Epstein-Barr Virus (EBV)-Associated Haemophagocytic Syndrome
Lorenza Torti1, Luigi M. Larocca2, Giuseppina Massini1, Annarosa Cuccaro1, Elena Maiolo1, Rosaria Santangelo3, Maria Bianchi1, Mariano Alberto Pennisi4, Stefan Hohaus1 and Luciana Teofili1
Departments of Hematology1, Pathology2, Microbiology3 and Anaesthesiology4, Catholic University, Rome, Italy
Correspondence
to:
Luciana Teofili. Department of Hematology, Catholic University of Rome,
Largo Gemelli 8, I- 00168 Rome. Tel. 39-6-30154180, Fax 39-6-3055153.
E-mail: lteofili@rm.unicatt.it
Published: January 25, 2012
Received: November 11, 2011
Accepted: December 16, 2011
Mediterr J Hematol Infect Dis 2012, 4(1): e2012008, DOI 10.4084/MJHID.2012.008
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
We
describe the case of a 17- year old female who developed
fatal haemophagocytic syndrome (HPS) one month following acute
infection caused by Epstein-Barr virus (EBV). Despite initiation of
treatment and reduction of EBV load, laboratory signs of HPS as severe
cytopenia, hypofibrinogenemia, hyperferritinemia and
hypertriglyceridemia persisted, and the patient died of multiorgan
failure. HPS is a rare, but life-threatening complication of EBV
infection.
Introduction
The haemophagocytic syndrome (HPS) represents a severe and aggressive hyperinflammatory disease with major diagnostic and therapeutic difficulties.[1-3] HPS is a spectrum of inherited and acquired conditions with disturbed immune regulation which results into a dysregulated activation and proliferation of lymphocytes. Haemophagocytosis is part of a sepsis-like syndrome caused by severe hypercytokinemia as result of a highly stimulated but ineffective immune response. Cardinal symptoms are prolonged fever, cytopenia affecting at least two of three lineages, hepatosplenomegaly and coagulopathy with hypofibrinogenemia. Diagnostic criteria for this disorder are shown in Table 1 edited by the Histiocyte Society.[2]
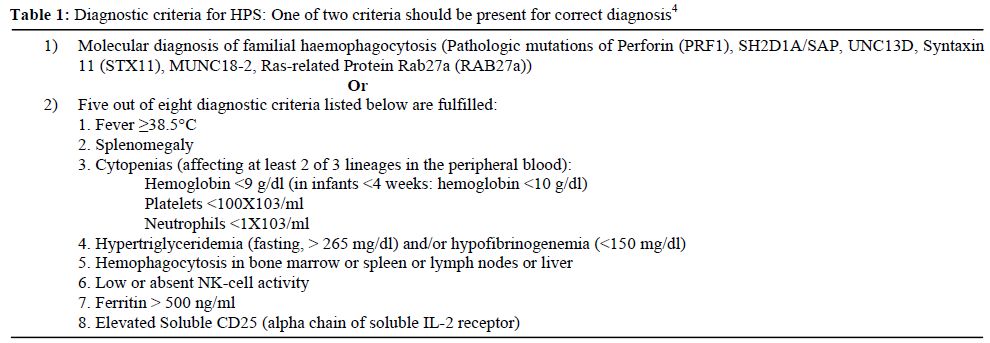
Table 1. Diagnostic criteria for HPS: One of two criteria should be present for correct diagnosis4
HPS may be the consequence of a familial immune dysregulatory disorder or be associated with a number of different infections, autoimmune disorders or malignancies, like lymphomas or carcinomas.[4,5] Frequent triggers are infectious agents, especially virus of the herpes group.[6] Among the infectious agents associated with HPS there are EBV, Cytomegalovirus, Parvovirus, Varicella zoster, Herpes-simplex as well as Herpes-Virus 8 and HIV infection alone or in combination. EBV is the most common infectious agent.
EBV-associated haemophagocytic syndrome has a relatively high incidence in Asian countries and particularly in Japan.[3] Here we present a case of EBV-associated haemophagocytic syndrome in a young Italian women that was fatal despite prompt medical treatment.
