Review Articles
Caterina Giovanna Valentini*, Luana Fianchi, Maria Teresa Voso, Morena Caira, Giuseppe Leone and Livio Pagano
Hematology Institute,
Catholic University, Rome, Italy
Correspondence
to:
Caterina Giovanna Valentini, MD. Istituto di Ematologia, UniversitÓ
Cattolica del Sacro Cuore, Largo Francesco Vito 1, 00168 Rome, Italy.
Tel : +39-0630154180, Fax +39-063051343. E-mail: : giovannavalentini@libero.it
Published: December 22, 2011
Received: December 7, 2011
Accepted: December 17, 2011
Medit J Hemat Infect Dis 2011, 3(1): e2011069, DOI 10.4084/MJHID.2011.069
This article is available from: http://www.mjhid.org/article/view/9724
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Breast
cancer is the most frequent cancer among women and the leading cause of
death among middle-aged women. Early detection by mammography screening
and improvement of therapeutic options have increased breast cancer
survival rates, with the consequence that late side effects of cancer
treatment become increasingly important. In particular, patients
treated with adjuvant chemotherapy regimens, commonly including
alkylating agents and anthracyclines, are at increased risk of
developing leukemia, further enhanced by the use of radiotherapy. In
the last few years also the use of growth factors seems to increase the
risk of secondary leukemia. The purpose of this review is to update
epidemiology of therapy-related myeloid neoplasms occurring in breast
cancer patients.
Epidemiology and Mortality Of Breast Cancer
Excluding skin cancers, breast cancer (BC) is the most common malignancy among women in developed country, accounting for about one-third of all new cancer cases in the United States (n= 230480; 30%), and it is the second leading cause of cancer death among women. Despite the high incidence, the mortality rate is low (15%), and, as a result of early diagnosis and the increasing use of adjuvant therapy, there is a rising number of long-term survivors.[1,2] Several studies have reported an increased incidence of acute myeloid leukemia (AML) after treatment of BC, with evidence of a dose-intensity relationship. It is estimated that 1 every 20 patients will develop a secondary non-breast cancer after 10 years, which corresponds to a 22% increase of relative risk, particularly for secondary AML and myelodisplastic syndromes (MDS).[3-5]
Differences Between Therapy-Related and Secondary Acute Myeloid Leukemia
Therapy-related acute myeloid leukemia or myelodisplastic syndromes (t-AML/MDS) are collectively known as therapy-related myeloid neoplasms (t-MN), included among “Acute myeloid leukemias and related precursor neoplasms” in the 2008 WHO classification.[6] The term “therapy-related” leukemia is descriptive and based on patient’s history of exposure to cytotoxic agents. The latency between primary diagnosis and therapy-related disease ranges from few months to several years, with a median of about two years, depending in part on the cumulative dose and/or the dose-intensity of the preceeding cytotoxic therapy, as well on the exposure to specific agents.[7-9] Besides "therapy-related" forms, there are AML/MDS defined as “second malignancy” arising as a second cancer after a previous diagnosis of a neoplasm treated with surgery alone.
Currently accounting for 10-20% of all cases of AML,10 the outcome of patients with t-AML compared with that of de novo AML, has been historically poor, with a higher frequency of poor-risk cytogenetics and shorter survival times.[11-13] Patients are often poor candidates for intensive AML therapy because of protracted damage from prior cytotoxic therapy and, in some cases, for the persistence of their primary disorder. Moreover, t-AML is relatively resistant to conventional therapies used for de novo leukemias.
Pathogenesis of t-MN After Breast Cancer
Chemotherapy with DNA-targeted antiproliferative drugs in the adjuvant setting has contributed to significant progress in the management of BC, substantially increasing the number of long-term survivors. As the risk of developing cancer increases with age, longer survival is associated with an increased probability of new cancer occurrence, particularly of developing t-AML/MDS. In the majority of cases they are represented by AML, but a secondary acute lymphoblastic leukemia is possible, although less common.[14,15] In the last decades the type of solid tumors preceding t-MN has changed: among 3026 newly diagnosed AML, there were 142 of 200 t-MN with a previous history of solid cancer, with BC representing the most common neoplasm (52%). The median latency between diagnosis of primary malignancy and the occurrence of t-AML was four years, and was shorter in patients younger at the time of primary malignancy diagnosis or treated with anthracyclines and/or topoisomerase-II inhibitors.[10]
Several studies have reported an increased risk for AML in BC patients treated with adjuvant therapy (Table 1 and Table 2), but it remains unclear if t-AML represents a truly stochastic event or if individual susceptibility plays a role.[16] Already 40 years ago Metcalf et al demonstrated a correlation between acute leukemia and BC and hypothesized common risk factors for both diseases.[17] This observation has been confirmed in various series of patients showing that there is an increased risk of developing AML in patients with BC treated with surgery alone, or with family history of BC, so that individual susceptibility for development of multiple tumors and a possible association between the two diseases must be hypothesized.[18-20] It is currently difficult to define individual susceptibility, because only few pathological conditions, above all constitutional and genetically determined, are known to predispose to leukemia. The interaction between the genotoxic effects of chemotherapy or ionizing radiation and the “host” is influenced, among others, by genetic polymorphism in drug metabolism and DNA repair processes, which may increase individual susceptibility to these agents. Furthermore, the observation of secondary leukemias in patients who did not receive chemio- or radiotherapy for their primary tumor suggests the existence of a common predisposing condition, possibly a general cancer susceptibility.
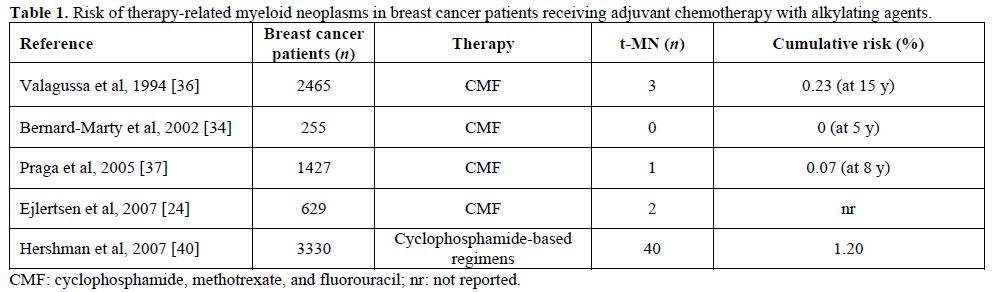
Table 1. Risk of therapy-related myeloid neoplasms in breast cancer patients receiving adjuvant chemotherapy with alkylating agents.
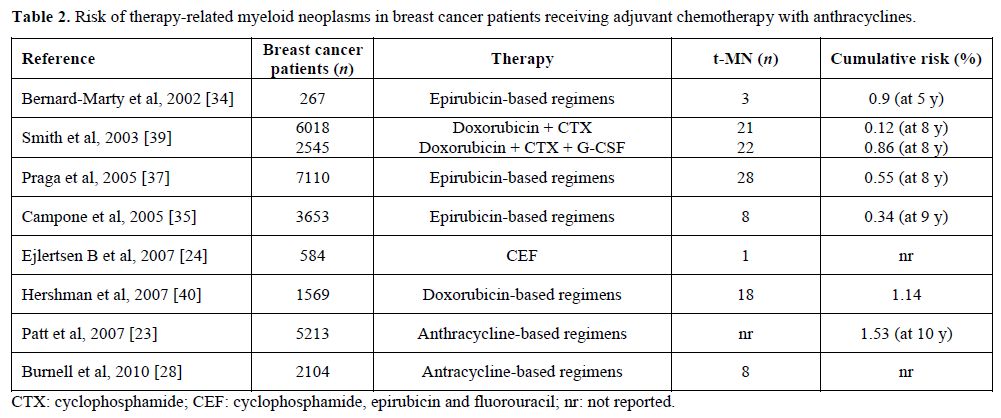
Table 2. Risk of therapy-related myeloid neoplasms in breast cancer patients receiving adjuvant chemotherapy with anthracyclines.
