Modulating Effect of the −158 Gγ (C→T) Xmn1 Polymorphism in Indian Sickle Cell Patients
Sanjay Pandey1, Sweta Pandey1, Rahasya Mani Mishra2 and Renu Saxena1
1 Department of Hematology, AIIMS, New Delhi, India
2 Department of Environmental Biology, APS University Rewa, India
2 Department of Environmental Biology, APS University Rewa, India
Correspondence
to:
Dr. Renu Saxena, Professor and Head. Department of Haematology,
I.R.C.H. Building (1st floor), All India Institute of Medical Sciences,
Ansari Nagar, New Delhi – 110 029, India. Tel: 91-011-26594670, Fax: 91-011-26588663. E-mail: renusax@hotmail.com
Published: January 15, 2012
Received: September 11, 2011
Accepted: November 26, 2011
Mediterr J Hematol Infect Dis 2012, 4(1): e20120 , DOI 10.4084/MJHID.2012.001
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Xmn1
polymorphism is a known factor, which increases fetal haemoglobin
production. Among the inherited disorders of blood, thalassaemia and
Sickle Cell Diseases contributes to a major bulk of genetic diseases in
India. Our aim was to verify the role of the Xmn1 polymorphism as a
modulating factor in sickle cell patients and frequency of the
polymorphism in Indian sickle cell patients. 60 sickle homozygous and
75 sickle beta thalassemia patients were included and 5 ml blood sample
was collected from them. Screening of sickle patients was done by HPLC.
An automated cell analyzer SYSMEX (K-4500 Model) was used to analyze
the Complete Blood Count of patients. Xmn1 polymorphism analysis was
done by PCR-RFLP and one-way ANOVA test was applied to analysis
of variance between groups. Among the sickle patients 27 were
heterozygous (+/-) and 19 were homozygous (+/+) while 30 were
heterozygous (+/-) and 24 were homozygous (+/+) in sickle β-thalassemia
patients. Extremely significant differences (p-value <0.001) of
hematological parameters seen among patients with Xmn1 carrier and
without the Xmn1 carrier. In our cases the clinical symptoms were
barely visible and higher HbF level with Xmn1 carriers were found.
Presence of Xmn1 polymorphism in sickle cell patients with higher HbF
were phenotypically distinguished in the sickle cell patients. We
conclude that the phenotypes of Indian sickle cell patients were
greatly influenced by Xmn1polymorphism.
Introduction
The C-T substitution at position –158 of the Gy globin gene, referred to as the Xmn1-gpolymorphism, is a common sequence variant in all population groups, present at a frequency of 0.32 to 0.35.[1] Clinical studies have shown that under conditions of hematopoietic stress, for example in homozygous b-thalassemia and sickle cell disease, the presence of the Xmn1- Gg site favors a higher Hb F response. This could explain why the same mutations on different b chromosomal backgrounds are associated with disease of different clinical severity.[2,3] Increased levels of fetal hemoglobin (Hb F or a2 γ2) are of no consequence in healthy adults, but confer major clinical benefits in patients with sickle cell anemia (SCA) and β- thalassemia, diseases that represent major public health problems.[4] Fetal hemoglobin (Hb F or a2 γ2) is predominant in red cells of the fetus and the newborn baby, and is largely replaced after birth by adult hemoglobin (α2β2). The two types of γ chains of Hb F (Gγ and Aγ) differ at position 136 (glycine versus alanine) and are produced by closely-linked genes of the β -globin gene cluster. In normal adults, red cells have less than 1% Hb F, and Gγ accounts for some 40% of total γ chain.[5] Genetic variation of Gγ values has been observed in sickle cell anemia (SS) patients, whose increased Hb F levels facilitate such studies. Although most have 40% Gγ, some have Gγ values of 60% to 70%.