Case Presentation.
A 17 year-old white woman was admitted to our hospital for fever, fatigue and abdominal discomfort. Her medical history showed no relevant data; in particular no recurrent infectious episodes were documented except for the common childhood diseases. One month before the admission, the patient suffered from infectious mononucleosis. She had developed a widespread skin rash during antibiotic therapy with amoxicillin given for fever. A that time, the monospot test resulted positive and the patient received corticosteroids with symptoms improvement.
On admission, the physical examination showed splenomegaly without palpable lymphadenopathy. Laboratory evaluation revealed a severe pancytopenia: platelet count was 17x 106/mmc, hemoglobin was 7,2 g/dl and she had a leucocyte count of 230/mmc. Lactate dehydrogenase was strongly increased to 1570 UI/l, while GPT was 217 U/l and bilirubin was elevated to 4,6mg/dl (Table 2). Clotting tests showed severe hypofibrinogenemia with a normal value of international normalized ratio (INR) and of activated partial thromboplastin time (aPTT). A bone marrow aspiration for the suspect of an acute hematological proliferative disease was performed, but was not diagnostic. Ferritin levels were high (6421 ng/ml). Serological studies for HIV 1,2, hepatitis B and C, cytomegalovirus, parvovirus were negative. Both IgG and IgM anti- viral capsid antigen (VCA) EBV antibodies were positive, while anti-EBV nuclear antigen were negative, indicating a recent primary EBV infection. Amplification of EBV-DNA from whole blood by polymerase chain reaction (PCR) yielded 930.000 copies/ml. As the patient presented signs of an acute colecystitis, she was treated with trans-hepatic drainage of the gall-bladder.
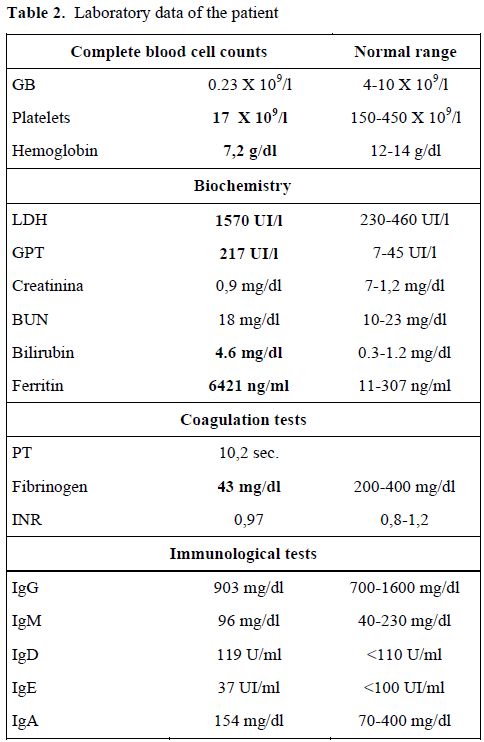
Table 2. Laboratory data of the patient
Acute acalcolous colecystitis (AAC) observed in our patient, reflected the severe inflammatory response associated with the primary EBV infection. In addition, the bile stasis can also been implicated in the gallbladder inflammation pathogenesis. Moreover, also EBV-induced hepatitis has been recognized as an important cause of cholestasis. Finally, another possible pathogenetic mechanism, may be the direct invasion of the gallbladder from the EBV-virus. AAC may develop during the course of acute EBV infection, especially in patients with cholestatic hepatitis.[6,7]
Clinical conditions of our patient rapidly deteriorated and she was transferred to the intensive care unit on day 2 following admission. Hemophagocytic syndrome was suspected and confirmed by a second bone marrow examination (Table 3). Bone marrow cytology and histology revealed several histiocytes with phagocytosis of erythrocytes and platelets (Figure 1). The bone marrow biopsy showed phagocytic cells with engulfed haemopoietic elements. The presence of large sized T lymphocytes in the biopsy raised the suspicion of EBV-related T-lymphoproliferative disease.