A population-based study from a French Cancer Registry evaluated the risk of developing a new primary invasive cancer during the first five years of follow up for 14353 cancer patients (breast, colorectal and prostate cancer), comparing with the expected numbers, based on primary cancer incidence rate using the standardized incidence ratio (SIR). Overall, 690 second cancers were registered, including 15 AML. In particular, among 5663 women treated for BC, 10 developed t-AML, which results into a greater risk than the general population (SIR=8.26, p<0.05).[21] AML risk after a prior BC was also examined in an Australian retrospective population-based study and this risk was compared to that of survivors after a prior diagnosis of hematological malignancies and other cancers combined. Among 183123 women diagnosed with BC, 158 (0.09%) subsequently developed AML, with the result that women with a prior diagnosis of BC were 2.6 times more likely to develop AML compared to the general female population (p<0.001). Although the incidence of AML rose sharply with age in all cohorts, the age-specific relative risk was highest in the 30-49 age groups and decreased with increasing age. An age-dependent risk of a subsequent diagnosis of AML was confirmed in women <50 years and in the range 50-64 years with previous BC, but not in those older than 65 years, if compared with the expected incidence of AML. A similar age-dependent pattern was observed for second BC and ovarian cancers; this association may be explained by either chemotherapy exposure or an interaction between therapy and genetic predisposition.[22] On the other hand, Patt et al evaluated the risk of AML in older women treated with modern schedules, demonstrating that while older women treated with adjuvant chemotherapy had more than 50% increased risk of AML, the absolute increase in risk at 10 years was low (1.8% in treated patients versus 1.2% among patients who did not received chemotherapy).[23]
Role of Adjuvant Chemotherapy
Adjuvant chemotherapy for BC has undergone major changes, expanding from node-positive women to lower risk patients. Anthracycline-containing regimens have shown superiority in comparison to cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Incorporation of taxanes (paclitaxel and docetaxel) into anthracyclines-based schedules yielded an additional benefit in both disease-free survival (DFS) and overall survival (OS) in most studies, and dose-dense drug administration have shown to be more effective than the conventional dosing schedule.[24-28] These novel therapeutic strategies have resulted into a considerable improvement of BC survival, but also into an increased t-AML/MDS rate.[29-35] Of note, it must be kept in mind that a under-reported incidence of overall t-MN in the different registries is likely, and difficult to accurately estimate, because of an inadequate coding, not specific for t-AML or t-MDS.
Alkylating agents. In the past, alkylating agents were the class of antineoplastic drugs unequivocally associated with t-MN (Table 1). The antineoplastic activity of these drugs is related to their ability to damage DNA by methylation or DNA inter-strand crosslinks formation, interfering with normal DNA replication. Alkylating agent-related AML typically develops after an average latency of 5-7 years, and overt leukemia is often (up to 70% of cases) proceeded by a dysplastic phase.[9,11] Fisher et al reported that the 10-year cumulative risk of AML was increased in patients treated with surgery followed by melphalan-based chemotherapy compared to those treated with surgery alone (1.29% versus 0.27%, respectively).[30] In the following years the leukemogenic potential of cyclophosphamide has emerged. Several studies indicated that the risk for developing AML/MDS among patients with early-stage BC treated with adjuvant chemotherapy containing standard dose cyclophosphamide is higher than that of the general population,[31,36] although the risk of developing t-MN in patients treated with melphalan is 10 times higher than that of patients who received cyclophosphamide.[32] In fact the risk for AML appears negligible in patients treated with CMF regimens, provided that cyclophosphamide is given at standard dose.
Anthracyclines. The latency period between exposure to anthracyclines and the onset of leukemia is usually about 2 years, and generally there is no previous myelodysplastic phase (Table 2). In order to assess the risk of developing AML and MDS after exposure to epirubicin-based regimen, Praga et al reviewed 7110 patients treated with epirubicin and cyclophosphamide in 19 randomized clinical trials in 2005. At a median follow up of eight years the cumulative probability of AML or MDS was 0.55%; however the risk increased in relation to the cumulative doses of both agents, ranging between 0.37% in patients received standard regimen and 4.97% for those treated with higher doses.[37] Similar results were obtained with doxorubicin-based regimen. In a large French case-control study, the risk of t-AML/MDS in women treated for BC was higher in those who received mitoxantrone-based chemotherapy than in those given anthracyclines.[38] Smith et al performed a combined analysis of six adjuvant studies conducted by the National Surgical Adjuvant Breast and Bowel Project group using regimens containing both doxorubicin and cyclophosphamide, and reported a 5-year incidence of AML ranging from 0.3% to 1.2%, with an increased risk for greater dose intensity.[39] The importance of dose intensity was also confirmed with the “intense dose-dense” regimen epirubicin, paclitaxel and cyclophosphamide every 2 weeks, which proved more effective than standard schedule epirubicin/cyclophosphamide and improved event-free and overall survivals, but was also more toxic with four cases (0.6% of patients) of t-AML/MDS.[26]
Antineoplastic activity of taxanes appears to be related to their ability to promote microtubular assembly and to inhibit microtubular disassembly. A SEER database analysis did not document an increased risk of secondary malignancies with these drugs.[23] A 7-year follow-up of a trial comparing doxorubicin/cyclophosphamide (AC) versus docetaxel/cyclophosphamide (TC) in early BC, reported no secondary leukemia in the TC arm, compared to two cases in 510 patients (0.4%) in the AC arm.[25]
Role of Granulocyte Colony-Stimulating Factors (G-CSF) and Radiotherapy
