AIDA induction 43 but she died in aplasia. The other two patients decided by themselves to discontinue further treatment. The first consolidation cycle varied according to the protocol that the patient followed: AIDA (n=39),43 PETHEMA (n=24),[35,44,45] European APL (n=7),[30,46-48] other (n=6). There were two deaths because of infection and one patient experienced severe organ toxicity after the 1st consolidation cycle and, therefore, 73 patients received the 2nd consolidation cycle. The patients followed the same protocols. One patient died after the 2nd consolidation because of infection, one developed severe organ toxicity, one relapsed, one developed myelodysplasia and two were considered unfit for any further chemotherapy. Two other patients were scheduled to receive the 3rd consolidation when the charts were reviewed. Therefore the 3rd consolidation cycle was given to 65 patients. No deaths or severe toxicity was noticed after the 3rd consolidation cycle.
Maintenance: A total of 64 patients received maintenance at a median time of 187 days (range, 89-303) from the start of the induction therapy.[24,28,49-51] Thirty five patients received ATRA alone (median, 8 cycles), 28 received ATRA+MTX+6-MP (median, 8 cycles) and one patient received only MTX+6-MP because her pseudotumor cerebri symptoms reappeared when ATRA was given. No other grade 3 hematologic or non-hematologic toxicity was noticed during maintenance. Fifteen patients are still on maintenance. Four patients discontinued maintenance because of relapse.
Outcomes
Early death: The early death rate was 14.9%. (Table 4) Significant predictors of increased risk of early death on univariate analysis were older age, leykocytosis, thrombocytopenia, higher Sanz score, DIC, major hemorrhage, infection at diagnosis and FLT3 ITD mutation (Table 5). No association of early death with morphologic subtype, secondary APL, transcript breakpoint, FLT3 TKD, karyotypic group or gender could be detected. In multivariate logistic regression, baseline characteristics that remained in the final model as independent predictors of early death were DIC, major bleeding and higher age (Table 5).[32,33,71]

Table 4. Early death. Main characteristics of patients.

Table 5. Univariate and multivariate logistic regression analysis of early death risk.
Response to treatment: As mentioned above, all 80 patients that completed induction therapy and were evaluated for response achieved CHR. Seven patients died before induction therapy was introduced and eight died early after induction. The cumulative incidence of CHR from the beginning of the induction therapy was
85.1% (95% CI, 76.1-90.9).(Figure 1) At the end of the consolidation therapy 73 of the 75 evaluable patients achieved molecular remission. Only two patients never achieved molecular remission. Both died after aggressive chemotherapy or allogeneic stem cell transplantation.[83,90] With a median follow-up of the surviving patients of 55 months (range, 1.3-182) the OS at 5 years was 78.4% (95% CI, 68.5-85.5). The OS for the patients of the low/intermediate risk group was 83.1% and that of the high risk group 62.9%. (Table 6, Figure 2) If we consider only the patients that received full induction therapy (i.e. until day 8, n=87), the OS at 5 years was 84.7% (95% CI, 75.1-90.8).

Figure 1. Cumulative incidence of hematologic response (N=94).

Table 6. OS, DFS and CIR estimates

Figure 2. Kaplan-Meier estimated Overall Survival curves. A: all patients (n=94). B: according to Sanz score.
Ten patients relapsed. In seven patients the relapse was molecular and in three hematologic. There was no case of extramedullary relapse. Most of these patients were treated with ATO.[91,92] Two patients died during salvage therapy. Six achieved molecular remission and proceeded to autologous stem cell transplantation. In all these six cases the graft was PML-RARα negative.[91,93] Five of these six patients are alive and in molecular remission for 2-124 months after the transplantation (median, 36). Three relapsed patients did not achieve molecular remission and proceeded to allogeneic stem cell transplantation. All had an HLA identical sibling. They are alive and in molecular remission [62, 70], and 118 months after the transplantation. The cumulative incidence of relapse (calculated from the date of CHR) was 18.3% (95% CI, 9.9-28.7). It was 14.8% for the low/intermediate risk group and 33.5% for the high risk group (univariate, HR=2.21, p=0.195). (Table 6, Figure 3) Two patients developed a myelodysplastic syndrome 4 months and 3 years after the diagnosis of APL.[36,37,44,51,94] Both are alive and in complete molecular remission concerning PML/RARα seven months and eight years since the diagnosis of MDS, respectively.
The DFS for all 80 patients that achieved CHR was 73.6%. The DFS of the patients belonging to the low/intermediate risk group was 75.1%, and of those belonging to the high risk group 66.5%. (Table 6, Figure 4)
The results of univariate and multivariate analysis concerning overall survival are shown in tables 7 and 8. The same factors were studied with univariate analysis for the DFS and the CIR. None proved to be significant (data not shown).

Figure 3. Relapse cumulative incidence. A: all patients (N=80). B: according to Sanz score.

Figure 4. Kaplan-Meier estimated Disease Free Survival curves. A: all patients (n=94). B: according to Sanz score.

Table 7. Characteristics and postremission outcome of APL patients (univariate analysis).

