Review Articles
Francesco Albano and Giorgina Specchia
Published: December 21, 2011
Received: October 30, 2011
Accepted: November 27, 2011
Mediterr J Hematol Infect Dis 2011, 3(1): e2011066, DOI 10.4084/MJHID.2011.066
This article is available from: http://www.mjhid.org/article/view/9480
Abstract
Acute leukemia may present in a variety of extramedullary tissues with or without bone marrow disease. Extramedullary involvement by acute leukemia is a relatively rare but clinically significant phenomenon that often poses diagnostic and therapeutic dilemmas. Myeloid sarcoma and leukemia cutis are two well-known EM manifestations. Extramedullary disease (EMD) in acute promyelocytic leukemia (APL) is particularly rare and shows special clinical and biological features.
How common is EMD in APL?
The combination of all-trans retinoic acid (ATRA) and anthracycline-based chemotherapy, together with maintenance treatment, has improved the outcome of APL. In fact, approximately 90% of patients with newly diagnosed APL achieve complete remission (CR).[1-2] and it is estimated that 70–80% of these patients will remain in remission.[1-3] However, approximately 20– 30% of patients will eventually relapse.[2] EMD is a rare complication in APL: it is estimated that about 3–5% of patients will suffer extramedullary relapse.[4-6] However, since the introduction of ATRA in the treatment of patients with APL, EMD disease has been increasingly reported; in fact, in the literature fewer than 25 well-documented cases had been described before 1995.[7] This is most likely in part due to the following reasons:
a) APL
patients may develop EMD more
frequently because they are achieving longer survival times thanks to
improved
treatment regimens.
b) It is
possible that the drugs
employed in the induction regimens (ATRA, anthracycline and arsenic
trioxide)
do not reach therapeutic concentrations at the anatomical sites where
EMD
eventually develops.
c) It is
also possible that ATRA
therapy might contribute to extramedullary relapses by modulating and
upregulating the expression of adhesion molecules on leukemic cells.
EMD commonly occurs within 1 year of achieving
CR, but it can appear at any time during the disease course and can be
isolated
or can precede systemic relapse.[2-6] As to cases of
EMD at APL
presentation, although a few anecdotal reports have been made this
observation
is very uncommon.[8]
What are the most frequent anatomical sites of EMD in APL?
The most
frequent site of EMD in APL patients is the central nervous
system (CNS) and at least 10% of hematologic relapses are accompanied
by CNS
involvement.[9] CNS relapse appears in around 1% of
APL patients and
may occur despite hematological remission.[6,10-12] The skin is the
second most common site of EMD.[2] The increased
frequency of EMD
especially in these two sites could be explained by some biological
effects of
ATRA induction treatment. In fact, ATRA-driven differentiation of APL
cells is
associated with a significant upregulation of cellular adhesion
molecules
expressed on the cell surface, like LFA-1 and VLA-4.[13]
The mechanism
of APL blasts adhesion to the endothelium may be further increased by
interleukin-1, via an effect which may be mediated through an increased
expression of ICAM-1 and VCAM-1 on the endothelial cell surface.[14] These
surface proteins have both been demonstrated on the CNS endothelium and
have
been implicated in the migration processes of leukocytes across the
blood–brain
barrier (BBB), through interactions with LFA-1 and VLA-4, respectively.[9]
Since both LFA-1 and VLA-4 are upregulated in APL blasts treated with
retinoids, it is reasonable to suppose that the upregulation of these
adhesion
molecules may promote passage across the BBB of ATRA-treated APL cells,
thereby
creating the conditions for a subsequent CNS relapse (Figure 1).