Recently, increasing numbers of women receiving adjuvant chemotherapy for BC have also received granulocyte stimulating factors to reduce the myelosuppressive effects of dose- intense chemotherapy. In vitro data suggest that G-CSF may increase the risk of AML/MDS, but its leukemogenic effect is still debated. An analysis of the SEER-medicare population-based database including 5510 women with BC treated with adjuvant chemotherapy, found that the addition of G-CSF is associated with a doubling of the risk of subsequent AML or MDS when compared with chemotherapy alone, even if the absolute risk is low.[40] In the analysis of six trials described by Smith et al and mentioned above, the incidence of therapy-related leukemia was sharply elevated in patients treated with intensified regimens that required G-CSF support (relative risk 6.16, p=0.0001).[39] On the other hand, Patt et al did not find an increased risk for AML in elderly (>65 years) BC patients, who received G-CSF during the first years after diagnosis as part of adjuvant therapy.[23] Similarly, in the Cancer and Leukemia Group B 9741 phase III trial, patients received dose-dense regimens plus filgrastim support, but had no increased risk of developing AML or MDS compared to those treated with the same regimen at conventional schedule without G-CSF.[27] Finally, a systematic review of 25 randomized clinical trials was recently conducted to evaluate the risk of AML or MDS in patients receiving chemotherapy for solid malignancies and lymphomas with or without the addition of G-CSF. At a median follow up of 54 months, the estimated relative risk for AML/MDS with G-CSF-supported chemotherapy was 1.92, with an estimate absolute increase in risk of 0.4%;41 however, although this increased risk, these data cannot distinguish between the potential causal effects as a result of the growth factor and of dose-intensified systemic chemotherapy, so that the potential toxicities of G-CSF needs further study.
Radiotherapy may also play a significant role (Table 3). A cohort study analyzing clinical records of BC patients with the aim of evaluating the long-term effect of radiotherapy on the risk of second cancers reported a total of 387 malignancies (7.3%) in 5248 women, with eight patients developing leukemia (0.15%), seven in the group treated with radiotherapy, versus one case only in the group not receiving radiotherapy. The relative risk adjusted for chemotherapy and hormone treatment was 6.67 (95% CI 0.76-58.00) and the median time from exposure was 4.5 years, with the suggestion of a raised incidence of leukemia within the first two or more years after radiotherapy.[42] Similarly, in a previous study, the risk of developing AML resulted four-fold increased with the use of radiotherapy (HR 4, 95% CI 1.4-11.8) and by seven folds when radiotherapy was combined to chemotherapy (HR 7.2, 95% CI 1.4-36.3).[33]
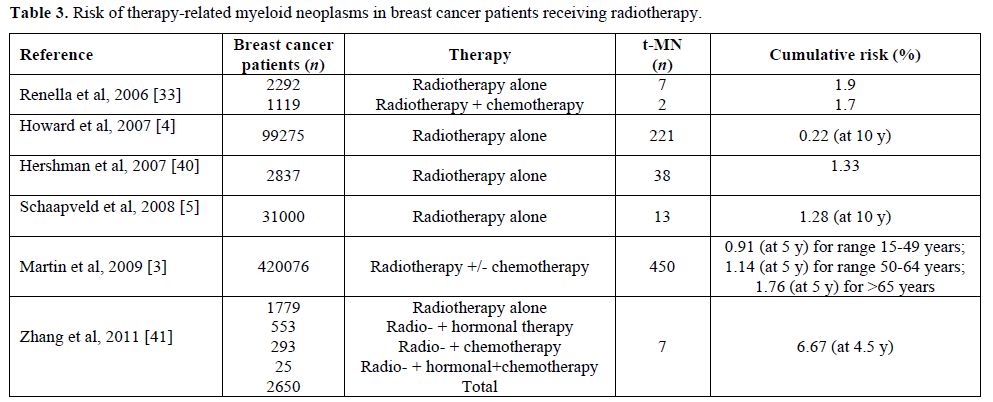
Table 3. Risk of therapy-related myeloid neoplasms in breast cancer patients receiving radiotherapy.
Summary and Final Remarks
Excluding skin cancers, breast cancer (BC) is the most common malignancy among women in developed country, accounting for about one-third of all new cancer cases in the United States (n= 230480; 30%), and it is the second leading cause of cancer death among women. Despite the high incidence, the mortality rate is low (15%), and, as a result of early diagnosis and the increasing use of adjuvant therapy, there is a rising number of long-term survivors.[1,2] Several studies have reported an increased incidence of acute myeloid leukemia (AML) after treatment of BC, with evidence of a dose-intensity relationship. It is estimated that 1 every 20 patients will develop a secondary non-breast cancer after 10 years, which corresponds to a 22% increase of relative risk, particularly for secondary AML and myelodisplastic syndromes (MDS).[3-5]
Differences Between Therapy-Related and Secondary Acute Myeloid Leukemia
Therapy-related acute myeloid leukemia or myelodisplastic syndromes (t-AML/MDS) are collectively known as therapy-related myeloid neoplasms (t-MN), included among “Acute myeloid leukemias and related precursor neoplasms” in the 2008 WHO classification.[6] The term “therapy-related” leukemia is descriptive and based on patient’s history of exposure to cytotoxic agents. The latency between primary diagnosis and therapy-related disease ranges from few months to several years, with a median of about two years, depending in part on the cumulative dose and/or the dose-intensity of the preceeding cytotoxic therapy, as well on the exposure to specific agents.[7-9] Besides "therapy-related" forms, there are AML/MDS defined as “second malignancy” arising as a second cancer after a previous diagnosis of a neoplasm treated with surgery alone.
Currently accounting for 10-20% of all cases of AML,10 the outcome of patients with t-AML compared with that of de novo AML, has been historically poor, with a higher frequency of poor-risk cytogenetics and shorter survival times.[11-13] Patients are often poor candidates for intensive AML therapy because of protracted damage from prior cytotoxic therapy and, in some cases, for the persistence of their primary disorder. Moreover, t-AML is relatively resistant to conventional therapies used for de novo leukemias.
Pathogenesis of t-MN After Breast Cancer
Chemotherapy with DNA-targeted antiproliferative drugs in the adjuvant setting has contributed to significant progress in the management of BC, substantially increasing the number of long-term survivors. As the risk of developing cancer increases with age, longer survival is associated with an increased probability of new cancer occurrence, particularly of developing t-AML/MDS. In the majority of cases they are represented by AML, but a secondary acute lymphoblastic leukemia is possible, although less common.[14,15] In the last decades the type of solid tumors preceding t-MN has changed: among 3026 newly diagnosed AML, there were 142 of 200 t-MN with a previous history of solid cancer, with BC representing the most common neoplasm (52%). The median latency between diagnosis of primary malignancy and the occurrence of t-AML was four years, and was shorter in patients younger at the time of primary malignancy diagnosis or treated with anthracyclines and/or topoisomerase-II inhibitors.[10]
Several studies have reported an increased risk for AML in BC patients treated with adjuvant therapy (Table 1 and Table 2), but it remains unclear if t-AML represents a truly stochastic event or if individual susceptibility plays a role.[16] Already 40 years ago Metcalf et al demonstrated a correlation between acute leukemia and BC and hypothesized common risk factors for both diseases.[17] This observation has been confirmed in various series of patients showing that there is an increased risk of developing AML in patients with BC treated with surgery alone, or with family history of BC, so that individual susceptibility for development of multiple tumors and a possible association between the two diseases must be hypothesized.[18-20] It is currently difficult to define individual susceptibility, because only few pathological conditions, above all constitutional and genetically determined, are known to predispose to leukemia. The interaction between the genotoxic effects of chemotherapy or ionizing radiation and the “host” is influenced, among others, by genetic polymorphism in drug metabolism and DNA repair processes, which may increase individual susceptibility to these agents. Furthermore, the observation of secondary leukemias in patients who did not receive chemio- or radiotherapy for their primary tumor suggests the existence of a common predisposing condition, possibly a general cancer susceptibility.