Table 8. Multivariate Cox regression analysis of Overall Survival .
Discussion
In this study we present the clinical, immunophenotypic, cytogenetic, and molecular findings as well as the clinical course of 95 Greek APL patients from 6 hospitals in Greece and Cyprus. Since there is not a national registry for this particular type of AML in Greece, the present study represents the largest series ever reported.
Demographics do not seem to differ substantially from previous literature reports.[43,45,51] Immunophenotyping is a useful tool for the correct diagnosis. Intermediate to high side scatter, strong CD33 expression and MPO positivity, CD34+/-, CD13++, CD15+ dim and lack of HLA-DR expression were characteristic findings in almost all cases studied. The only patient showing HLA-DR expression was found to be PML-RARα negative since her cytogenetic abnormality was t(11;17).[58-61] We noticed a strong correlation between the expression of lymphoid
markers and the development of differentiation syndrome since 7/8 cases expressing lymphoid markers developed the syndrome. By far the most frequent lymphoid marker expressed was CD2, a cell adhesion molecule. An explanation for such a correlation might be the adherence of the cells expressing such molecules to the capillary endothelium with consequent inflammation.[95] There were only two cases with CD56 expression, a rather low incidence and, therefore, no conclusions about its significance can be drawn.[ 96]
Taking into account that cytogenetically cryptic PML-RARα rearrangements are observed in 4%-6% of APL cases, we found a rather high number of cases with normal cytogenetics, 9/68 (13.2%). Chance and ‘technical reasons’ are the only explanations we can offer for this finding.[5,16,26] Additional chromosomal abnormalities were found in 15/59 cases (28.8%) and trisomy 8 was by far the most frequent abnormality. These findings are in accordance to other reports. The presence of additional chromosomal abnormalities did not influence the response to treatment. We therefore agree with the suggestion that ACAs is not a reason to intensify treatment.[11-15] The prognostic significance of ider(17q) and three way translocations of t(15;17) are currently unknown due to their low incidence.[41] In addition, the third chromosome that is involved in the three way translocation to the t(15;17) varies. One of the two variant translocations involving chromosomes 15, 17 and 18 was associated with a submicroscopic deletion of the 5’ part of the RARα gene, as evidenced by FISH.[97] Because only limited number of studies has examined such deletions associated with the PML-RARa fusion gene, their significance and their involvement in the pathogenesis of the disease if any, remain unclear. One of our patients manifested t(8;21) in addition to the t(15;17). Although the patient received induction therapy according to the AIDA protocol and subsequently two cycles of high dose cytarabine with idarubicin and ATRA he did not achieve molecular remission. The co-existence of t(15;17) and t(8;21) in a single leukemic clone is a very rare finding and the results of the few reported cases are conflicting. However, it has been proposed that PML-RARa and AML1/ETO fusion proteins may mutually affect their pathogenetic mechanism, rendering the cells resistant to ATRA which is in agreement with the resistance of our patient to ATRA based treatment.[38,80,81]
In the Greek origin patients the L isoform of the PML-RARa was the most frequent and this is in agreement with previous reports. In contrast, in the Cyprus origin patients we noticed a very high frequency of the S isoform.[5,17,18] We can offer no explanation for this finding and it definitely needs confirmation. The presence of FLT3 mutations was associated with various adverse features and generally with intermediate/high risk score, as previously reported. It seems therefore that these mutations are inherently connected with the biology of the disease. We observed one patient showing the FLT3 TKD permanently for 4 years while being in complete molecular remission. Such a finding supports the suggestion of not using FLT3 mutations as a marker for minimal residual disease in APL.[22,69,98,99]
One important finding of our study was the considerable early death rate. Actually there were 14/94 early deaths (14.9%), with half of these patients dying within the first few days before they could receive full induction treatment. Hemorrhage was the main cause of early death. (Table 4)[32,33,35,71] Such a figure is increased compared to the numbers reported in the clinical trials of APL (3%-10%). However, population based studies have confirmed that the early death rate reported in clinical trials does not correspond to real life circumstances and worryingly, such a finding has not appreciably changed in the ATRA era. Delays in referring the patients, delays in establishing diagnosis, and failure to promptly begin specific treatment on clinical suspicion and to provide aggressive supportive therapy are factors explaining such mortality and are the targets of the interventions urgently needed. Moreover, our data suggest that much of the prognostic effect of significant variables on OS outcome largely stems from the effect of these variables on the risk of early death. (Tables 5, 7, and 8) This is also supported by the observation that these variables had no discernible effect on disease-free survival or relapse incidence. As regards patients surviving induction treatment the response was excellent. All 80 evaluable patients achieved complete hematologic remission. At the end of the consolidation therapy 73/75 achieved molecular remission. These results are in accordance to the relevant reports of the protocols that our patients followed (AIDA0493, the LPA1999 and LPA2005 PETHEMA and the European APL1993 and APL2000). [30,35,43-51] Only two patients showed primary resistance, i.e. they never achieved molecular remission. Both died after being treated with aggressive chemotherapy or allogeneic stem cell transplantation. We observed 10 relapses (cumulative incidence of relapse 18.3%, 95% CI 9.9%-28.7%) (Figure 3). Because of the close monitoring most relapses were molecular and therefore the patients were salvaged rather early. The salvage treatment (for the majority ATO and autologous or allogeneic stem cell transplantation) was successful: five patients remain in molecular remission for 2-124 months (median, 36) after autologous stem cell transplantation and three patients for 62-118 months after allogeneic stem cell transplantation. These results further support the importance of the close molecular monitoring after the first line treatment is completed.[65,100-102] No CNS relapse was observed in the cohort of our patients in none of whom CNS prophylaxis was given.[27,52] There were two patients that developed myelodysplastic syndrome, an incidence not different from the one reported. [27,36,37,40,44,51,94] We were unable to confirm the value of various well established risk factors for the patients’ outcome. Most probable explanations are the rather small numbers in our study as well as the risk adapted therapy that was provided to most of our patients. In summary, our data confirm the excellent results of the current treatment of the disease, provided that the patient will survive the early period after the disease has been diagnosed.
References
- Hillestad LK. Acute promyelocytic leukemia.
Acta Med Scand 1957;159:189-194 http://dx.doi.org/10.1111/j.0954-6820.1957.tb00124.x

- Bernard J, Mathe G, Boulay J, Ceoard B,
Chome J. Acute promyelocytic leukemia: a study made on 20 cases.
Schweiz Med Wochenschr 1959;89:604-608 PMid:13799642

- Bennett JM, Catowsky D, Daniel MT, Flandrin
G, Galton D, Gralnick M, Sultan C. Proposals for the classification of
the acute leukemias: French-American-British (FAB) cooperative group.
Br J Haematol 1976;33(4):451-458http://dx.doi.org/10.1111/j.1365-2141.1976.tb03563.x
PMid:188440

- Bennet JM, Catowsky D, Daniel MT, Flandrin
G, Galton D, Gralnick M, Sultan C. A variant form of hypergranular
promyelocytic leukemia (M3). Br J Haematol 1980;44(1):169-170 http://dx.doi.org/10.1111/j.1365-2141.1980.tb01195.x
PMid:6929699

- Lo Coco F, Diverio D, Falini B, Biondi A,
Nervi C, and Pelicci PG. Genetic Diagnosis and Molecular Monitoring in
the Management of Acute Promyelocytic Leukemia. Blood 1999;94:12-22
PMid:10381493

- Rowlay JD, Golomb HM, Dougherty C. 15/17
translocation, a consistent chromosomal change in acute promyelocytic
leukemia. Lancet 1977;1:549-550 http://dx.doi.org/10.1016/S0140-6736(77)91415-5

- Larson RA, Kondo K, Vardiman JW, Butler
ARE, Golomb HM, Rowley JD: Evidence for a (15;17) translocation in
every patient with acute promyelocytic leukemia. Am J Med 1984;76:827 http://dx.doi.org/10.1016/0002-9343(84)90994-X

- Kakizuka A, Miller WH Jr, Umesono K,
Warrell RP Jr, Frankel SR, Murty VV, Dmitrovsky E, Evans RM:
Chromosomal translocation t(15;17) in human acute promyelocytic
leukemia fuses RAR alpha with a novel putative transcription factor,
PML. Cell 1991;66:663-74 http://dx.doi.org/10.1016/0092-8674(91)90112-C

- Grignani F, Ferrucci PF, Testa U, Talamo G,
Fagioli M, Alcalay M, Mencarelli A, Grignani F, Peschle C, Nicoletti I,
Pelicci PG: The acute promyelocytic leukemia-specific PML-RARa fusion
protein inhibits differentiation and promotes survival of myeloid
precursor cells. Cell 1993;74:423-31 http://dx.doi.org/10.1016/0092-8674(93)80044-F