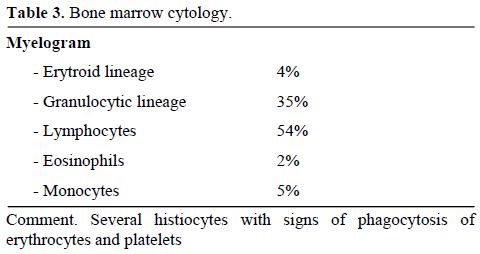
Table 3. Bone marrow cytology.
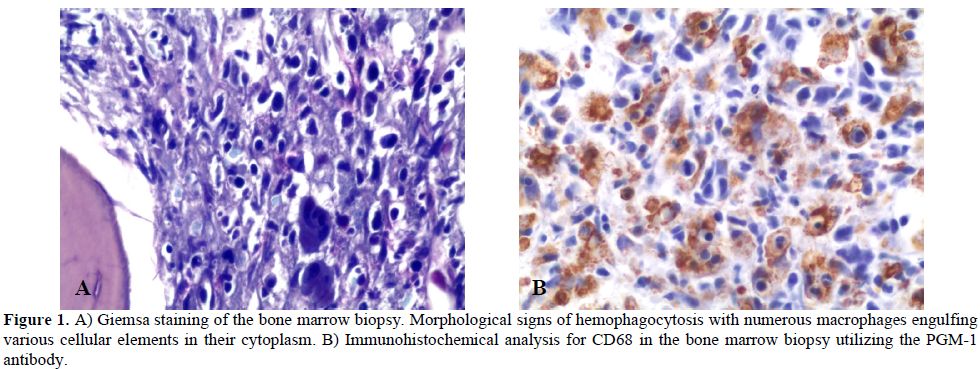
Figure 1. A) Giemsa staining of the bone marrow biopsy. Morphological signs of hemophagocytosis with numerous macrophages engulfing various cellular elements in their cytoplasm. B) Immunohistochemical analysis for CD68 in the bone marrow biopsy utilizing the PGM-1 antibody.
The patient was immediately started on high doses of corticosteroids and immunoglobulins (1g/kg for two days). As the clinical conditions did not improve, treatment with etoposide was started according to the HLH-94 protocol. Etoposide was administered in a dose of 150 mg/m2, on day 9 and day 12. EBV copy numbers slowly decreased, and laboratory signs of HPS as severe hypofibrinogenemia and hyperferrtinemia improved (Figure 2), but pancytopenia persisted and the patient was supported with massive transfusions of blood and fresh frozen plasma, granulocyte-colony stimulating factor (G-CSF), and antibiotics. The patient died in multiorgan failure on day 15 following admission.
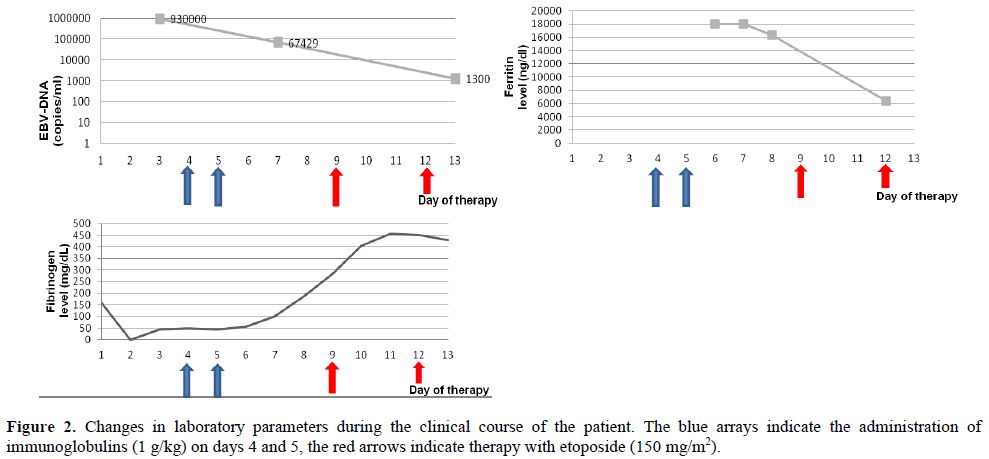
Figure 2. Changes in laboratory parameters during the clinical course of the patient. The blue arrays indicate the administration of immunoglobulins (1 g/kg) on days 4 and 5, the red arrows indicate therapy with etoposide (150 mg/m2).