Table 1. Risk of therapy-related myeloid neoplasms in breast cancer patients receiving adjuvant chemotherapy with alkylating agents.

Table 2. Risk of therapy-related myeloid neoplasms in breast cancer patients receiving adjuvant chemotherapy with anthracyclines.
A population-based study from a French Cancer Registry evaluated the risk of developing a new primary invasive cancer during the first five years of follow up for 14353 cancer patients (breast, colorectal and prostate cancer), comparing with the expected numbers, based on primary cancer incidence rate using the standardized incidence ratio (SIR). Overall, 690 second cancers were registered, including 15 AML. In particular, among 5663 women treated for BC, 10 developed t-AML, which results into a greater risk than the general population (SIR=8.26, p<0.05).[21] AML risk after a prior BC was also examined in an Australian retrospective population-based study and this risk was compared to that of survivors after a prior diagnosis of hematological malignancies and other cancers combined. Among 183123 women diagnosed with BC, 158 (0.09%) subsequently developed AML, with the result that women with a prior diagnosis of BC were 2.6 times more likely to develop AML compared to the general female population (p<0.001). Although the incidence of AML rose sharply with age in all cohorts, the age-specific relative risk was highest in the 30-49 age groups and decreased with increasing age. An age-dependent risk of a subsequent diagnosis of AML was confirmed in women <50 years and in the range 50-64 years with previous BC, but not in those older than 65 years, if compared with the expected incidence of AML. A similar age-dependent pattern was observed for second BC and ovarian cancers; this association may be explained by either chemotherapy exposure or an interaction between therapy and genetic predisposition.[22] On the other hand, Patt et al evaluated the risk of AML in older women treated with modern schedules, demonstrating that while older women treated with adjuvant chemotherapy had more than 50% increased risk of AML, the absolute increase in risk at 10 years was low (1.8% in treated patients versus 1.2% among patients who did not received chemotherapy).[23]
Role of Adjuvant Chemotherapy
Adjuvant chemotherapy for BC has undergone major changes, expanding from node-positive women to lower risk patients. Anthracycline-containing regimens have shown superiority in comparison to cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Incorporation of taxanes (paclitaxel and docetaxel) into anthracyclines-based schedules yielded an additional benefit in both disease-free survival (DFS) and overall survival (OS) in most studies, and dose-dense drug administration have shown to be more effective than the conventional dosing schedule.[24-28] These novel therapeutic strategies have resulted into a considerable improvement of BC survival, but also into an increased t-AML/MDS rate.[29-35] Of note, it must be kept in mind that a under-reported incidence of overall t-MN in the different registries is likely, and difficult to accurately estimate, because of an inadequate coding, not specific for t-AML or t-MDS.
Alkylating agents. In the past, alkylating agents were the class of antineoplastic drugs unequivocally associated with t-MN (Table 1). The antineoplastic activity of these drugs is related to their ability to damage DNA by methylation or DNA inter-strand crosslinks formation, interfering with normal DNA replication. Alkylating agent-related AML typically develops after an average latency of 5-7 years, and overt leukemia is often (up to 70% of cases) proceeded by a dysplastic phase.[9,11] Fisher et al reported that the 10-year cumulative risk of AML was increased in patients treated with surgery followed by melphalan-based chemotherapy compared to those treated with surgery alone (1.29% versus 0.27%, respectively).[30] In the following years the leukemogenic potential of cyclophosphamide has emerged. Several studies indicated that the risk for developing AML/MDS among patients with early-stage BC treated with adjuvant chemotherapy containing standard dose cyclophosphamide is higher than that of the general population,[31,36] although the risk of developing t-MN in patients treated with melphalan is 10 times higher than that of patients who received cyclophosphamide.[32] In fact the risk for AML appears negligible in patients treated with CMF regimens, provided that cyclophosphamide is given at standard dose.
Anthracyclines. The latency period between exposure to anthracyclines and the onset of leukemia is usually about 2 years, and generally there is no previous myelodysplastic phase (Table 2). In order to assess the risk of developing AML and MDS after exposure to epirubicin-based regimen, Praga et al reviewed 7110 patients treated with epirubicin and cyclophosphamide in 19 randomized clinical trials in 2005. At a median follow up of eight years the cumulative probability of AML or MDS was 0.55%; however the risk increased in relation to the cumulative doses of both agents, ranging between 0.37% in patients received standard regimen and 4.97% for those treated with higher doses.[37] Similar results were obtained with doxorubicin-based regimen. In a large French case-control study, the risk of t-AML/MDS in women treated for BC was higher in those who received mitoxantrone-based chemotherapy than in those given anthracyclines.[38] Smith et al performed a combined analysis of six adjuvant studies conducted by the National Surgical Adjuvant Breast and Bowel Project group using regimens containing both doxorubicin and cyclophosphamide, and reported a 5-year incidence of AML ranging from 0.3% to 1.2%, with an increased risk for greater dose intensity.[39] The importance of dose intensity was also confirmed with the “intense dose-dense” regimen epirubicin, paclitaxel and cyclophosphamide every 2 weeks, which proved more effective than standard schedule epirubicin/cyclophosphamide and improved event-free and overall survivals, but was also more toxic with four cases (0.6% of patients) of t-AML/MDS.[26]
Antineoplastic activity of taxanes appears to be related to their ability to promote microtubular assembly and to inhibit microtubular disassembly. A SEER database analysis did not document an increased risk of secondary malignancies with these drugs.[23] A 7-year follow-up of a trial comparing doxorubicin/cyclophosphamide (AC) versus docetaxel/cyclophosphamide (TC) in early BC, reported no secondary leukemia in the TC arm, compared to two cases in 510 patients (0.4%) in the AC arm.[25]
Role of Granulocyte Colony-Stimulating Factors (G-CSF) and Radiotherapy
Recently, increasing numbers of women receiving adjuvant chemotherapy for BC have also received granulocyte stimulating factors to reduce the myelosuppressive effects of dose- intense chemotherapy. In vitro data suggest that G-CSF may increase the risk of AML/MDS, but its leukemogenic effect is still debated. An analysis of the SEER-medicare population-based database including 5510 women with BC treated with adjuvant chemotherapy, found that the addition of G-CSF is associated with a doubling of the risk of subsequent AML or MDS when compared with chemotherapy alone, even if the absolute risk is low.[40] In the analysis of six trials described by Smith et al and mentioned above, the incidence of therapy-related leukemia was sharply elevated in patients treated with intensified regimens that required G-CSF support (relative risk 6.16, p=0.0001).[39] On the other hand, Patt et al did not find an increased risk for AML in elderly (>65 years) BC patients, who received G-CSF during the first years after diagnosis as part of adjuvant therapy.[23] Similarly, in the Cancer and Leukemia Group B 9741 phase III trial, patients received dose-dense regimens plus filgrastim support, but had no increased risk of developing AML or MDS compared to those treated with the same regimen at conventional schedule without G-CSF.[27] Finally, a systematic review of 25 randomized clinical trials was recently conducted to evaluate the risk of AML or MDS in patients receiving chemotherapy for solid malignancies and lymphomas with or without the addition of G-CSF. At a median follow up of 54 months, the estimated relative risk for AML/MDS with G-CSF-supported chemotherapy was 1.92, with an estimate absolute increase in risk of 0.4%;41 however, although this increased risk, these data cannot distinguish between the potential causal effects as a result of the growth factor and of dose-intensified systemic chemotherapy, so that the potential toxicities of G-CSF needs further study.
Radiotherapy may also play a significant role (Table 3). A cohort study analyzing clinical records of BC patients with the aim of evaluating the long-term effect of radiotherapy on the risk of second cancers reported a total of 387 malignancies (7.3%) in 5248 women, with eight patients developing leukemia (0.15%), seven in the group treated with radiotherapy, versus one case only in the group not receiving radiotherapy. The relative risk adjusted for chemotherapy and hormone treatment was 6.67 (95% CI 0.76-58.00) and the median time from exposure was 4.5 years, with the suggestion of a raised incidence of leukemia within the first two or more years after radiotherapy.[42] Similarly, in a previous study, the risk of developing AML resulted four-fold increased with the use of radiotherapy (HR 4, 95% CI 1.4-11.8) and by seven folds when radiotherapy was combined to chemotherapy (HR 7.2, 95% CI 1.4-36.3).[33]