[6] About 5% of black sickle cell patients are heterozygous for the normal -Gγ -Aγ and a mutant -Gγ -Aγ chromosome with both genes producing Gγ globin; they have Gγ values of about 70%.[7-9]Approximately 1/6 black sickle cell and 2/3 black β -thalassemia heterozygotes have high Gγ values of about 60%. It has been estimated that in India with a population of 100 million at the millennium (2000) and a birth rate of 25/1000, there would be about 45 million carriers and about 9000 infants born each year with haemoglobinopathies.[10] Among the genetic factors known to affect HbF production are DNA sequence variations within the β -globin gene cluster. In particular, the (C-T) variation at position – 158 upstream of the Gγ globin gene, which is detectable by the restriction enzyme Xmn1. The sequence variation has been shown to increase Hb F levels in β-thalassaemia anemia.[11,12,13] There is a paucity of data for the effect of the Xmn1 polymorphism on the phenotype of Indian sickle cell patient; thus our aim was to evaluate the role of the Xmn1 polymorphism as a modulating factor in sickle cell patients and its frequency.
Material and Methods.
Subjects were 60 sickles homozygous and 75 sickle beta thalassemia patients (29 patients were HbSβ+ while 46 patients were HbSβ0). About 5 ml blood sample was collected from hematology outpatient department AIIMS; after taking their consent. Study was approved from institutional ethical committee. Clinical evaluation was done during physical examination (visually appeared) as well as laboratory evaluation. Age of onset of splenomegaly and jaundice was <10 year and the presence of anemia was evaluated through hemogram analysis. Complete blood count and red cell indices were measured by automated cell analyzer (SYSMEX K-4500, Kobe Japan). Quantitative assessment of hemoglobin Hb F, Hb A, Hb A2 and Hb S and diagnosis of HbSS and HbSβ-thalassemia was performed by high performance liquid chromatography (HPLC-Bio-Rad-VariantTM Bio Rad, CA, USA). DNA extraction done by phenol chloroform method and DNA quantification done by nano drop spectrophotometer. Xmn1 polymorphism analysis was done by PCR-RFLP method as per Sutton et al. [14]. A one-way ANOVA test was applied to analysis of variance between groups. P-value <0.05 were considered statistical significant. Presence of clinical symptoms with and without Xmn1polymorphism was used to for comparison of clinical features.
Result.
Sixty sickle homozygous (35 male and 25 female with mean age 11.32±7.61 years) and 75 sickle β-thalassemia (57 male and 18 female with mean age12±8.33 years) patients were characterized. Out of 60 sickle homozygous patients; 27 (45%) were heterozygous (+/-) and 19 (31.67%) were homozygous (+/+) while 30 (40%) were heterozygous and 24 (32%) were homozygous in sickle β-thalassemia for Xmn1 polymorphism. Fourteen (23.33%) patient in sickle homozygous and 21 (28%) patients in sickle β- thalassemia were normal for Xmn1 polymorphism. The frequency of the Xmn1 polymorphism was higher among sickle homozygous than sickle β-thalassemia patients. Clinical severity were improved with homozygous (+/+) Xmn1 polymorphism in sickle cell anemia as well as sickle β-thalassemia patients. Reticulocytes, haemoglobin and red cell indices were higher in Xmn1carriers than non carriers and found extremely statistically significant (p-value <0.001). The explanation to the improvement of RBCs in Xmn1 carriers is that the anemia was less and overall red cell indices and Hb value was improved. Hyperpigmentation was found in a few cases. Splenomegaly , gall stone, painful crisis, jaundice and frequency of blood transfusion in relation with HbF and Xmn1 polymorphism were lesser in Xmn1 carriers in comparison to non-carriers and found statistically significant (p-value <0.001). Details of haematological parameters and clinical parameters of HbSS and HbSβ-thalassemia are given in table 1, 2, 3 and 4 respectively.