Moreover, ATRA also stimulates keratinocytes to
proliferate and upregulate their expression of ICAMs.[9]
It has been
suggested that the migration of leukemic cells into the skin and other
tissues
during ATRA induction treatment may leave a reservoir of viable
leukemic cells
in these sites, that eventually may proliferate and cause EMD. These
biological
events could account for the clinical observation of a preferential
skin
localization of APL cells relapsing after ATRA treatment. Moreover, a
high
frequency of EMD in APL may also be related to the ATRA-induced
upregulation of
G-CSF receptors in APL cells, making them more sensitive to endogenous
or
exogenous G-CSF effects.[15] Other described sites of
EMD in APL
include: the testes, sites of vascular access, external ear and
auditory canal,
lung, pleura, heart, lymph nodes, mediastinum, thymus, spine, breast,
pelvis,
mandible and gingiva, bowel. Since in patients affected by ATRA
syndrome APL
cells infiltrate multiple tissues and organs, it has been hypothesized
that
ATRA could promote the migration of differentiating blasts into several
tissues,
constituting a reservoir of viable leukemic cells. These cells could
later
proliferate and result in an extramedullary recurrence.[16-17]
However,
the issue as to whether ATRA promotes EMD in APL is still highly
controversial,
since several studies have reached different conclusions.[5-7,18-19]
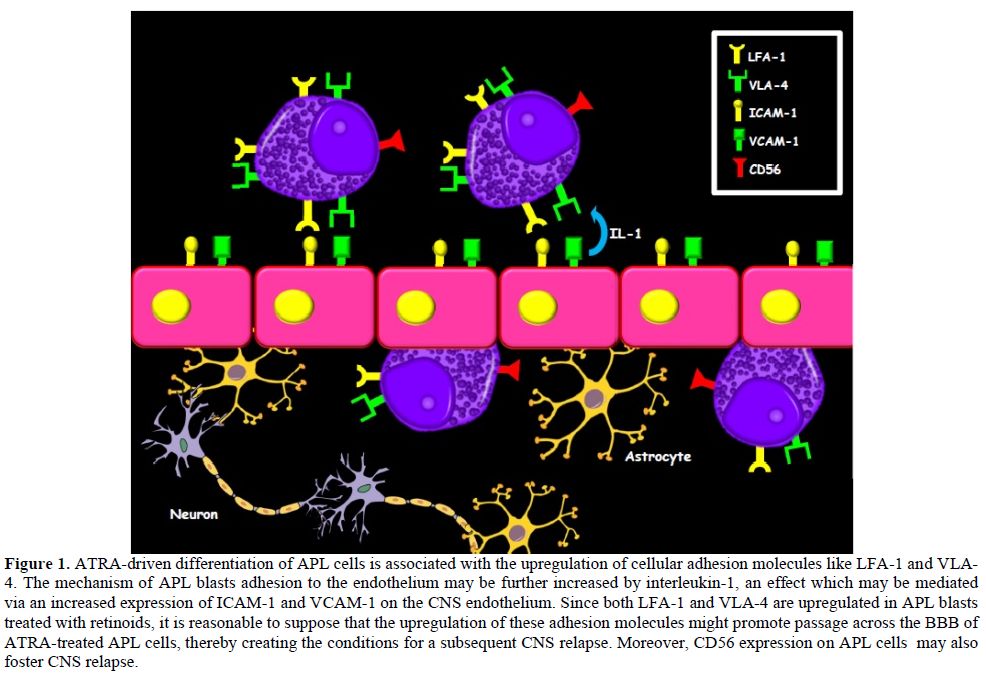
Figure 1. ATRA-driven differentiation of APL cells is associated with the upregulation of cellular adhesion molecules like LFA-1 and VLA-4. The mechanism of APL blasts adhesion to the endothelium may be further increased by interleukin-1, an effect which may be mediated via an increased expression of ICAM-1 and VCAM-1 on the CNS endothelium. Since both LFA-1 and VLA-4 are upregulated in APL blasts treated with retinoids, it is reasonable to suppose that the upregulation of these adhesion molecules might promote passage across the BBB of ATRA-treated APL cells, thereby creating the conditions for a subsequent CNS relapse. Moreover, CD56 expression on APL cells may also foster CNS relapse.
Are
there risk factors for an EMD onset in APL?
Several
factors have been associated with a higher risk of extramedullary
relapse such as younger age (<45 years), a high WBC count at
diagnosis,
microgranular morphology, expression of CD2 and/or CD56, PML-RARa bcr3
isoform expression, ATRA syndrome, monotherapy regimens, and the
use of therapy schedules that exclude
cytarabine.[24,12,20,21]
Moreover, two recent studies [4,22] reported
a significantly higher incidence of CNS involvement in patients with an
initial
WBC of more than 10 x109/L. In addition to
hyperleukocytosis, the
PETHEMA study also identified a previous CNS hemorrhage during
induction as an
independent risk factor for CNS relapse.[22] It has
recently been
demonstrated that CD56+ APL has a greater risk of extramedullary
relapse.[21]
The higher frequency of coexpression of stem cell (CD117) and NK-cell
antigens
(CD2, CD7) in CD56+ APL cells suggests that in some of these cases APL
might
have arisen in progenitors that did not undergo lineage restriction.[23]
Therefore, it is possible that CD56+ APL may emerge from a more
immature,
undifferentiated and pluripotent leukemic stem cell that is less
sensitive to
the combination of ATRA and anthracyclines. This could explain the
higher
frequency of extramedullary relapse in these cases.[21,24-25]
Which is the best therapy? Because of
the rarity of the disease, the prognosis
of patients with EMD in APL is still unclear. The
GIMEMA study[6] reported that the
outcome was similar to
that of patients who experienced isolated bone marrow relapse, whereas
in the
joint study by the PETHEMA and the European APL groups [4]it
was
found that patients with an