Table 3. Risk of therapy-related myeloid neoplasms in breast cancer patients receiving radiotherapy.
Summary and Final Remarks
- In BC survivors, there is a small but significant and increasing number of secondary myeloid neoplasms after adjuvant chemotherapy, particularly after treatment with alkylating agents and/or topoisomerase II inhibitors, that lead to two distinctly and different forms of t-MN.
- The risk of leukemia appears very low if the cumulative dose of anthracyclines and cyclophosphamide is not very high. Clinical trials attempting to improve therapeutic benefit by dose escalation need to take into account the increased risk for leukemia, when assessing potential benefits and risks.
- The incidence of t-MN appears to be also increased in patients treated for BC with surgery alone, and these cases are not “therapy-related”. Thus, t-MN may be part of a cancer-risk syndrome involving BC, and possibly a general cancer susceptibility.
- A raised risk of t-MN is associated with radiotherapy, particularly for women treated after the menopause.
- The concurrent use of G-CSFs as supportive care in order to deliver intensive adjuvant chemotherapy could further enhance this risk, so that their use should be limited to the settings with available strong evidence.
References
- Siegel R, Ward E, Brawley O, Jemal A.
Cancer statistics, 2011: the impact of eliminating socioeconomic and
racial disparities on premature cancer deaths. CA Cancer J Clin.
2011;61(4):212-36. http://dx.doi.org/10.3322/caac.20121
PMid:21685461