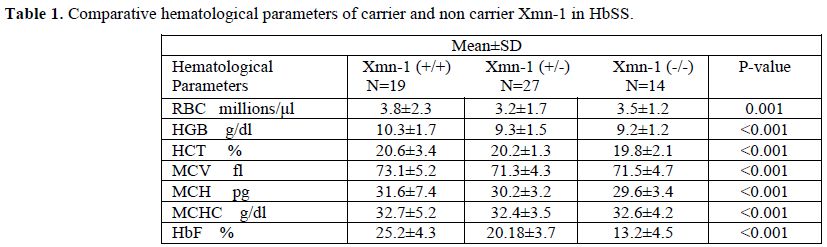
Table 1. Comparative hematological parameters of carrier and non carrier Xmn-1 in HbSS.
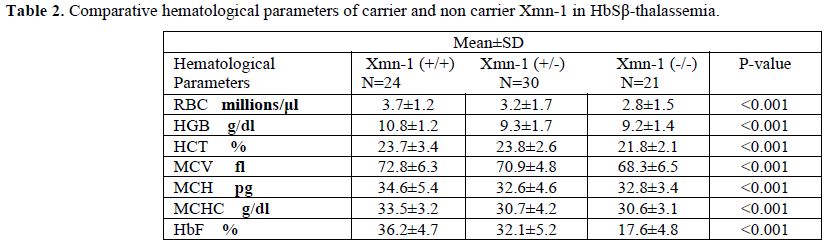
Table 2. Comparative hematological parameters of carrier and non carrier Xmn-1 in HbSβ-thalassemia.
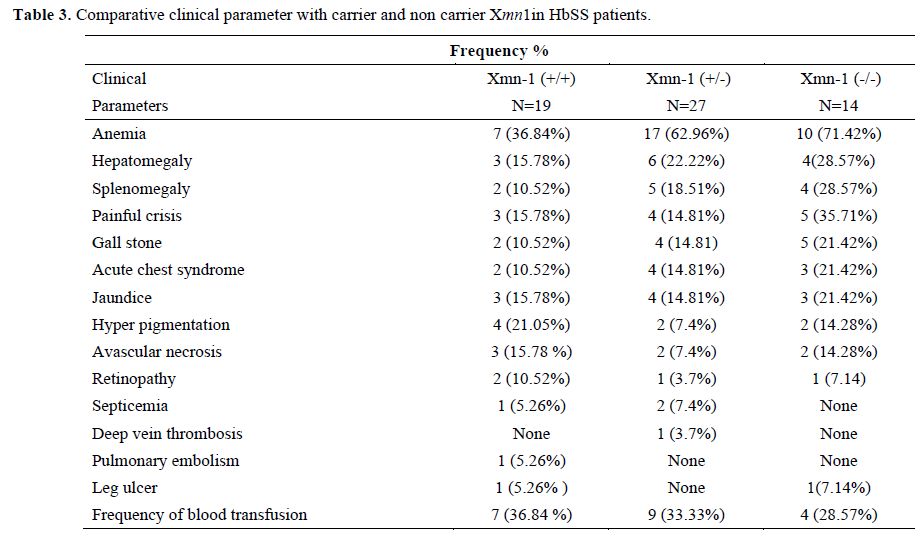
Table 3. Comparative clinical parameter with carrier and non carrier Xmn1in HbSS patients.
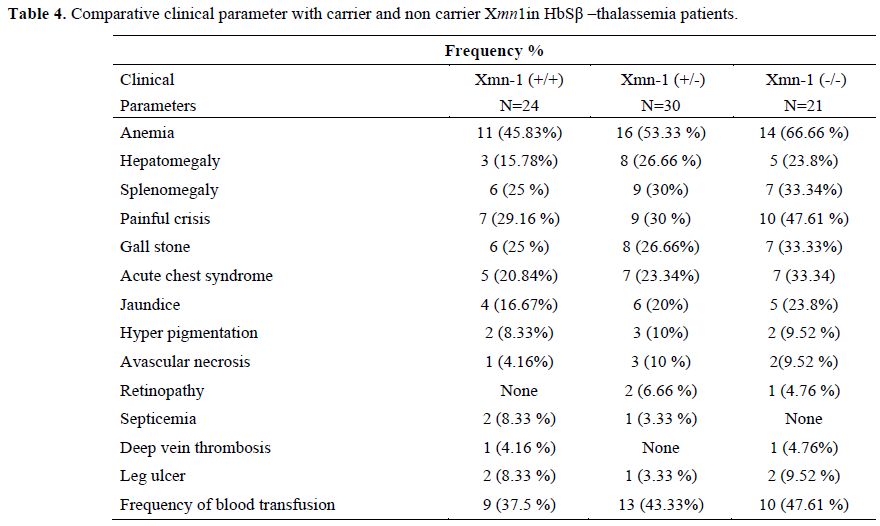
Table 4. Comparative clinical parameter with carrier and non carrier Xmn1in HbSβ –thalassemia patients.
Discussion.
Fetal hemoglobin (HbF) genes are genetically regulated and the level of HbF and its distribution among sickle erythrocytes is highly variable. Hb F is the major genetic modulator of the hematological and clinical features of sickle cell disease with the Senegal and Saudi-Indian haplotype which gives its beneficial effects to the pateints.[15,16] Many epidemiological studies suggested that disease complications most closely linked to sickle vasoocclusion and blood viscosity were robustly related to HbF concentration while complications associated with the intensity of hemolysis were less affected. Although HbF is the protective factor for leg ulcers, one complication closely associated with hyper-hemolysis.[17,18] The effect of -158 C > T mutation on expression of Gγ globin gene has been the subject of considerable interest. The association of some β-globin mutations with Xmn1 site with elevated HbF expression has been previously published. The role of increased HbF response as an ameliorating factor has become evident in patients who were mildly affected despite being homozygotes or compound heterozygotes for β0 or β+ thalassaemia.[19] Association of Xmn1 and frequency of blood transfusion was reported in thalassemic patients.[20,21]
The strong association of Xmn1 site with the Arab Indian haplotype is thought to be associated with high fetal hemoglobin concentration and confer a benign course of the disease. However, the clinical presentation of sickle cell disease in different regions in our country is highly variable.[22] In our cases, hematological parameters of sickle homozygous and sickle β-thalassemia an improved condition with Xmn1 carriers while non carriers of Xmn1polymorphism were found worsened. Our cases of sickle cell patient showed the presence of C-T variation at position -158 in the Gγ gene, affect hematologically as well as clinically and increased production of HbF. Xmn1 carriers of HbSβ-thalassemia patients had higher HbF than HbSS patients. The clinical features of HbS-β thalassemia are extremely variable, ranging from a completely asymptomatic state to a severe disorder similar to homozygous sickle cell disease. This heterogeneity is likely to be due to the presence of different β-thalassemia alleles or interaction with modulating genetic factors like associated α-thalassemia and/or a gene for raised HbF production (Xmn1 polymorphism).[23] Heterozygosity for presence of Xmn1 site polymorphism is also likely to influence phenotype[24] and Xmn1 polymorphism absence reduction is associated with acquired HbF elevation.