extramedullary relapse had a poorer outcome. EMD can occur in isolation
or
associated with bone marrow involvement as a first relapse, but also
after one
or more hematologic relapses. The molecular status in the peripheral
blood/bone
marrow did not seem to predict the possibility of EMD relapse.[5,6] Management
of relapse in the CNS and other extramedullary sites in APL patients is
a
challenging issue on which there is a strong need for further data. The optimal management of APL
patients in different
situations has not been critically assessed.[26]
Because the majority
of CNS relapses occurs in APL patients with hyperleukocytosis,4
CNS
prophylaxis for patients in this particular high-risk setting may be
appropriate.[26] In these cases CNS prophylaxis
should be performed
after the achievement of CR because lumbar puncture at presentation and
during
induction is extremely hazardous. However, the benefit of this kind of
strategy
has not yet been clearly established.
The role of
ATRA and arsenic
trioxide in the therapeutic management of CNS relapse is still unclear
because
it is not known whether these drugs cross the BBB; nevertheless, some
authors
have reported responses to these agents in patients with meningeal
disease.[27-28]
This may be due to the EMD disrupting the BBB. Arsenic trioxide has
also been
reported to cross the BBB and may be useful as a therapeutic agent to
control
CNS relapse.[29] On the other hand, some reports have
confirmed that
although arsenic crosses the BBB when administered intravenously, the
concentration in CSF is probably not sufficient to treat meningeal
leukemia.[30-31]
Recently, as induction treatment of CNS relapse, the European
LeukemiaNet
recommendations [26] proposed a schedule of weekly
triple intrathecal
therapy (ITT) with methotrexate, hydrocortisone, and cytarabine until
complete
clearance of blasts in the cerebrospinal fluid (CSF), followed by 6 to
10 more
spaced-out ITT treatments as consolidation. In these cases systemic
treatment
should also be given because CNS disease is almost invariably
associated with
hematologic or molecular relapse in the marrow. Chemotherapy regimens
with high
CNS penetrance, such as high-dose cytarabine, have been used in this
situation.
In patients responding to treatment, allogeneic or autologous
transplant is
then recommended as consolidation treatment, together with craniospinal
irradiation.
It was demonstrated that cytarabine during consolidation treatment
significantly reduced the relapse rate in high-risk APL patients.[32-33]Because of the limited numbers of EMD events
reported in these studies, it is
very hard to draw firm conclusions regarding the best schedule of
cytarabine to
use in the consolidation regimen to prevent the EMD in APL. In cases of
promyelocytic sarcoma, wherever it is localized, radiation and
intensive
systemic therapy might be considered. Recently, successful treatment of
relapsed and refractory EMD with Tamibarotene,[34] a
synthetic
retinoid approved in Japan for use in relapsed/refractory APL, has been
reported.[35] Tamibarotene is 10 times more potent
than ATRA as an
inducer of HL-60 and NB-4 leukemia cell lines differentiation. While
tamibarotene has displayed a significant activity in bone
marrow-relapsed APL,
its efficacy in EMD needs to be confirmed in further studies.
Conclusions.
At present, there are still many open issues on EMD in APL patients. However, some aspects are becoming clearer. An improved understanding of the biological mechanisms that underlie EMD should allow us to devise more effective prophylaxis and induction therapeutic strategies against this severe clinical presentation.
References
- Wang ZY, Chen Z. Acute promyelocytic
leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–15 http://dx.doi.org/10.1182/blood-2007-07-102798
PMid:18299451