- Desantis C, Siegel R, Bandi P, Jemal A.
Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61(6):409-18. http://dx.doi.org/10.3322/caac.20134
PMid:21969133

- Martin MG, Welch JS, Luo J, Ellis MJ,
Graubert TA, Walter MJ. Therapy related acute myeloid leukemia in
breast cancer survivors, a population-based study. Breast Cancer Res
Treat. 2009;118(3):593-8. http://dx.doi.org/10.1007/s10549-009-0376-3
PMid:19322652

- Howard RA, Gilbert ES, Chen BE, et al.
Leukemia following breast cancer: an international population-based
study of 376,825 women. Breast Cancer Res Treat. 2007;105(3):359-68. http://dx.doi.org/10.1007/s10549-006-9460-0
PMid:17221155

- Schaapveld M, Visser O, Louwman MJ, et al.
Risk of new primary nonbreast cancers after breast cancer treatment: a
Dutch population-based study. J Clin Oncol. 2008;26(8):1239-46. http://dx.doi.org/10.1200/JCO.2007.11.9081
PMid:18323547

- Vardiman JW, Thiele J, Arber RD, et al. The
2008 revision of the WHO classification of myeloid neoplasms and acute
leukemia: rationale and important changes. 2009; Blood (114): 937-51. http://dx.doi.org/10.1182/blood-2009-03-209262
PMid:19357394

- Larson RA and Le Beau MM. Prognosis and
therapy when acute promyelocytic leukemia and other "good risk" acute
myeloid leukemias occur as a therapy-related myeloid neoplasm. Mediterr
J Hematol Infect Dis. 2011;3(1):e2011032. http://dx.doi.org/10.4084/mjhid.2011.032
PMid:21869918 PMCid:3152454