Discussion.
Haemophagocytic syndrome (HPS) is the most severe complication of infectious mononucleosis. HPS is a highly fatal disease if untreated or treated late. Diagnosing HPS is a challenge, as there is no single simple diagnostic test, and it remains a syndromic disorder with a combination of typical findings being often initially absent but developing with progressive disease. Because of diagnostic difficulties, diagnosis of HPS is often delayed and only established when the patient is already critically ill and transferred to the intensive care unit, as in our case.
As EBV responds poorly to antiviral agents, treatment is aimed at controlling the lymphocyte/macrophage activation and proliferation. In addition to corticosteroids and immunoglobulins, treatment of EBV-associated HPS often requires administration of etoposide.[8,9] Repetitive administration of a myelosuppressive agent in an already severe cytopenic patient with a high risk for additional infectious complications is not trivial, and should be limited to centers experienced in treatment of acute haematological diseases.
Since HPS is rare, no data are available from randomized controlled clinical trials testing potential different treatment approaches. As T cell dysregulation appears to be involved in the pathogenesis of both primary and secondary HPS, treatment with anti-thymocyte globulins (ATG) has been reported as an alternative to etoposide-based therapy in a single center study on patients with familial HPS.[10] An on-going trial of the Histiocyte Society studies the early addition of cyclosporine to the etoposide-based regimen.[11] An additional therapy for patients with progressive EBV-associated HPS may be rituximab, at it can eliminate EBV-infected B cells.[12]
Bone marrow histology raised the suspicion of a T cell lymphoma in our patient. Lymphomas of T-cell and NK-cell origin are among the most common neoplasias associated with secondary malignancy-associated HPS.[4,5,11] This association has been more frequently been reported in Asian countries where the incidence of EBV-associated lymphomas appear to be significantly higher. In fact some studies describe fulminant EBV-T cell lymphoproliferative disorders following acute EBV-infection.[13] However, the suspicion in our patient was not substantiated by other signs of a lymphoproliferative disease, although we cannot rule out that the EBV-induced lymphoproliferation in our patient was already indication of transformation into a malignant disease. In this case, only the assessment of the monoclonal nature of the EBV-infected T cell expansion could have definitely indicate the transformation in lymphoma.
In conclusion, our case highlights that hemophagocytic syndrome can complicate acute EBV-infection, and should raise the awareness for this rare life-threatening syndrome in order to promptly start appropriate treatment.
The haemophagocytic syndrome (HPS) represents a severe and aggressive hyperinflammatory disease with major diagnostic and therapeutic difficulties.[1-3] HPS is a spectrum of inherited and acquired conditions with disturbed immune regulation which results into a dysregulated activation and proliferation of lymphocytes. Haemophagocytosis is part of a sepsis-like syndrome caused by severe hypercytokinemia as result of a highly stimulated but ineffective immune response. Cardinal symptoms are prolonged fever, cytopenia affecting at least two of three lineages, hepatosplenomegaly and coagulopathy with hypofibrinogenemia. Diagnostic criteria for this disorder are shown in Table 1 edited by the Histiocyte Society.[2]

Table 1. Diagnostic criteria for HPS: One of two criteria should be present for correct diagnosis4
HPS may be the consequence of a familial immune dysregulatory disorder or be associated with a number of different infections, autoimmune disorders or malignancies, like lymphomas or carcinomas.[4,5] Frequent triggers are infectious agents, especially virus of the herpes group.[6] Among the infectious agents associated with HPS there are EBV, Cytomegalovirus, Parvovirus, Varicella zoster, Herpes-simplex as well as Herpes-Virus 8 and HIV infection alone or in combination. EBV is the most common infectious agent.