- Tallman MS. Treatment of relapsed or
refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol.
2007;20:57–65 http://dx.doi.org/10.1016/j.beha.2006.11.002

- Lengfelder E, Saussele S, Weisser A,
Buchner T, Hehlmann R. Treatment concepts of acute promyelocytic
leukemia. Crit Rev Oncol Hematol. 2005;56:261–74 http://dx.doi.org/10.1016/j.critrevonc.2004.08.009
PMid:16236522

- de Botton S, Sanz MA, Chevret S, Dombret H,
Martin G, Thomas X, Mediavilla JD, Recher C, Ades L, Quesnel B, Brault
P, Fey M, Wandt H, Machover D, Guerci A, Maloisel F, Stoppa AM, Rayon
C, Ribera JM, Chomienne C, Degos L, Fenaux P; European APL Group;
PETHEMA Group. Extramedullary relapse in acute promyelocytic leukemia
treated with all-trans retinoic acid and chemotherapy. Leukemia.
2006;20:35–41 http://dx.doi.org/10.1038/sj.leu.2404006
PMid:16307026

- Vega-Ruiz A, Faderl S, Estrov Z, Pierce S,
Cortes J, Kantarjian H, Ravandi F. Incidence of extramedullary disease
in patients with acute promyelocytic leukemia: a single-institution
experience. Int J Hematol. 2009;89:489-496http://dx.doi.org/10.1007/s12185-009-0291-8
PMid:19340529

- Specchia G, Lo Coco F, Vignetti M, Avvisati
G, Fazi P, Albano F, Di Raimondo F, Martino B, Ferrara F, Selleri C,
Liso V, Mandelli F. Extramedullary involvement at relapse in acute
Incidence of extramedullary disease in acute promyelocytic leukemia
patients promyelocytic leucemia patients treated or not with all-trans
retinoic acid: a report by the Gruppo Italiano Malattie Ematologiche
dell’Adulto. J Clin Oncol. 2001;19:4023–8 PMid:11600603

- Wiernik PH, De Bellis R, Muxi P, Dutcher JP. Extramedullary acute promyelocytic leukemia. Cancer. 1996 78:2510-2514 http://dx.doi.org/10.1002/(SICI)1097-0142(19961215)78:12<2510::AID-CNCR10>3.0.CO;2-Z
- Worch J, Ritter J, Fruhwald MC.
Presentation of acute promyelocytic leukemia as granulocytic sarcoma.
Pediatr Blood Cancer. 2008;50:657–60 http://dx.doi.org/10.1002/pbc.21190