[25] Gilmans[13] data are consistent with the hypothesis that T at position - 1 58 causes the high HbF values. There is a paucity of data in relation of Xmn1polymorphism and phenotypic effect on Indian sickilers. However a study on HbEβ-thalassemia report the phenotypic effect of Xmn1 polymorphism17 while another study report none of the association in clinical severity and presence of Xmn1 polymorphism in thalassemia intermedia patients.[26] Raina et al.[27] concluded that the presence of Xmn1 polymorphism and IVS 1-1 mutation leads to a milder phenotypic presentation causing a delay in onset of blood transfusions but dose not effect the amount of blood received /kg/year. However α-thalassemia also influence on the level of HbF in patients with sickle cell disease.[28,29] In our cases the frequency of Xmn1 polymorphism found higher amongst sickle cell anemia patient in comparison to sickle β thalassemia. Sickle homozygous and sickle β-thalassemia patients showed clinical variation and this could be due to the association of Xmn1polymorphism, either in homozygous or heterozygous state. Presence of Xmn1polymorphism in sickle patients with higher HbF that improve phenotypic presentation in the sickle cell patients. A study from Western Iran in β- thalassemia patients report the presence of Xmn1 polymorphic site on both chromosomes (+/+) the level of Hb F tended to be increased compared to the absence of Xmn1 (−/−) and the presence of this polymorphic site caused a positive influence on Hb F production and the Gγ percent which could improve the clinical symptoms of β-thalassemia patients.[30] Our finding in sickle cell disease patients was similar with the study. Thus we conclude that the phenotypes of Indian sickle cell patients were greatly influenced by Xmn1polymorphism.
Acknowledgements.
Sincere thanks to technical staff of department of hematology AIIMS, for expert assistance.
Financial Support.
This study supported by ICMR & Hematology Department AIIMS, New Delhi.
The C-T substitution at position –158 of the Gy globin gene, referred to as the Xmn1-gpolymorphism, is a common sequence variant in all population groups, present at a frequency of 0.32 to 0.35.[1] Clinical studies have shown that under conditions of hematopoietic stress, for example in homozygous b-thalassemia and sickle cell disease, the presence of the Xmn1- Gg site favors a higher Hb F response. This could explain why the same mutations on different b chromosomal backgrounds are associated with disease of different clinical severity.[2,3] Increased levels of fetal hemoglobin (Hb F or a2 γ2) are of no consequence in healthy adults, but confer major clinical benefits in patients with sickle cell anemia (SCA) and β- thalassemia, diseases that represent major public health problems.[4] Fetal hemoglobin (Hb F or a2 γ2) is predominant in red cells of the fetus and the newborn baby, and is largely replaced after birth by adult hemoglobin (α2β2). The two types of γ chains of Hb F (Gγ and Aγ) differ at position 136 (glycine versus alanine) and are produced by closely-linked genes of the β -globin gene cluster. In normal adults, red cells have less than 1% Hb F, and Gγ accounts for some 40% of total γ chain.[5] Genetic variation of Gγ values has been observed in sickle cell anemia (SS) patients, whose increased Hb F levels facilitate such studies. Although most have 40% Gγ, some have Gγ values of 60% to 70%.[6] About 5% of black sickle cell patients are heterozygous for the normal -Gγ -Aγ and a mutant -Gγ -Aγ chromosome with both genes producing Gγ globin; they have Gγ values of about 70%.