- Leone G, Fianchi L, Voso MT.
Therapy-related myeloid neoplasms. Curr Opin Oncol. 2011;23(6):672-80. http://dx.doi.org/10.1097/CCO.0b013e32834bcc2a
PMid:21918440

- Azim HA Jr, de Azambuja E, Colozza M, Bines
J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in
breast cancer. Ann Oncol. 2011;22(9):1939-47. http://dx.doi.org/10.1093/annonc/mdq683
PMid:21289366

- Smith MA, Rubinstein L, Anderson JR,
Arthur D, Catalano PJ, Freidlin B, Heyn R, Khayat A, Krailo M, Land VJ,
Miser J, Shuster J, Vena. Secondary leukemia or myelodysplastic
syndrome after treatment with epipodophyllotoxins. J Clin Oncol 1999;
17, 569-577. PMid:10080601

- Larson RA. Etiology and management of
therapy-related myeloid leukemia. Hematology Am Soc Hematol Educ
Program. 2007:453-9.http://dx.doi.org/10.1182/asheducation-2007.1.453
PMid:18024664

- Smith SM, Le Beau MM, Huo D, et al.
Clinical-cytogenetic associations in 306 patients with therapy-related
myelodysplasia and myeloid leukemia: the University of Chicago series.
Blood, 2003; 102: 43-52. http://dx.doi.org/10.1182/blood-2002-11-3343
PMid:12623843

- Schoch C, Kern W, Schnittger S, Hiddemann
W, Haferlach T. Karyotype is an independent prognostic parameter in
therapy-related acute myeloid leukemia (t-AML): an analysis of 93
patients with t-AML in comparison to 1091 patients with de novo AML.
Leukemia. 2004 Jan;18(1):120-5. http://dx.doi.org/10.1038/sj.leu.2403187
PMid:14586477

- Kayser S, D÷hner K, Krauter J, et al. The
impact of therapy-related acute myeloid leukemia (AML) on outcome in
2853 adult patients with newly diagnosed AML. Blood
2011;117(7):2137-45. http://dx.doi.org/10.1182/blood-2010-08-301713
PMid:21127174

- Pagano L, Pulsoni A, Mele L, et al. Acute
myeloid leukemia in patients previously diagnosed with breast cancer:
experience of the GIMEMA group. Ann Oncol. 2001;12(2):203-7. http://dx.doi.org/10.1023/A:1008318816244
PMid:11300325

- Leone G, Fianchi L, Pagano L, Voso MT.
Incidence and susceptibility to therapy-related myeloid neoplasms. Chem
Biol Interact. 2010;184(1-2):39-45. http://dx.doi.org/10.1016/j.cbi.2009.12.013
PMid:20026017

- Metcalf FD, Moore MAS, Warner NL. Colony
formation in vitro by myelomonocytic leukemia cells. J Natl Cancer Inst
1969; 43: 983-1001. PMid:5259326

- Leone G, Pagano L, Ben-Yehuda D, Voso MT.
Therapy-related leukemia and myelodysplasia: susceptibility and
incidence. Haematologica. 2007;92(10):1389-98. http://dx.doi.org/10.3324/haematol.11034
PMid:17768113

- Rosner F, Carey RW, Zarrabi MH. Breast
cancer and acute leukemia: report of 24 cases and review of the
literature. Am J Med 1978; 4: 151-72.

- Rauscher GH, Sandler DP, Poole C, et al.
Is family history of breast cancer a marker of susceptibility to
exposures in the incidence of de novo adult acute leukemia? Cancer
Epidemiol Biomarkers Prev. 2003;12(4):289-94. PMid:12692102

- Cluze C, Delafosse P, Seigneurin A,
Colonna M. Incidence of second cancer within 5 years of diagnosis of a
breast, prostate or colorectal cancer: a population-based study. Eur J
Cancer Prev. 2009;18(5):343-8. http://dx.doi.org/10.1097/CEJ.0b013e32832abd76
PMid:19436213

- Beadle G, Baade P, Fritschi L. Acute
myeloid leukemia after breast cancer: a population-based comparison
with hematological malignancies and other cancers. Ann Oncol.
2009;20(1):103-9. http://dx.doi.org/10.1093/annonc/mdn530
PMid:18647961

- Patt DA, Duan Z, Fang S, Hortobagyi GN,
Giordano SH. Acute myeloid leukemia after adjuvant breast cancer
therapy in older women: understanding risk. J Clin Oncol. 2007
1;25(25):3871-6.

- Ejlertsen B, Mouridsen HT, Jensen MB, et
al. Improved outcome from substituting methotrexate with epirubicin:
results from a randomised comparison of CMF versus CEF in patients with
primary breast cancer. Eur J Cancer. 2007;43(5):877-84. http://dx.doi.org/10.1016/j.ejca.2007.01.009
PMid:17306974

- Jones S, Holmes FA, O'Shaughnessy J, et
al. Docetaxel With Cyclophosphamide Is Associated With an Overall
Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year
Follow-Up of US Oncology Research Trial 9735. J Clin Oncol.
2009;27(8):1177-83. http://dx.doi.org/10.1200/JCO.2008.18.4028
PMid:19204201

- Moebus V, Jackisch C, Lueck HJ,et al.
Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel,
and cyclophosphamide compared with conventionally scheduled
chemotherapy in high-risk primary breast cancer: mature results of an
AGO phase III study. J Clin Oncol. 2010;28(17):2874-80. http://dx.doi.org/10.1200/JCO.2009.24.7643 PMid:20458045