EBV-associated haemophagocytic syndrome has a relatively high incidence in Asian countries and particularly in Japan.[3] Here we present a case of EBV-associated haemophagocytic syndrome in a young Italian women that was fatal despite prompt medical treatment.
Case Presentation.
A 17 year-old white woman was admitted to our hospital for fever, fatigue and abdominal discomfort. Her medical history showed no relevant data; in particular no recurrent infectious episodes were documented except for the common childhood diseases. One month before the admission, the patient suffered from infectious mononucleosis. She had developed a widespread skin rash during antibiotic therapy with amoxicillin given for fever. A that time, the monospot test resulted positive and the patient received corticosteroids with symptoms improvement.
On admission, the physical examination showed splenomegaly without palpable lymphadenopathy. Laboratory evaluation revealed a severe pancytopenia: platelet count was 17x 106/mmc, hemoglobin was 7,2 g/dl and she had a leucocyte count of 230/mmc. Lactate dehydrogenase was strongly increased to 1570 UI/l, while GPT was 217 U/l and bilirubin was elevated to 4,6mg/dl (Table 2). Clotting tests showed severe hypofibrinogenemia with a normal value of international normalized ratio (INR) and of activated partial thromboplastin time (aPTT). A bone marrow aspiration for the suspect of an acute hematological proliferative disease was performed, but was not diagnostic. Ferritin levels were high (6421 ng/ml). Serological studies for HIV 1,2, hepatitis B and C, cytomegalovirus, parvovirus were negative. Both IgG and IgM anti- viral capsid antigen (VCA) EBV antibodies were positive, while anti-EBV nuclear antigen were negative, indicating a recent primary EBV infection. Amplification of EBV-DNA from whole blood by polymerase chain reaction (PCR) yielded 930.000 copies/ml. As the patient presented signs of an acute colecystitis, she was treated with trans-hepatic drainage of the gall-bladder.

Table 2. Laboratory data of the patient
Acute acalcolous colecystitis (AAC) observed in our patient, reflected the severe inflammatory response associated with the primary EBV infection. In addition, the bile stasis can also been implicated in the gallbladder inflammation pathogenesis. Moreover, also EBV-induced hepatitis has been recognized as an important cause of cholestasis. Finally, another possible pathogenetic mechanism, may be the direct invasion of the gallbladder from the EBV-virus. AAC may develop during the course of acute EBV infection, especially in patients with cholestatic hepatitis.[6,7]
Clinical conditions of our patient rapidly deteriorated and she was transferred to the intensive care unit on day 2 following admission. Hemophagocytic syndrome was suspected and confirmed by a second bone marrow examination (Table 3). Bone marrow cytology and histology revealed several histiocytes with phagocytosis of erythrocytes and platelets (Figure 1). The bone marrow biopsy showed phagocytic cells with engulfed haemopoietic elements. The presence of large sized T lymphocytes in the biopsy raised the suspicion of EBV-related T-lymphoproliferative disease.

Table 3. Bone marrow cytology.

Figure 1. A) Giemsa staining of the bone marrow biopsy. Morphological signs of hemophagocytosis with numerous macrophages engulfing various cellular elements in their cytoplasm. B) Immunohistochemical analysis for CD68 in the bone marrow biopsy utilizing the PGM-1 antibody.
The patient was immediately started on high doses of corticosteroids and immunoglobulins (1g/kg for two days). As the clinical conditions did not improve, treatment with etoposide was started according to the HLH-94 protocol. Etoposide was administered in a dose of 150 mg/m2, on day 9 and day 12. EBV copy numbers slowly decreased, and laboratory signs of HPS as severe hypofibrinogenemia and hyperferrtinemia improved (Figure 2), but pancytopenia persisted and the patient was supported with massive transfusions of blood and fresh frozen plasma, granulocyte-colony stimulating factor (G-CSF), and antibiotics. The patient died in multiorgan failure on day 15 following admission.

Figure 2. Changes in laboratory parameters during the clinical course of the patient. The blue arrays indicate the administration of immunoglobulins (1 g/kg) on days 4 and 5, the red arrows indicate therapy with etoposide (150 mg/m2).