[7-9]Approximately 1/6 black sickle cell and 2/3 black β -thalassemia heterozygotes have high Gγ values of about 60%. It has been estimated that in India with a population of 100 million at the millennium (2000) and a birth rate of 25/1000, there would be about 45 million carriers and about 9000 infants born each year with haemoglobinopathies.[10] Among the genetic factors known to affect HbF production are DNA sequence variations within the β -globin gene cluster. In particular, the (C-T) variation at position – 158 upstream of the Gγ globin gene, which is detectable by the restriction enzyme Xmn1. The sequence variation has been shown to increase Hb F levels in β-thalassaemia anemia.[11,12,13] There is a paucity of data for the effect of the Xmn1 polymorphism on the phenotype of Indian sickle cell patient; thus our aim was to evaluate the role of the Xmn1 polymorphism as a modulating factor in sickle cell patients and its frequency.
Material and Methods.
Subjects were 60 sickles homozygous and 75 sickle beta thalassemia patients (29 patients were HbSβ+ while 46 patients were HbSβ0). About 5 ml blood sample was collected from hematology outpatient department AIIMS; after taking their consent. Study was approved from institutional ethical committee. Clinical evaluation was done during physical examination (visually appeared) as well as laboratory evaluation. Age of onset of splenomegaly and jaundice was <10 year and the presence of anemia was evaluated through hemogram analysis. Complete blood count and red cell indices were measured by automated cell analyzer (SYSMEX K-4500, Kobe Japan). Quantitative assessment of hemoglobin Hb F, Hb A, Hb A2 and Hb S and diagnosis of HbSS and HbSβ-thalassemia was performed by high performance liquid chromatography (HPLC-Bio-Rad-VariantTM Bio Rad, CA, USA). DNA extraction done by phenol chloroform method and DNA quantification done by nano drop spectrophotometer. Xmn1 polymorphism analysis was done by PCR-RFLP method as per Sutton et al. [14]. A one-way ANOVA test was applied to analysis of variance between groups. P-value <0.05 were considered statistical significant. Presence of clinical symptoms with and without Xmn1polymorphism was used to for comparison of clinical features.
Result.
Sixty sickle homozygous (35 male and 25 female with mean age 11.32±7.61 years) and 75 sickle β-thalassemia (57 male and 18 female with mean age12±8.33 years) patients were characterized. Out of 60 sickle homozygous patients; 27 (45%) were heterozygous (+/-) and 19 (31.67%) were homozygous (+/+) while 30 (40%) were heterozygous and 24 (32%) were homozygous in sickle β-thalassemia for Xmn1 polymorphism. Fourteen (23.33%) patient in sickle homozygous and 21 (28%) patients in sickle β- thalassemia were normal for Xmn1 polymorphism. The frequency of the Xmn1 polymorphism was higher among sickle homozygous than sickle β-thalassemia patients. Clinical severity were improved with homozygous (+/+) Xmn1 polymorphism in sickle cell anemia as well as sickle β-thalassemia patients. Reticulocytes, haemoglobin and red cell indices were higher in Xmn1carriers than non carriers and found extremely statistically significant (p-value <0.001). The explanation to the improvement of RBCs in Xmn1 carriers is that the anemia was less and overall red cell indices and Hb value was improved. Hyperpigmentation was found in a few cases. Splenomegaly , gall stone, painful crisis, jaundice and frequency of blood transfusion in relation with HbF and Xmn1 polymorphism were lesser in Xmn1 carriers in comparison to non-carriers and found statistically significant (p-value <0.001). Details of haematological parameters and clinical parameters of HbSS and HbSβ-thalassemia are given in table 1, 2, 3 and 4 respectively.