- Evans GD, Grimwade DJ. Extramedullary
disease in acute promyelocytic leukemia. Leuk Lymphoma. 1999;33:219-229
PMid:10221502

- Liso V, Specchia G, Pogliani EM, Palumbo
G, Mininni D, Rossi V, Teruzzi E, Mestice A, Coppi MR, Biondi A.
Extramedullary involvement in patients with acute promyelocytic
leukemia: a report of seven cases. Cancer. 1998; 83: 1522–1528 http://dx.doi.org/10.1002/(SICI)1097-0142(19981015)83:8<1522::AID-CNCR6>3.0.CO;2-4

- Burry LD, Seki JT. CNS relapses of acute
promyelocytic leukemia after all-trans retinoic acid. Ann Pharmacother
2002; 36: 1900–1906http://dx.doi.org/10.1345/aph.1A471
PMid:12452754

- Breccia M, Carmosino I, Diverio D, De
Santis S, De Propris MS, Romano A, Petti MC, Mandelli F, Lo-Coco F.
Early detection of meningeal localization in acute promyelocytic
leukaemia patients with high presenting leucocyte count. Br J Haematol
2003; 120: 266–270 http://dx.doi.org/10.1046/j.1365-2141.2003.04056.x
PMid:12542484

- Di Noto R, Lo Prado C, Schiavone EM,
Ferrara F, Manzo C, Vacca C, Del Vecchio L. All-trans retinoic acid
(ATRA) and the regulation of adhesion molecules in acute myeloid
leukemia. Leuk Lymphoma 1996; 21: 201–209 http://dx.doi.org/10.3109/10428199209067601
PMid:8726400

- Marchetti M, Falanga A, Giovanelli S,
Oldani E, Barbui T. All-trans-retinoic acid increases adhesion to
endothelium of the human promyelocytic leukaemia cell line NB4. Br J
Haematol 1996; 93: 360–366. http://dx.doi.org/10.1046/j.1365-2141.1996.4911029.x
PMid:8639429

- de Gentile A, Toubert ME, Dubois C,
Krawice I, Schlageter MH, Balitrand N, Castaigne S, Degos L, Rain JD,
Najean Y. Induction of high-affinity GM-CSF receptors during all-trans
retinoic acid treatment of acute promyelocytic leukemia. Leukemia 1994;
8: 1758–1762 PMid:7934172

- Raanani P, Shpillberg O, Ben-Bassat I.
Extramedullary disease and targeted therapies for hematological
malignancies—is the association real? Ann Oncol. 2007;18:7–12 http://dx.doi.org/10.1093/annonc/mdl129
PMid:16790518

- Ko BS, Tang GL, Chen YC, Yao M, Wang CH,
Shen MC, Tien HF. Extramedullary relapse after all-trans retinoic acid
treatment in acute promyelocytic leukemia. The occurrence of retinoic
acid syndrome is a risk factor. Leukemia 1999; 13: 1406–1408 http://dx.doi.org/10.1038/sj.leu.2401495
PMid:10482992

- Tsimberidou AM, Estey E, Whitman GJ,
Dryden MJ, Ratnam S, Pierce S, Faderl S, Giles F, Kantarjian HM,
Garcia-Manero G. Extramedullary relapse in a patient with acute
promyelocytic leukemia: successful treatment with arsenic trioxide,
all-trans retinoic acid and gemtuzumab ozogamicin therapies. Leuk Res
2004;28: 991–994 http://dx.doi.org/10.1016/j.leukres.2004.01.004
PMid:15234578

- Ohno R, Asou N, Ohnishi K. Treatment of
acute promyelocytic leukemia: strategy toward further increase of cure
rate. Leukemia 2003; 17: 1454–1463 http://dx.doi.org/10.1038/sj.leu.2403031
PMid:12886231