- Redner RL. Variations on a theme: the
alternate translocations in APL. Leukemia 2002;16(10):1927-32 http://dx.doi.org/10.1038/sj.leu.2402720
PMid:12357344

- Schoch C, Haase D, Haferlach T, Freund M,
Link H, Lengfelder E, Löffler H, Büchner T, Fonatsch C: Incidence and
implication of additional chromosome aberrations in acute promyelocytic
leukaemia with translocation t(15;17)(q22;q21): a report on 50
patients. Br J Haematol 1996;94:493-500 http://dx.doi.org/10.1046/j.1365-2141.1996.d01-1829.x
PMid:8790148

- De Botton S, Chevret S, Sanz M, Dombret H,
Thomas X, Guerci A, Fey M, Rayon C, Huguet F, Sotto J, Gardin C,
Makhoul PC, Travade P, Solary E, Fegueux N, Bordessoule D, San Miguel
J, Link H, Desablens B, Stamatoulas A, Deconinck E, Geiser K, Hess U,
Maloisel F, Castaigne S, Preudhomme C, Chomienne C, Degos L, Fenaux P.
Additional chromosomal abnormalities in patients with acute
promyelocytic leukaemia (APL) do not confer poor prognosis: results of
APL 93 trial. Br J Haematol 2000;111:801-806 http://dx.doi.org/10.1046/j.1365-2141.2000.02442.x
PMid:11122141

- Hernández JM, Martín G, Gutiérrez NC,
Cervera J, Ferro MT, Calasanz MJ, Martínez-Climent JA, Luño E, Tormo M,
Rayón C, Díaz-Mediavilla J, González M, González-San Miguel JD,
Pérez-Equiza K, Rivas C, Esteve J, Alvarez Mdel C, Odriozola J, Ribera
JM, Sanz MA; PETHA Cooperative Group, Spain: Additional cytogenetic
changes do not influence the outcome of patients with newly diagnosed
acute promyelocytic leukemia treated with an ATRA plus anthracyclin
based protocol. A report of the Spanish group PETHEMA. Haematologica
2001;86:807-13. PMid:11522536

- Cervera J, Montesinos P, Hernández-Rivas
JM, Calasanz MJ, Aventín A, Ferro MT, Luño E, Sánchez J, Vellenga E,
Rayón C, Milone G, de la Serna J, Rivas C, González JD, Tormo M, Amutio
E, González M, Brunet S, Lowenberg B, Sanz MA. Additional chromosome
abnormalities in patients with acute promyelocytic leukemia treated
with all-trans retinoic acid and chemotherapy. Haematologica
2010;95(3):424-31. Epub 2009 Nov 10 http://dx.doi.org/10.3324/haematol.2009.013243
PMid:19903674 PMCid:2833072

- Ono T, Takeshita A, Iwanaga M, Asou N,
Naoe T, Ohno R; Japan Adult Leukemia Study Group. Impact of additional
chromosomal abnormalities in patients with acute promyelocytic
leukemia: 10-year results of the Japan Adult Leukemia Study Group APL97
study. Haematologica 2011;96(1):174-176. Epub 2010 Sep 30 http://dx.doi.org/10.3324/haematol.2010.030205
PMid:20884714 PMCid:3012784

- Grimwade D, Howe K, Langabeer S, Davies L,
Oliver F, Walker H, Swirsky D, Wheatley K, Goldstone A, Burnett A,
Solomon E. Establishing the presence of the t(15;17) in suspected acute
promyelocytic leukaemia: cytogenetic, molecular and PML
immunofluorescence assessment of patients entered into the M.R.C.ATRA
trial. M.R.C. Adult Leukaemia Working Party. Br J Haematol
1996;94:557–573 PMid:8790159

- David Grimwade. The significance of
minimal residual disease in patients with t(15;17). Best Pract Res Clin
Haematol 2002; 15(1):137-158 http://dx.doi.org/10.1053/beha.2002.0189

- Reiter A, Saussele S, Grimwade D, Wiemels
JL, Segal MR, Lafage-Pochitaloff M, Walz C, Weisser A, Hochhaus A,
Willer A, Reichert A, Buchner T, Lengfelder E, Hehlmann R, and Cross
NCP. Genomic Anatomy of the Specific Reciprocal Translocation t(15;17)
in Acute Promyelocytic Leukemia. Genes, Chromosomes & Cancer
2003;36:175–188 http://dx.doi.org/10.1002/gcc.10154
PMid:12508246

- Kottaridis PD, Gale RE, Frew ME, Harrison
G, Langabeer SE, Belton AA, Walker H, Wheatley K, Bowen DT, Burnett AK,
Goldstone AH, and Linch DC. The presence of a FLT3 internal tandem
duplication in patients with acute myeloid leukemia (AML) adds
important prognostic information to cytogenetic risk group and response
to the first cycle of chemotherapy: analysis of 854 patients from the
United Kingdom Medical Research Council AML 10 and 12 trials. Blood
2001; 98:1752-1759 http://dx.doi.org/10.1182/blood.V98.6.1752PMid:11535508

- Schnittger S, Schoch C, Dugas M, Kern W,
Staib P, Wuchter C, Löffler H, Sauerland CM, Serve H, Büchner T,
Haferlach T, and Hiddemann W. Analysis of FLT3 length mutations in 1003
patients with acute myeloid leukemia: correlation to cytogenetics, FAB
subtype, and prognosis in the AMLCG study and usefulness as a marker
for the detection of minimal residual disease. Blood 2002;100:59-66 http://dx.doi.org/10.1182/blood.V100.1.59
PMid:12070009

- Kottaridis PD, Gale RE, Langabeer SE, Frew
ME, Bowen DT, and Linch DC. Studies of FLT3 mutations in paired
presentation and relapse samples from patients with acute myeloid
leukemia: implications for the role of FLT3 mutations in
leukemogenesis, minimal residual disease detection, and possible
therapy with FLT3 inhibitors. Blood 2002; 100:2393-2398 http://dx.doi.org/10.1182/blood-2002-02-0420
PMid:12239147

- Beitinjaneh A, Jang S, Roukoz H, Majhail
NS. Prognostic significance of FLT3 internal tandem duplication and
tyrosine kinase domain mutations in acute promyelocytic leukemia: a
systematic review. Leuk Res 2010;34(7):831-836. Epub 2010 Jan 21 http://dx.doi.org/10.1016/j.leukres.2010.01.001
PMid:20096459

- Mistry AR, Pedersen EW, Solomon E,
Grimwade D. The molecular pathogenesis of acute promyelocytic leukemia:
implications for the clinical management of the disease. Blood Rev
2003;17(2):71-97 http://dx.doi.org/10.1016/S0268-960X(02)00075-9