Table 1. Comparative hematological parameters of carrier and non carrier Xmn-1 in HbSS.

Table 2. Comparative hematological parameters of carrier and non carrier Xmn-1 in HbSβ-thalassemia.

Table 3. Comparative clinical parameter with carrier and non carrier Xmn1in HbSS patients.

Table 4. Comparative clinical parameter with carrier and non carrier Xmn1in HbSβ –thalassemia patients.
Discussion.
Fetal hemoglobin (HbF) genes are genetically regulated and the level of HbF and its distribution among sickle erythrocytes is highly variable. Hb F is the major genetic modulator of the hematological and clinical features of sickle cell disease with the Senegal and Saudi-Indian haplotype which gives its beneficial effects to the pateints.[15,16] Many epidemiological studies suggested that disease complications most closely linked to sickle vasoocclusion and blood viscosity were robustly related to HbF concentration while complications associated with the intensity of hemolysis were less affected. Although HbF is the protective factor for leg ulcers, one complication closely associated with hyper-hemolysis.[17,18] The effect of -158 C > T mutation on expression of Gγ globin gene has been the subject of considerable interest. The association of some β-globin mutations with Xmn1 site with elevated HbF expression has been previously published. The role of increased HbF response as an ameliorating factor has become evident in patients who were mildly affected despite being homozygotes or compound heterozygotes for β0 or β+ thalassaemia.[19] Association of Xmn1 and frequency of blood transfusion was reported in thalassemic patients.[20,21]
The strong association of Xmn1 site with the Arab Indian haplotype is thought to be associated with high fetal hemoglobin concentration and confer a benign course of the disease. However, the clinical presentation of sickle cell disease in different regions in our country is highly variable.[22] In our cases, hematological parameters of sickle homozygous and sickle β-thalassemia an improved condition with Xmn1 carriers while non carriers of Xmn1polymorphism were found worsened. Our cases of sickle cell patient showed the presence of C-T variation at position -158 in the Gγ gene, affect hematologically as well as clinically and increased production of HbF. Xmn1 carriers of HbSβ-thalassemia patients had higher HbF than HbSS patients. The clinical features of HbS-β thalassemia are extremely variable, ranging from a completely asymptomatic state to a severe disorder similar to homozygous sickle cell disease. This heterogeneity is likely to be due to the presence of different β-thalassemia alleles or interaction with modulating genetic factors like associated α-thalassemia and/or a gene for raised HbF production (Xmn1 polymorphism).[23] Heterozygosity for presence of Xmn1 site polymorphism is also likely to influence phenotype[24] and Xmn1 polymorphism absence reduction is associated with acquired HbF elevation.[25] Gilmans[13] data are consistent with the hypothesis that T at position - 1 58 causes the high HbF values. There is a paucity of data in relation of Xmn1polymorphism and phenotypic effect on Indian sickilers. However a study on HbEβ-thalassemia report the phenotypic effect of Xmn1 polymorphism17 while another study report none of the association in clinical severity and presence of Xmn1 polymorphism in thalassemia intermedia patients.[26] Raina et al.[27] concluded that the presence of Xmn1 polymorphism and IVS 1-1 mutation leads to a milder phenotypic presentation causing a delay in onset of blood transfusions but dose not effect the amount of blood received /kg/year. However α-thalassemia also influence on the level of HbF in patients with sickle cell disease.[28,29] In our cases the frequency of Xmn1 polymorphism found higher amongst sickle cell anemia patient in comparison to sickle β thalassemia. Sickle homozygous and sickle β-thalassemia patients showed clinical variation and this could be due to the association of Xmn1polymorphism, either in homozygous or heterozygous state. Presence of Xmn1polymorphism in sickle patients with higher HbF that improve phenotypic presentation in the sickle cell patients. A study from Western Iran in β- thalassemia patients report the presence of Xmn1 polymorphic site on both chromosomes (+/+) the level of Hb F tended to be increased compared to the absence of Xmn1 (−/−) and the presence of this polymorphic site caused a positive influence on Hb F production and the Gγ percent which could improve the clinical symptoms of β-thalassemia patients.[30] Our finding in sickle cell disease patients was similar with the study. Thus we conclude that the phenotypes of Indian sickle cell patients were greatly influenced by Xmn1polymorphism.
Acknowledgements.
Sincere thanks to technical staff of department of hematology AIIMS, for expert assistance.
Financial Support.
This study supported by ICMR & Hematology Department AIIMS, New Delhi.
References
- Garner C, Tatu T, Game L, Cardon LR,Spector TD, Farrall M, et al. A candidate gene study of F cell levels in sibling pairs using a joint linkage and association analysis. GeneScreen 2000;1:9-14. http://dx.doi.org/10.1046/j.1466-9218.2000.00001.x
- Thein SL, Wainscoat JS, Sampietro M, Old
JM, Cappellini D, Fiorelli G, et al. Association of thalassaemia
intermedia with a β-globin gene haplotype. Br JHaematol 1987;65:367-73.
PMid:18492615