- Ades L, Sanz Miguel A, Chevret S,
Montesinos P, Chevallier P, Raffoux E, Vellenga E, Guerci A, Pigneux A,
Huguet F, Rayon C, Stoppa AM, de la Serna J, Cahn JY, Meyer-Monard S,
Pabst T, Thomas X, de Botton S, Parody R, Bergua J, Lamy T, Vekhoff A,
Negri S, Ifrah N, Dombret H, Ferrant A, Bron D, Degos L, Fenaux P.
Treatment of newly diagnosed acute promyelocytic leukemia (APL): a
comparison of French–Belgian–Swiss and Pethema results. Blood.
2008;111:1078–84 http://dx.doi.org/10.1182/blood-2007-07-099978
PMid:17975017

- Montesinos P, Rayón C, Vellenga E, Brunet
S, González J, González M, Holowiecka A, Esteve J, Bergua J, González
JD, Rivas C, Tormo M, Rubio V, Bueno J, Manso F, Milone G, de la Serna
J, Pérez I, Pérez-Encinas M, Krsnik I, Ribera JM, Escoda L, Lowenberg
B, Sanz MA; PETHEMA; HOVON Groups. Clinical significance of CD56
expression in patients with acute promyelocytic leukemia treated with
all-trans retinoic acid and anthracycline-based regimens. Blood. 2011;
117:1799-805 http://dx.doi.org/10.1182/blood-2010-04-277434
PMid:21148082

- Montesinos P, Díaz-Mediavilla J, Debèn G,
Prates V, Tormo M, Rubio V, Pérez I, Fernández I, Viguria M, Rayón C,
González J, de la Serna J, Esteve J, Bergua JM, Rivas C, González M,
González JD, Negri S, Brunet S, Lowenberg B, Sanz MA. Central nervous
system involvement at first relapse in patients with acute
promyelocytic leukemia treated with all-trans retinoic acid and
anthracycline monochemotherapy without intrathecal prophylaxis.
Haematologica. 2009; 94:1242-1249, http://dx.doi.org/10.3324/haematol.2009.007872
PMid:19608685 PMCid:2738716

- Albano F, Mestice A, Pannunzio A, Lanza F,
Martino B, Pastore D, Ferrara F, Carluccio P, Nobile F, Castoldi G,
Liso V, Specchia G. The biological characteristics of CD34+ CD2+ adult
acute promyelocytic leukemia and the CD34 CD2 hypergranular (M3) and
microgranular (M3v) phenotypes. Haematologica. 2006; 91:311-316.
PMid:16531253

- Ferrara F, Morabito F, Martino B, Specchia
G, Liso V, Nobile F, Boccuni P, Di Noto R, Pane F, Annunziata M,
Schiavone EM, De Simone M, Guglielmi C, Del Vecchio L, Lo Coco F. CD56
expression is an indicator of poor clinical outcome in patients with
acute promyelocytic leukemia treated with simultaneous
all-trans-retinoic acid and chemotherapy. J Clin Oncol.
2000;18:1295-1300 PMid:10715300

- Ito S, Ishida Y,Oyake T,Satoh M, Aoki Y,
Kowata S, Uchiyama T, Enomoto S, Sugawara T, Numaoka H, Suzuki K, Murai
K. Clinical and biological significance of CD56 antigen expression in
acute promyelocytic leukemia. Leuk Lymphoma. 2004; 45:1783-1789. http://dx.doi.org/10.1080/10428190410001683624

- Sanz MA, Grimwade D, Tallman MS, Lowenberg
B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Büchner T, Döhner H,
Burnett AK, Lo Coco F. Management of acute promyelocytic leukemia:
recommendations from an expert panel on behalf of the European
LeukemiaNet. Blood.2009; 26;113:1875-91. http://dx.doi.org/10.1182/blood-2008-04-150250
PMid:18812465

- Patriarca F, Fili C, Antonella G, Sperotto
A, Prosdocimo S, Fanin R. Activity of all-trans-retinoic acid in a case
of central nervous system extramedullary relapse of acute promyelocytic
leukemia. Eur J Haematol. 2002; 68:310–3http://dx.doi.org/10.1034/j.1600-0609.2002.01660.x
PMid:12144538