- Lengfelder E, Saussele S, Weisser A,
Buchner T, Hehlmann R: Treatment concepts of acute promyelocytic
leukemia. Crit Rev Oncol Hematol 2005;56:261-274 http://dx.doi.org/10.1016/j.critrevonc.2004.08.009
PMid:16236522

- Sirulnik LA, Stone RM: Acute promyelocytic
leukemia: current strategies for the treatment of newly diagnosed
disease. Clin Adv Hematol Oncol 2005;429:391-397

- Wang ZY, Chen Z. Acute promyelocytic
leukemia: from highly fatal to highly curable. Blood
2008;111(5):2505-2515 http://dx.doi.org/10.1182/blood-2007-07-102798
PMid:18299451

- Sanz MA, Grimwade D, Tallman MS, Lowenberg
R, Fenaux P, Estey EH, Naoe T, Lengfelder E, Büchner T, Döhner H,
Burnett AK, and Lo-Coco F. Management of acute promyelocytic leukemia:
recommendations from an expert panel on behalf of the European Leukemia
Net. Blood 2009;113:1875-1891 http://dx.doi.org/10.1182/blood-2008-04-150250
PMid:18812465

- Tallman MS and Altman JK. How I treat
acute promyelocytic leukemia. Blood 2009;114:5126-5135 http://dx.doi.org/10.1182/blood-2009-07-216457
PMid:19797519

- Powell BL, Moser B, Stock W, Gallagher RE,
Willman CL, Stone RM, Rowe JM, Coutre S, Feusner JH, Gregory J, Couban
S, Appelbaum FR, Tallman MS, and Larson RA. Arsenic trioxide improves
event-free and overall survival for adults with acute promyelocytic
leukemia: North American Leukemia Intergroup Study C9710. Blood
2010;116(19): 3751- 3757http://dx.doi.org/10.1182/blood-2010-02-269621
PMid:20705755 PMCid:2981533

- Adès L, Sanz MA, Chevret S, Montesinos P,
Chevallier P, Raffoux E, Vellenga E, Guerci A, Pigneux A, Huguet F,
Rayon C, Stoppa AM, de la Serna J, Cahn JY, Meyer-Monard S, Pabst T,
Thomas X, de Botton S, Parody R, Bergua J, Lamy T, Vekhoff A, Negri S,
Ifrah N, Dombret H, Ferrant A, Bron D, Degos L, and Fenaux P. Treatment
of newly diagnosed acute promyelocytic leukemia (APL): a comparison of
French-Belgian-Swiss and PETHEMA results. Blood 2008;111:1078-1084
PMid:17975017

- Sanz MA, Lo-Coco F. Modern approaches to
treating acute promyelocytic leukemia. J Clin Oncol 2011;29(5):495-503 http://dx.doi.org/10.1200/JCO.2010.32.1067
PMid:21220600

- Lehmann S, Ravn A, Carlsson l, Antunovic
p, Deneberg S, Mollgard L, Rangert Derolf A, stockelberg D, Tibefelt U,
Wahlin A, Wennstrom L, Hoglund M and Juliusson G. Continuing high early
death rate in acute promyelocytic leukemia: a population-based report
from the Swedish Adult Acute Leukemia Registry. Leukemia
2011;25:1128-1134 http://dx.doi.org/10.1038/leu.2011.78
PMid:21502956

- Park JH, Qiao B, Panageas KS, Schymura MJ,
Jurcic JG, Rosenblat TL, Altman JK, Douer D, Rowe JM, and Tallman MS.
Early death rate in acute promyelocytic leukemia remains high despite
all-trans retinoic acid. Blood 2011;118(5):1248-1254 http://dx.doi.org/10.1182/blood-2011-04-346437
PMid:21653939

- Sanz MA, Martin G, Rayon C, Esteve J,
Gonzalez M, Diaz-Mediavilla J, Bolufer P, Barragan E, Terol MJ,
Gonzalez JD, Colomer D, Chillon C, Rivas C, Gomez T, Ribera JM,
Bornstein R, Roman J, Calasanz MJ, Arias J, Alvarez C, Ramos F, and
Deben G for the PETHEMA Group. A Modified AIDA Protocol With
Anthracycline-Based Consolidation Results in High Antileukemic Efficacy
and Reduced Toxicity in Newly Diagnosed PML/RARa-Positive Acute
Promyelocytic Leukemia. Blood 1999;94(9): 3015-3021 PMid:10556184

- Sanz MA, Martin G, Gonzalez M, Leon A,
Rayon C, Rivas C, Colomer D, Amutio E, Capote FJ, Milone GA, de la
Serna J, Roman J, Barragan E, Bergua J, Escoda L, Parody R, Negri S,
Calasanz MJ, and Bolufer P. Risk adapted treatment of acute
promyelocytic leukemia with all-trans retinoic acid and anthracycline
monochemotherapy: a multicenter study by the PETHEMA Group. Blood
2004;103(4):1237-1243 http://dx.doi.org/10.1182/blood-2003-07-2462
PMid:14576047

- Latagliata R, Petti MC, Fenu S, Mancini M,
Spiriti MAM, Breccia M, Brunetti GA, Avvisati G, Lo Coco F and Mandelli
F. Therapy-related myelodysplastic syndrome-acute myelogenous leukaemia
in patients treated for acute promyelocytic leukaemia: an emerging
problem. Blood 2002;99(3):822-824 http://dx.doi.org/10.1182/blood.V99.3.822
PMid:11806982

- Zompi S, Viguie F. Therapy-related acute
myeloid leukaemia and myelodysplasia after successful treatment of
acute promyelocytic leukaemia. Leuk Lymphoma 2002;43:275-280 http://dx.doi.org/10.1080/10428190290006044

- Stavroyianni N, Yataganas X, Abazis D,
Pangalos C, Meletis J. Acute promyelocytic leukemia relapsing into
FAB-M2 acute myeloid leukemia with trisomy 8. Cancer Genet Cytogenet
2000;117(1):82-83 http://dx.doi.org/10.1016/S0165-4608(99)00132-6

- Matsouka P, Sambani C, Giannakoulas N,
Symeonidis A, Zoumbos N. Polyploidy in acute promyelocytic leukemia
without the 15:17 translocation. Haematologica 2001;86(12):1312-1313
PMid:11726325

- Athanasiadou A, Saloum R, Zorbas I,
Tsompanakou A, Batsis I, Fassas A, Anagnostopoulos A. Therapy-related
myelodysplastic syndrome with monosomy 5 and 7 following successful
therapy for acute promyelocytic leukemia with anthracyclines. Leuk
Lymphoma 2002;43(12):2409-2411 http://dx.doi.org/10.1080/1042819021000040143