- Citron ML, Berry DA, Cirrincione C, et al.
Randomized trial of dose-dense versus conventionally scheduled and
sequential versus concurrent combination chemotherapy as postoperative
adjuvant treatment of node-positive primary breast cancer: first report
of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J
Clin Oncol. 2003;21(8):1431-9. http://dx.doi.org/10.1200/JCO.2003.09.081
PMid:12668651

- Burnell M, Levine MN, Chapman JA, et al.
Cyclophosphamide, epirubicin, and fluorouracil versus dose-dense
epirubicin and cyclophosphamide followed by paclitaxel versus
doxorubicin and cyclophosphamide followed by paclitaxel in
node-positive or high-risk node-negative breast cancer. J Clin Oncol.
2010;28(1):77-82. http://dx.doi.org/10.1200/JCO.2009.22.1077
PMid:19901117 PMCid:2799234

- Smith RE. Risk for the development of
treatment-related acute myelocytic leukemia and myelodysplastic
syndrome among patients with breast cancer: review of the literature
and the National Surgical Adjuvant Breast and Bowel Project experience.
Clin Breast Cancer. 2003;4(4):273-9. http://dx.doi.org/10.3816/CBC.2003.n.032
PMid:14651772

- Fisher B, Rockette H, Fisher ER, et al.
Leukemia after breast cancer patients following adjuvant chemotherapy
or postoperative radiotherapy. The NSABP experience. J Clin Oncol 1985;
3: 1640-58. PMid:3906049

- Tallman MS, Gray R, Bennett JM, et al.
Leukemogenic potential of adjuvant chemotherapy for early-stage breast
cancer: the Eastern Cooperative Oncology Group experience. J Clin
Oncol. 1995;13(7):1557-63. PMid:7602344

- Curtis RE, Boice JD Jr, Stovall M, et al.
Risk of leukemia after chemotherapy and radiation treatment for breast
cancer. N Engl J Med. 1992;326(26):1745-51. http://dx.doi.org/10.1056/NEJM199206253262605
PMid:1594016

- Renella R, Verkooijen HM, Fioretta G, et
al. Increased risk of acute myeloid leukaemia after treatment for
breast cancer. Breast. 2006;15(5):614-9. http://dx.doi.org/10.1016/j.breast.2005.11.007
PMid:16386906

- Bernard-Marty C, Mano M, Paesmans M, et
al. Second malignancies following adjuvant chemotherapy: 6-year results
from a Belgian randomized study comparing cyclophosphamide,
methotrexate and 5-fluorouracil (CMF) with an anthracycline-based
regimen in adjuvant treatment of node-positive breast cancer patients.
Ann Oncol. 2003;14(5):693-8. http://dx.doi.org/10.1093/annonc/mdg204
PMid:12702521

- Campone M, RochÚ H, Kerbrat P, et al.
Secondary leukemia after epirubicin-based adjuvant chemotherapy in
operable breast cancer patients: 16 years experience of the French
Adjuvant Study Group. Ann Oncol. 2005;16(8):1343-51. http://dx.doi.org/10.1093/annonc/mdi251
PMid:15905306

- Valagussa P, Moliterni A, Terenziani M,
Zambetti M, Bonadonna G. Second malignancies following CMF-based
adjuvant chemotherapy in resectable breast cancer. Ann Oncol.
1994;5(9):803-8. PMid:7848882

- Praga C, Bergh J, Bliss J, et al. Risk of
acute myeloid leukemia and myelodysplastic syndrome in trials of
adjuvant epirubicin for early breast cancer: correlation with doses of
epirubicin and cyclophosphamide. J Clin Oncol. 2005;23(18):4179-91. http://dx.doi.org/10.1200/JCO.2005.05.029
PMid:15961765

- Le Deley MC, Suzan F, Cutuli B, et al.
Anthracyclines, mitoxantrone, radiotherapy, and granulocyte
colony-stimulating factor: risk factors for leukemia and
myelodysplastic syndrome after breast cancer. J Clin Oncol.
2007;25(3):292-300. http://dx.doi.org/10.1200/JCO.2006.05.9048
PMid:17159192

- Smith RE, Bryant J, DeCillis A, Anderson
S. Acute myeloid leukemia and myelodysplastic syndrome after
doxorubicin-cyclophosphamide adjuvant therapy for operable breast
cancer: the National Surgical Adjuvant Breast and Bowel Project
Experience. J Clin Oncol. 2003;21(7):1195-204. http://dx.doi.org/10.1200/JCO.2003.03.114 PMid:12663705

- Hershman D, Neugut AI, Jacobson JS, et al.
Acute myeloid leukemia or myelodysplastic syndrome following use of
granulocyte colony-stimulating factors during breast cancer adjuvant
chemotherapy. J Natl Cancer Inst. 2007;99(3):196-205. http://dx.doi.org/10.1093/jnci/djk028
PMid:17284714

- Lyman GH, Dale DC, Wolff DA, et al. Acute
myeloid leukemia or myelodysplastic syndrome in randomized controlled
clinical trials of cancer chemotherapy with granulocyte
colony-stimulating factor: a systematic review. J Clin Oncol.
2010;28(17):2914-24. http://dx.doi.org/10.1200/JCO.2009.25.8723
PMid:20385991

- Zhang W, Becciolini A, Biggeri A, Pacini
P, Muirhead CR. Second malignancies in breast cancer patients following
radiotherapy: a study in Florence, Italy. Breast Cancer Res.
2011;13(2):R38. http://dx.doi.org/10.1186/bcr2860
PMid:21463502 PMCid:3219201