- Labie D, Pagnier J, Lapoumeroulie C,
Rouabhi F, Dunda-Belkhodja O, Chardin P, et al. Common haplotype
dependency of high G y-globin gene expression and high Hb F levels in β
thalassemia and sickle cell anemia patients. Proc Natl Acad Sci USA
1985; 82:2111-4. http://dx.doi.org/10.1073/pnas.82.7.2111

- Swee Lay Thein, Stephan Menzel, Mark
Lathrop,Chad Garner.Control of fetal hemoglobin: new insights emerging
from genomics and clinical implications. Hum. Mol. Genet. 2009; 18 :
R216–R223 http://dx.doi.org/10.1093/hmg/ddp401 PMid:19808799 PMCid:2758709

- Huisman THJ, Harris H, Gravely M, Schroeder
WA, Shelton JR. Shelton JB, Evans L: The chemical heterogeneity of the
fetal hemoglobin in normal newborn infants and in adults.J Mol Cell
Biochem. 1977; 17:45-55. http://dx.doi.org/10.1007/BF01732554 PMid:904619

- Gardiner MB, Reese AL, Headlee ME, Huisman
THJ: The heterogeneity of the gama chain of fetal hemoglobin in Hb S
heterozygotes. Blood. 1982; 60: 513-18. PMid:18459486

- Powars PA, Altay C, Huisman THJ, Smithies
O: Two novel arrangements of the human fetal globin genes: G-gama and A
- gama-A gama. NucI Acids Res. 1984:12:7023-34 http://dx.doi.org/10.1093/nar/12.18.7023 PMid:6091051 PMCid:320140

- Gilman JG, Huisman THJ: Two independent
genetic factors in the beta-globin gene cluster are associated with
high G levels in the HbF of SS patients. Blood 1984; 64:452-7.
PMid:6204701

- Harano T, Reese AL, Ryan R. Abraham BL,
Huisman THJ: Five haplotypes in black beta -thalassaemia heterozygotes:
Three are associated with high and two with low G values in fetal
haemoglobin.Br J Haematol. 1985; 59:333-42. PMid:19040842

- Balgir R.S. The burden of haemoglobinopathies in India and the challenges ahead. Curr Sci 2000; 79: 1536-47.

- Verma I.C. Burden of genetic disorders in India. Indian J Pediatr 2000; 67: 893-98. http://dx.doi.org/10.1007/BF02723953

- International Committee for
Standardization in Haemotology. Recommendations for selected methods
for quantitative estimation of HbA2 and HbA2 reference preparation.
Brit J Haemat 1978; 38: 573-78. http://dx.doi.org/10.1111/j.1365-2141.1978.tb01082.x PMid:646955

- Gilman JG, Huisman THJ. DNA sequence
variation associated with elevated fetal Gg globin production
Blood.1985; 66: 783-87. PMid:2412616

- Sutton M, Bouhassira EE, Nagel RL.
Polymerase chain reaction amplification applied to the determination of
beta like globin gene cluster haplotypes. Am J Hematol. 1989; 32: 66-69
http://dx.doi.org/10.1002/ajh.2830320113 PMid:2757004

- Akinsheye I, Alsultan A, Solovieff N, Ngo
D, Baldwin CT, Sebastiani P, Chui DVH and Steinberg MH. Fetal
hemoglobin in sickle cell anemia. Blood 2011;118:19-27;
doi:10.1182/blood-2011-03-325258 http://dx.doi.org/10.1182/blood-2011-03-325258 PMid:21490337

- Julie Makani,Stephan Menzel,Siana
Nkya,Sharon E. Cox,Emma Drasar,Deogratius Soka,et. al. Genetics of
fetal hemoglobin in Tanzanian and British patients with sickle cell
anemia. Blood 2011; 117:1390-1392 http://dx.doi.org/10.1182/blood-2010-08-302703 PMid:21068433