- Manola KN, Karakosta M, Sambani C,
Terzoudi G, Pagoni M, Gatsa E, Papaioannou M. Isochromosome
der(17)(q10)t(15;17) in acute promyelocytic leukemia resulting in an
additional copy of the RARA-PML fusion gene: report of 4 cases and
review of the literature. Acta Haematol 2010;123(3):162-170 http://dx.doi.org/10.1159/000294959
PMid:20224268

- Sanz MA, Lo Coco F, Martin G, Avvisati G,
Rayon C, Barbui T, Diaz-Mediavilla J, Fioritoni G, Gonzalez JD, Liso V,
Esteve J, Ferrara F, Bolufer P, Bernasconi C, Gonzalez M, Rodeghiero F,
Colomer D, Petti MC, Jose M. Ribera, and Franco Mandelli for the
Spanish PETHEMA and the Italian GIMEMA Cooperative GroupsDefinition of
relapse risk and role of non-anthracycline drugs for consolidation in
patients with acute promyelocytic leukemia: a joint study of the
PETHEMA and GIMEMA cooperative groups. Blood 2000;96(4):1247-1253
PMid:10942364

- Avvisati G, Lo-Coco F, Paoloni FP, Petti
MC, Diverio D, Vignetti M, Latagliata R, Specchia G, Baccarani M, Di
Bona E, Fioritoni G, Marmont F, Rambaldi A, Di Raimondo F, Kropp MG,
Pizzolo G, Pogliani EM, Rossi G, Cantore N, Nobile F, Gabbas A, Ferrara
F, Fazi P, Amadori S, Mandelli F, and for the GIMEMA, AIEOP, and EORTC
Cooperative Groups. AIDA 0493 protocol for newly diagnosed acute
promyelocytic leukemia: very long-term results and role of maintenance.
Blood 2011;117:4716-4725 http://dx.doi.org/10.1182/blood-2010-08-302950
PMid:21385856

- Sanz MA, Montesinos P, Vellenga E, Rayon
C, de la Serna J, Parody R, Bergua JM, Leon A, Negri S, Gonzalez M,
Rivas C, Esteve J, Milone G, Gonzalez JD, Amutio E, Brunet S,
García-Larana J, Colomer D, Calasanz MJ, Chillon C, Barragan E, Bolufer
P, and Lowenberg Bob. Risk-adapted treatment of acute promyelocytic
leukemia with all-trans retinoicacid and anthracycline
monochemotherapy: long-term outcome of the LPA99 multicenter study by
the PETHEMA Group. Blood 2008;112(8):3130-3134 http://dx.doi.org/10.1182/blood-2008-05-159632
PMid:18664623

- Sanz MA, Montesinos P, Rayón C, Holowiecka
A, de la Serna J, Milone G, de Lisa E, Brunet S, Rubio V, Ribera JM,
Rivas C, Krsnik I, Bergua J, González J, Díaz-Mediavilla J, Rojas R,
Manso F, Ossenkoppele G, González JD, and Lowenberg B. Risk-adapted
treatment of acute promyelocytic leukemia based on all-trans retinoic
acid and anthracycline with addition of cytarabine in consolidation
therapy for high-risk patients: further improvements in treatment
outcome. Blood 2010;115(25):5137-5146 http://dx.doi.org/10.1182/blood-2010-01-266007
PMid:20393132

- Fenaux P, Le Deley MC, Castaigne S,
Archimbaud E, Chomienne C, Link H, Guerci A, Duarte M, Daniel MT, Bowen
D, Huebner G, Bauters F, Fegueux N, Fey M, Sanz M, Lowenberg B,
Maloisel F, Auzanneau G, Sadoun A, Gardin C, Bastion Y, Ganser A, Jacky
E, Dombret H, Chastang C, Degos L, and the European APL 91 Group.
Effect of all transretinoic acid in newly diagnosed acute promyelocytic
leukemia: results of a multicenter randomized trial. European APL 91
Group. Blood 1993;82(11):3241-3249 PMid:8241496

- Fenaux P, Chastang C, Chevret S, Sanz M,
Dombret H, Archimbaud E, Fey M, Rayon C, Huguet F, Sotto JJ, Gardin C,
Makhoul PC, Travade P, Solary E, Fegueux N, Bordessoule D, San Miguel
J, Link H, Desablens B, Stamatoullas A, E. Maloisel DF, Castaigne S,
Preudhomme C, and Degos L for the European APL Group. A Randomized
Comparison of All Transretinoic Acid (ATRA) Followed by Chemotherapy
and ATRA Plus Chemotherapy and the Role of Maintenance Therapy in Newly
Diagnosed Acute Promyelocytic Leukemia. Blood 1999;94(4):1192-1200
PMid:10438706

- Ades L, Chevret S, Raffoux E, et al. Is
cytarabine useful in the treatment of acute promyelocytic leukemia?
results of a randomized trial from the European Acute Promyelocytic
Leukemia Group. J Clin Oncol 2006;24:5703-5710 http://dx.doi.org/10.1200/JCO.2006.08.1596
PMid:17116939

- Avvisati G, Petti M, Lo Coco F, Testi A, Fazi P. AIDA : the Italian way of treating acute promyelocytic leukemia (APL), final act [abstract]. Blood 2003;102:142a. Abstract#487
- Asou N, Kishimoto Y, Kiyoi H, Okada M,
Kawai Y, Tsuzuki M, Horikawa K, Matsuda M, Shinagawa K, Kobayashi T,
Ohtake S, Nishimura M, Takahashi M, Yagasaki F, Takeshita A, Kimura Y,
Iwanaga M, Naoe T, and Ohno R, for the Japan Adult Leukemia Study Group
A randomized study with or without intensified maintenance chemotherapy
in patients with acute promyelocytic leukemia who have become negative
for PML-RAR_ transcript after consolidation therapy: The Japan Adult
Leukemia Study Group (JALSG) APL97 study. Blood 2007;110(1):59-66 http://dx.doi.org/10.1182/blood-2006-08-043992
PMid:17374742

- Adès L, Guerci A, Raffoux E, Sanz M,
Chevallier P, Lapusan S, Recher C, Thomas X, Rayon C, Castaigne S,
Tournilhac O, de Botton S, Ifrah N, Cahn JY, Solary E, Gardin C, Fegeux
N, Bordessoule D, Ferrant A, Meyer-Monard S, Vey N, Dombret H, Degos L,
Chevret S, Fenaux P, and for the European APL Group. Very long-term
outcome of acute promyelocytic leukemia after treatment with all-trans
retinoic acid and chemotherapy: the European APL Group experience.
Blood 2010;115:1690-1696 http://dx.doi.org/10.1182/blood-2009-07-233387
PMid:20018913

- Evans GD, Grimwade DJ. Extramedullary
disease in acute promyelocytic leukemia. Leuk Lymphoma 1999;33:219-229
PMid:10221502

- E. Patatanian and D. F. Thompson. Retinoic
acid syndrome: a review. J Clin Pharm Therap 2008;33:331–338 http://dx.doi.org/10.1111/j.1365-2710.2008.00935.x
PMid:18613850