- Burry LD, Seki JT. CNS relapses of acute
promyelocytic leukemia after all-trans retinoic acid. Ann Pharmacother.
2002;36:1900–6 http://dx.doi.org/10.1345/aph.1A471
PMid:12452754

- Kiguchi T, Yoshino Y, Yuan B, Yoshizawa S,
Kitahara T, Akahane D, Gotoh M, Kaise T, Toyoda H, Ohyashiki K.
Speciation of arsenic trioxide penetrates into cerebrospinal fluid in
patients with acute promyelocytic leukemia. Leuk Res. 2010; 34:403-5 http://dx.doi.org/10.1016/j.leukres.2009.08.001
PMid:19733394

- Knipp S, Gatterman N, Schapira M,
Käferstein H, Germing U. Arsenic in the cerebrospinal fluid of a
patient receiving arsenic trioxide for relapsed acute promyelocytic
leukemia with CNS involvement. Leuk Res. 2007;31:1585–7http://dx.doi.org/10.1016/j.leukres.2007.03.007
PMid:17416415

- Helwig A, Klemm M, Schuttig R, Röllig C,
Wassilew N, Ehninger G, Illmer T. Arsenic-induced APL differentiation
in cerebrospinal fluid. Leuk Res. 2007; 31:703–5 http://dx.doi.org/10.1016/j.leukres.2006.06.011
PMid:16876245

- Lo-Coco F, Avvisati G, Vignetti M, Breccia
M, Gallo E, Rambaldi A, Paoloni F, Fioritoni G, Ferrara F, Specchia G,
Cimino G, Diverio D, Borlenghi E, Martinelli G, Di Raimondo F, Di Bona
E, Fazi P, Peta A, Bosi A, Carella AM, Fabbiano F, Pogliani EM, Petti
MC, Amadori S, Mandelli F; Italian GIMEMA Cooperative Group. Front-line
treatment of acute promyelocytic leukemia with AIDA induction followed
by risk-adapted consolidation for adults younger than 61 years: results
of the AIDA-2000 trial of the GIMEMA Group. Blood 2010; 116:3171-9 http://dx.doi.org/10.1182/blood-2010-03-276196
PMid:20644121

- Sanz MA, Montesinos P, Rayón C, Holowiecka
A, de la Serna J, Milone G, de Lisa E, Brunet S, Rubio V, Ribera JM,
Rivas C, Krsnik I, Bergua J, González J, Díaz-Mediavilla J, Rojas R,
Manso F, Ossenkoppele G, González JD, Lowenberg B; PETHEMA and HOVON
Groups. Risk-adapted treatment of acute promyelocytic leukemia based on
all-trans retinoic acid and anthracycline with addition of cytarabine
in consolidation therapy for high-risk patients: further improvements
in treatment outcome. Blood. 2010;115:5137-46 http://dx.doi.org/10.1182/blood-2010-01-266007
PMid:20393132

- Naina HV, Levitt D, Vusirikala M, Anderson
LD Jr, Scaglioni PP, Kirk A, Collins RH Jr. Successful treatment of
relapsed and refractory extramedullary acute promyelocytic leukemia
with tamibarotene. J Clin Oncol. 2011; 29:e534-6 http://dx.doi.org/10.1200/JCO.2011.34.8953
PMid:21482998

- Tobita T, Takeshita A, Kitamura K, Ohnishi
K, Yanagi M, Hiraoka A, Karasuno T, Takeuchi M, Miyawaki S, Ueda R,
Naoe T, Ohno R. Treatment with a new synthetic retinoid, Am80, of acute
promyelocytic leukemia relapsed from complete remission induced by
all-trans retinoic acid. Blood. 1997; 90:967-973 PMid:9242525