- Kato GJ, Gladwin MT, Steinberg MH.
Deconstructing sickle cell disease: Reappraisal of the role of
hemolysis in the development of clinical subphenotypes. Blood Rev.
2007;21:37-47. http://dx.doi.org/10.1016/j.blre.2006.07.001 PMid:17084951 PMCid:2048670

- Nolan VG, Adewoye A, Baldwin C, et al.
Sickle cell leg ulcers: associations with haemolysis and SNPs in
Klotho, TEK and genes of the TGF-beta/BMP pathway. Br J
Haematol.2006;133:570-578. http://dx.doi.org/10.1111/j.1365-2141.2006.06074.x PMid:16681647 PMCid:1679888

- Dedoussis GV, Mandilara GD, Boussiv M,
Loutradis A. HbF production in b-thalassaemia heterozygotes for the
IVSII-1 G-A β0-globin mutation. Implication of the haplotype and the
Gg-158 C-T mutation on the HbF level. Am J Hematol 2000; 64: 151-55. http://dx.doi.org/10.1002/1096-8652(200007)64:3<151::AID-AJH2>3.3.CO;2-O

- Winichagoon P, Fucharoen S, Chen P, Wasi
P. Genetic factors affecting clinical severity in b-thalassaemia
syndromes. J Pediatr Hematol Oncol 2000; 22: 573-80. PMid:11322807

- Kultar A, Kultar F, Wilson JB, Headlee MG,
Huisman THJ. Quantification of haemoglobin componenets by
Highperformance cation exchange liquid chromatography.Am J hematol
1984; 17: 39-53. PMid:12383737

- Garner C, Tatu T, Reittie JE, Littlewood
T, Darley J, Cervino S. et al. Genetic influenceson F cells and other
hematologic variables: a twin heritability study. Blood.
2000:95:342–346. PMid:10607722

- Serjeant GR. Sickle cell- β thalassemia. In: Serjeant GR, editor. Sickle Cell Disease. 3rd ed. Oxford: Oxford University Press; 2001.
- Panigrahi I, Agarwal S, Gupta T, Singhal
P, Pradhan M. Hemoglobin E-beta Thalassemia: Factors Affecting
Phenotype.Indian pediatrics.2005; 42:357-62 PMid:15876597

- Shimmoto MM, Vicari P, Fernandes AC,
Guimaraes GS, Figueiredo MS. XmnI polymorphism is associated with fetal
hemoglobin levels in hypoplastic syndromes. Sao Paulo Med. J. 2006; 124
:110-11 http://dx.doi.org/10.1590/S1516-31802006000200012 PMid:16878196

- Oberoi S, Das R, Panigrahi I, Kaur J,
Marwaha RK. Xmn1-(G) γ polymorphism and clinical predictors of severity
of disease in β-thalassemia intermedia. Pediatr Blood Cancer. 2011.
doi: 10.1002/pbc.23175. http://dx.doi.org/10.1002/pbc.23175

- Raina Aditya, Verma IC, Renu Saxena,
Dinesh Kaul, Khanna VK. Relation of Xmn-1 Polymorphism & Five
Common Indian Mutations of Thalassaemia with Phenotypic Presentation in
β-thalassaemia. JK Science. 2006; 8: 139-143

- Embury SH, Dozy AM, Miller J, Davis JR Jr,
Kleman KM, Preisler H.et al. Concurrent sickle-cell anemia and
thalassemia: Effect on severity of anemia. N Engl J Med 1982;
306:270-74. http://dx.doi.org/10.1056/NEJM198202043060504 PMid:6172710

- Schroeder WA, Powars DR, Kay LM. β-cluster
haplotypes, α-gene status and hematologic data from SS, SC, and Sβ
-thalassemia patients in Southern California. Hemoglobin 1989; 13:325-
53. http://dx.doi.org/10.3109/03630268909003397 PMid:2473969

- Hooshang Nemati, Zohreh Rahimi, Gholamreza
Bahrami.The Xmn1 polymorphic site 5′ to the Gγ gene and its correlation
to the Gγ:Aγ ratio, age at first blood transfusion and clinical
features in β-Thalassemia patients from Western Iran. Mol. Biol.
Rep.2010;37:159-164 http://dx.doi.org/10.1007/s11033-009-9566-7 PMid:19444645