- Tallman MS, Andersen JW, Schiffer CA,
Appelbaum FR, Feusner JH, Woods WG, Ogden A, Weinsten H, Shepherd L,
Willman C, Bloomfield CD, Rowe JM and Wiernik PH. All-trans retinoic
acid in acute promyelocytic leukemia: long-term outcome and prognostic
factor analysis from the North American Intergroup protocol. Blood
2002;100:4298-4302 http://dx.doi.org/10.1182/blood-2002-02-0632
PMid:12393590

- Wiley JS, Firkin FC. Reduction of
pulmonary toxicity by prednisolone prophylaxis during all-trans
retinoic acid treatment of acute promyelocytic leukemia. Leukemia
1995;9:774-778 PMid:7769839

- G Avvisati, F Lo Coco, D Diverio, M Falda,
F Ferrara, M Lazzarino, D Russo, MC Petti, and F MandelliAIDA (All
trans retinoic acid plus idarubicin) in newly diagnosed acute
promyelocytifc leukemia: a GIMEMA pilot study. Blood
1996;88(4):1390-1398 PMid:8695858

- Mandelli F, Diverio D, Avvisati G, Luciano
A, Barbui T, Bernasconi C, Broccia G, Cerri R, Falda M, Fioritoni G,
Leoni F, Liso V, Petti MC, Rodeghiero F, Saglio G, Vegna ML, Visani G,
Jehn U, Willemze R, Muus P, Pelicci PG, Biondi A, and Lo Coco F for the
Gruppo Italiano Malattie Ematologiche Maligne dell’ Adulto and
Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative
Groups. Molecular remission in PML/RARa-positive acute promyelocytic
leukemia by combined all-trans retinoic acid and idarubicin (AIDA)
therapy. Blood. 1997;90(3):1014-1021 PMid:9242531

- Orfao A, Chillon MC, Bortoluci AM,
Lopez-Berges MC, Garcia-Sanz R, Gonzalez M, Tabernero MD, García-Marcos
MA, Rasillo AI,* Hernández-Rivas J, San Miguel Jesús F. The flow
cytometric pattern of CD34, CD15 and CD13 expression in acute
myeloblastic leukemia is highly characteristic of the presence of
PML-RARalpha gene rearrangements. Haematologica 1999;84:405–412.
PMid:10329918

- Kakkas I, Psarra K, Kapsimali V,
Garofalaki M, Pagoni M, Apostolidis I, Karmiris T, Nikiforakis E,
Papasteriades C. Diagnostic value of acute promyelocytic leukaemia flow
cytometry immunophenotyping. Haema 2005;8(4):641-643

- Di Noto R, Mirabelli P and Del Vecchio L.
Flow cytometry analysis of acute promyelocytic leukemia: the power of
surface hematology. Leukemia 2007;21: 4–8. http://dx.doi.org/10.1038/sj.leu.2404412
PMid:17167527

- MC Bene, T Nebe, P Bettelheim, B Buldini,
H Bumbea, W Kern, F Lacombe, P Lemez, I Marinov, E Matutes, M Maynadie,
U Oelschlagel, A Orfao, R Schabath, M Solenthaler, G
Tschurtschenthaler, AM Vladareanu, G Zini, GC Faure and A Porwit.
Immunophenotyping of acute leukemia and lymphoproliferative disorders:
a consensus proposal of the European LeukemiaNet Work Package 10.
Leukemia 2011;25:567–574 http://dx.doi.org/10.1038/leu.2010.312
PMid:21252983

- van Dongen JJM, Macintyre EA, Gabert JA,
Delabesse E, Rossi V, Saglio G, Gottardi E, Rambaldi A, Dotti G,
Griesinger F, Parreira A, Gameiro P, Gonzalez Diaz M, Malec M, Langerak
AW, San Miguel JF and Biondi A. Standardized RT-PCR analysis of fusion
gene transcripts from chromosome aberrations in acute leukemia for
detection of minimal residual disease Leukemia 1999;13:1901–1928 http://dx.doi.org/10.1038/sj.leu.2401592
PMid:10602411

- van der Velden VHJ, Hochhaus A, Cazzaniga
G, Szczepanski T, Gabert J and van Dongen JJM. Detection of minimal
residual disease in hematologic malignancies by real-time quantitative
PCR: principles, approaches, and laboratory aspects, Leukemia
2003;17:1013–1034 http://dx.doi.org/10.1038/sj.leu.2402922
PMid:12764363

- Gabert J, Beillard E, van der Velden VHJ,
Bi W, Grimwade D, Pallisgaard N, Barbany G, Cazzaniga G, Cayuela JM,
Cave H, Pane F, Aerts JLE, De Micheli D, Thirion X, Pradel V, Gonzalez
M, Viehmann S, Malec M, Saglio G and van Dongen JJM. Standardization
and quality control studies of ‘real-time’ quantitative reverse
transcriptase polymerase chain reaction of fusion gene transcripts for
residual disease detection in leukemia – A Europe Against Cancer
Program. Leukemia 2003;17:2318–2357 http://dx.doi.org/10.1038/sj.leu.2403135
PMid:14562125

- Lo Coco F, Breccia M, Diverio D. The
importance of molecular monitoring in acute promyelocytic leukemia.
Best Pract Res Clin Haematol 2003;16(3):503-520 http://dx.doi.org/10.1016/S1521-6926(03)00041-0

- Santamaría C, Chillón MC, Fernández C,
Martín-Jiménez P, Balanzategui A, Sanz RG, San Miguel JF,
Marcos-Gonzalez González. Using quantification of the PML-RARa
transcript to stratify the risk of relapse in patients with acute
promyelocytic leukemia. Haematologica 2007; 92:315-322 http://dx.doi.org/10.3324/haematol.10734
PMid:17339180

- Garofalaki M, Pagoni M, Tziotziou I, Nikolou E, Nikiforakis E. The use of RQ-PCR according to "Europe against cancer (EAC)" program for the diagnosis of APL patients. 5th international symposium on acute promyelocytic leukemia, Rome 24-26 September 2009
- Garofalaki M, Pagoni M, Tziotziou I, Nikolou E, Balta A, Vagia M, Tzannou I, Baltadakis I, Nikiforakis E. Real-time quantitative (RQ)-PCR by the europe against cancer (EAC) protocol in acute promyelocytic leukemia (APL), International symposium on minimal residual cancer, September 16-19, 2009, Athens Greece, Oral presentation, abstract #027 p.91)
- Pagoni M, Garofalaki M, Tziotziou I, Nikolou E, Panitsas F, Bika I, Vourtsi A, Delimpassi S, Gigantes S, Harhalakis ?. Prognostic implication of FLT3 mutations in patients with acute promyelocytic leukemia, abstact# Acute leukemias XIII Biology and Treatment Strategies Munich, Germany February 27 - March 2, 2011
- Cheson BD, Cassileth PA, Head DR, Schiffer
CA, Bennet JM, Bloomfield CD, Brynning R, Gale RP, Grever MR, Keating
MJ, Sawitsky A, Strass S, Weinstein H, Woods WG: Report of the National
Cancer Institute-Sponsored Workshop on definition of diagnosis and
response in acute myeloid leukemia. J Clin Oncol 1990;8:813-819
PMid:2185339

- Lo-Coco F, Avvisati G, Vignetti M, Breccia
M, Gallo E, Rambaldi A, Paoloni F, Fioritoni G, Ferrara F, Specchia G,
Cimino G, Diverio D, Borlenghi E, Martinelli G, Di Raimondo F, Di Bona
E, Fazi P, Peta A, Bosi A, Carella AM, Fabbiano F, Pogliani EM, Petti
MC, Amadori S, Mandelli F, and for the Italian GIMEMA Cooperative Group
Front-line treatment of acute promyelocytic leukemia with AIDA
induction followed by risk-adapted consolidation for adults younger
than 61 years: results of the AIDA-2000 trial of the GIMEMA Group.
Blood 2010;116:3171-3179 http://dx.doi.org/10.1182/blood-2010-03-276196
PMid:20644121

- Frankel SR, Eardley A, Lauwers G, Weiss M,
and Warrell RP, Jr. The “retinoic Acid Syndrome” in Acute Promyelocytic
Leukemia. An Intern Med 1992;117:292-296

- De Botton S, Dombret H, Sanz M, San Miguel
J, Caillot D, Zittoun R, Gardembas M, Stamatoulas A, Conde E, Guerci A,
Gardin C, Geiser K, Cony Makhoul D, Reman O, de la Serna J, Lefrere F,
Chomienne C, Chastang C, Degos L, Fenaux P, and the European APL Group.
Incidence, Clinical Features, and Outcome of All Trans-Retinoic Acid
Syndrome in 413 Cases of Newly Diagnosed Acute Promyelocytic Leukemia.
Blood 1998;92: 2712-2718 PMid:9763554

- Beaumont M, Sanz M, Carli PM, Maloisel F,
Thomas X, Detourmignies L, Guerci A, Gratecos N, Rayon C, San Miguel J,
Odriozola J, Cahn JY, Huguet F, Vekhof A, Stamatoulas A, Dombret H,
Capote F, Esteve J, Stoppa AM, Fenaux P. Therapy-related acute
promyelocytic leukemia. J Clin Oncol 2003;21(11):2123-2137 http://dx.doi.org/10.1200/JCO.2003.09.072
PMid:12775738

- Douglas A. Woo, MD; Robert H. Collins, MD; Howard S. Rossman, DO; Olaf Stüve, MD; Elliot M. Frohman, MD Mitoxantrone-Associated Leukemia in Multiple Sclerosis: Case Studies. Int J MS Care 2008;10:41–46
- Mays AN, Osheroff N, Xiao Y, Wiemels JL,
Felix CA, Byl JA, Saravanamuttu K, Peniket A, Corser R, Chang C, Hoyle
C, Parker AN, Hasan SK, Lo-Coco F, Solomon E, Grimwade D. Evidence for
direct involvement of epirubicin in the formation of chromosomal
translocations in t(15;17) therapy-related acute promyelocytic
leukemia. Blood 2010;115(2):326-30 http://dx.doi.org/10.1182/blood-2009-07-235051
Mid:19884644
PMCid:2808156

- Montesinos P, González JD, González J,
Rayón C, de Lisa E, Amigo ML, Ossenkoppele GJ, Peñarrubia MJ,
Pérez-Encinas M, Bergua J, Debén G, Sayas MJ, de la Serna J, Ribera JM,
Bueno J, Milone G, Rivas C, Brunet S, Löwenberg B, Sanz M.
Therapy-related myeloid neoplasms in patients with acute promyelocytic
leukemia treated with all-trans-retinoic Acid and anthracycline-based
chemotherapy. J Clin Oncol 2010;28(24):3872-3879 http://dx.doi.org/10.1200/JCO.2010.29.2268
PMid:20625122

- Ammatuna E, Montesinos P, Hasan SK,
Ramadan SM, Esteve J, Hubmann M, Pagoni M, Grimwade D, Sanz MA, and
Lo-Coco F. Presenting features and treatment outcome of acute
promyelocytic leukemia arising after multiple sclerosis. Haematologica
2011;96(4):621-625 http://dx.doi.org/10.3324/haematol.2010.036657
PMid:21193421 PMCid:3069242

- Nikolay D. Dimov, L. Jeffrey Medeiros,
Farhad Ravandi, and Carlos E. Bueso-Ramos, Acute Promyelocytic Leukemia
at Time of Relapse Commonly Demonstrates Cytogenetic Evidence of Clonal
Evolution and Variability in Blast Immunophenotypic Features. Am J Clin
Pathol 2010;133:484-490 http://dx.doi.org/10.1309/AJCPJ7K0AWMBHMAI
PMid:20154288

- N Stavroyianni, T Kalmantis, X Yataganas.
Simultaneous PML/RARa and AML 1/ETO gene rearrangements in a patient
with acute myeloid leukemia. Leukemia 1999;13(8):1294-1295 http://dx.doi.org/10.1038/sj.leu.2401455
PMid:10450762

- Abreu e Lima RS, Baruffi MR, de Lima AS,
de Oliveira FM, de Figueiredo-Pontes LL, Tone LG, Rogatto SR, Falcao
RP, Ferrari Chauffaille Mde L, Rego EM. The co-expression of PML/RAR
alpha and AML1/ETO fusion genes is associated with ATRA resistance. Br
J Haematol 2005;128(3):407-409 http://dx.doi.org/10.1111/j.1365-2141.2004.05343.x
PMid:15667548

- Au WY, Fung A, Chim CS, Lie AK, Liang R,
Ma ES, Chan CH, Wong KF, Kwong YL. FLT-3 aberrations in acute
promyelocytic leukaemia: clinicopathological associations and
prognostic impact. Br J Haematol 2004;125(4):463-469 http://dx.doi.org/10.1111/j.1365-2141.2004.04935.x
PMid:15142116

- de la Serna J, Montesinos P, Vellenga E,
Rayon C, Parody R, Leon A, Esteve J, Bergua JM, Milone G, Deben G,
Rivas C, Gonzalez M, Tormo M, Diaz-Mediavilla J, Gonzalez JD, Negri S,
Amutio E, Brunet S, Lowenberg B, and Sanz MA. Causes and prognostic
factors of remission induction failure in patients with acute
promyelocytic leukemia treated with all-trans retinoic acid and
idarubicin. Blood 2008;111(7):3395- 3402 http://dx.doi.org/10.1182/blood-2007-07-100669
PMid:18195095

- Huang ME, Ye YC, Chen SR, Chai JR, Lu JX,
Zhoa L, Gu LJ, and Wang ZY. Use of all-trans retinoic acid in the
treatment of acute promyelocytic leukemia. Blood 1988;72:567-572
PMid:3165295

- Castaigne S, Chomienne C, Daniel MT,
Ballerini P, Berger R, Fenaux P, and Degos L. All-Trans Retinoic Acid
as a Differentiation Therapy for Acute Promyelocytic Leukemia. I.
Clinical Results. Blood 1990;76(9):1704-1709 PMid:2224119

- Raymond P. Warrell Jr, Peter Maslak, Anna
Eardliy, Glenn Heller, Wilson H. Miller Jr, and Stanley R Frankel.
Treatment of acute promyelocytic leukemia with all-trans retinoic acid:
an update of the New York experience. Leukemia 1994;8(6):929-933
PMid:8207986

- Peter H. Wiernik, Phillip L.C. Banks,
Delvyn C. Case Jr, Zalmen A. Arlin, Phillip O. Periman, Mary B. Todd,
Paul S. Ritch, Robert E. Enck, and Alan B. Weitberg Cytarabine Plus
Idarubicin or Daunorubicin as Induction and Consolidation Therapy for
Previously Untreated Adult Patients With Acute Myeloid Leukemia. Blood
1992;79(2):313-319 PMid:1730080

- Melnick A and Licht JD. Deconstructing a
Disease: RARa, Its Fusion Partners, and Their Roles in the Pathogenesis
of Acute Promyelocytic Leukemia. Blood 1999;93(10):3167-3215
PMid:10233871

- Sirulnik A, Melnick A, Zelent A, licht JD.
Molecular pathogenesis of acute promyelocytic leykaemia and APL
variants. Best Pract Res Clin Haematol. 2003;16(3):387-408 http://dx.doi.org/10.1016/S1521-6926(03)00062-8

- Breccia M, Diverio D, Noguera NI, Visani
G, Santoro A, Locatelli F, Damiani D, Marmont F, Vignetti M, Petti MC,
Lo Coco F. Clinico-biological features and outcome of acute
promyelocytic leukemia patients with persistent polymerase chain
reaction-detectable disease after AIDA front-line induction and
consolidation therapy. Haematologica 2004;89:29-33 PMid:14754603

- Lo Coco F, Diverio D, Avvisati G, Petti
MC, Meloni G, Pogliani EM, Biondi A, Rossi G, Carlo-Stella C, Selleri
C, Martino B, Specchia G, and Mandelli F. Therapy of Molecular Relapse
in Acute Promyelocytic Leukemia. Blood 1999;94:2225-2229 PMid:10498592

- Lengfelder E, Lo-Coco F, Montesinos P, Grimwade D, Ades L, Kishore B, Pagoni M, Holowiecka A, Fenaux P, Sanz M, on behalf of the European LeukemiaNet. Treatment of relapsed acute promyelocytic leukaemia (APL) with Arsenic Trioxide. First results of the European Registry of Patients with Relapsed APL. Abstract Blood (ASH Annual Meeting Abstracts) 2010;116: (Abstract#15) Oral Session
- Meloni G, Diverio D, Vignetti M, Avvisati
G, Capria S, Petti MC, Mandelli F, and Lo Coco F. Autologous Bone
Marrow Transplantation for Acute Promyelocytic Leukemia in Second
Remission: Prognostic Relevance of Pretransplant Minimal Residual
Disease Assessment by Reverse-Transcription Polymerase Chain Reaction
of the PML/RARa Fusion Gene. Blood 1997;90(3):1321-1325 PMid:9242568

- Lobe I, Rigal-Huguet F, Vekhoff A,
Desablens B, Bordessoule D, Mounier C, Ferrant A, Sanz M, Fey M,
Chomienne C, Chevret S, Degos L and Fenaux P for the European APL
group. Myelodysplastic syndrome after acute promyelocytic leukemia: the
European APL group experience. Leukemia 2003;17(8):1600-1604http://dx.doi.org/10.1038/sj.leu.2403034
PMid:12886249

- Larson RS, Brown DC, Sklar LA. Retinoic
acid induces aggregation of the acute promyelocytic leukemia cell line
NB-4 by utilization of LFA-1 and ICAM-2. Blood 1997;90(7):2747
PMid:9326242

- Ito S, Ishida Y, Oyake T, Satoh M, Aoki Y,
Kowata S, Uchiyama T, Enomoto S, Sugawara T, Numaoka H, Suzuki K, Murai
K. Clinical and biological significance of CD56 antigen expression in
acute promyelocytic leukaemia. Leuk Lymphoma 2004;45(9):1783-1789 http://dx.doi.org/10.1080/10428190410001683624

- Stavropoulou C, Georgakakos VN, Manola KN,
Pagoni M, Garofalaki M, Pantelias GE, Sambani C: 5'RARA submicroscopic
deletion from new variant translocation involving chromosomes 15, 17,
and 18, in a case of acute promyelocytic leukemia. Cancer Genet
Cytogenet 2008;182:50-5. http://dx.doi.org/10.1016/j.cancergencyto.2007.12.011
PMid:18328952

- Stock W, Najib K, Moser BK, Powell BL, Holowka N, Gulati K, Bloomfield CD, Larson RA, Sher D. High incidence of FLT3 mutations in adults with acute promyelocytic leukaemia (APL): Correlation with diagnostic features and treatment outcome (CALGB9710) abstract#7002, J Clin Oncol 2008;26:suppl abstr 7002
- Singh H, Werner L, Deangelo D, Ballen K,
Amrein P, Wadleigh M, Neuberg D, Fox E, Stone R, Attar E. Clinical
outcome of patients with acute promyelocytic leukemia and FLT3
mutations. Am J Hematol 2010;85(12):956-957 http://dx.doi.org/10.1002/ajh.21867
PMid:20981678

- Joseph G. Jurcic, Stephen D. Nimer, David
A. Scheinberg, Tony DeBlasio, Raymond P. Warrell, Jr, and Wilson H.
Miller, Jr. Prognostic significance of minimal residual disease
detection and PML/RAR-a isoform type: long-term follow-up in acute
promyelocytic leukemia. Blood 2001;98:2651-2656 PMid:11675334

- Grimwade D, Lo Coco F. Acute
promyelocytic leukemia: a model for the role of molecular diagnosis and
residual disease monitoring in directing treatment approach in acute
myeloid leukemia. Leukemia 2002;16(10):1959-1973http://dx.doi.org/10.1038/sj.leu.2402721
PMid:12357347

- Reiter A, Lengfelder E, Grimwade D.
Pathogenesis, diagnosis and monitoring of residual disease in acute
promyelocytic leukemia. Acta Haematol 2004;112(1-2):55-67 http://dx.doi.org/10.1159/000077560
PMid:15179005