Discussion.
Haemophagocytic syndrome (HPS) is the most severe complication of infectious mononucleosis. HPS is a highly fatal disease if untreated or treated late. Diagnosing HPS is a challenge, as there is no single simple diagnostic test, and it remains a syndromic disorder with a combination of typical findings being often initially absent but developing with progressive disease. Because of diagnostic difficulties, diagnosis of HPS is often delayed and only established when the patient is already critically ill and transferred to the intensive care unit, as in our case.
As EBV responds poorly to antiviral agents, treatment is aimed at controlling the lymphocyte/macrophage activation and proliferation. In addition to corticosteroids and immunoglobulins, treatment of EBV-associated HPS often requires administration of etoposide.[8,9] Repetitive administration of a myelosuppressive agent in an already severe cytopenic patient with a high risk for additional infectious complications is not trivial, and should be limited to centers experienced in treatment of acute haematological diseases.
Since HPS is rare, no data are available from randomized controlled clinical trials testing potential different treatment approaches. As T cell dysregulation appears to be involved in the pathogenesis of both primary and secondary HPS, treatment with anti-thymocyte globulins (ATG) has been reported as an alternative to etoposide-based therapy in a single center study on patients with familial HPS.[10] An on-going trial of the Histiocyte Society studies the early addition of cyclosporine to the etoposide-based regimen.[11] An additional therapy for patients with progressive EBV-associated HPS may be rituximab, at it can eliminate EBV-infected B cells.[12]
Bone marrow histology raised the suspicion of a T cell lymphoma in our patient. Lymphomas of T-cell and NK-cell origin are among the most common neoplasias associated with secondary malignancy-associated HPS.[4,5,11] This association has been more frequently been reported in Asian countries where the incidence of EBV-associated lymphomas appear to be significantly higher. In fact some studies describe fulminant EBV-T cell lymphoproliferative disorders following acute EBV-infection.[13] However, the suspicion in our patient was not substantiated by other signs of a lymphoproliferative disease, although we cannot rule out that the EBV-induced lymphoproliferation in our patient was already indication of transformation into a malignant disease. In this case, only the assessment of the monoclonal nature of the EBV-infected T cell expansion could have definitely indicate the transformation in lymphoma.
In conclusion, our case highlights that hemophagocytic syndrome can complicate acute EBV-infection, and should raise the awareness for this rare life-threatening syndrome in order to promptly start appropriate treatment.
References
- Fisman DN Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000 Nov-Dec;6(6):601-8. http://dx.doi.org/10.3201/eid0606.000608 PMid:11076718 PMCid:2640913

- Henter JI, Horne A, Aricó M, Egeler RM,
Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J,
Janka G HLH-2004: Diagnostic and therapeutic guidelines for
hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007
Feb;48(2):124-31. http://dx.doi.org/10.1002/pbc.21039

- Janka GE. Hemophagocytic syndromes. Blood Rev. 2007 Sep;21(5):245-53. http://dx.doi.org/10.1016/j.blre.2007.05.001 PMid:17590250

- Tong H, Ren Y, Liu H, Xiao F, Mai W, Meng
H, Qian W, Huang J, Mao L, Tong Y, Wang L, Qian J, Jin J. Clinical
characteristics of T-cell lymphoma associated with hemophagocytic
syndrome: comparison of T-cell lymphoma with and without hemophagocytic
syndrome. Leuk Lymphoma. 2008 Jan;49(1):81-7. http://dx.doi.org/10.1080/10428190701713630

- Gallipoli P, Drummond M, Leach M. Hemophagocytosis and relapsed peripheral T-cell lymphoma. Eur J Haematol. 2009 Mar;82(3):246. http://dx.doi.org/10.1111/j.1600-0609.2008.01167.x PMid:19018857

- Shaukat A, Tsai HT, Rutherford R, Anania
FA: Epstein–Barr virus induced hepatitis: an important cause of
cholestasis. Hepatol Res 2005; 33: 24–26. http://dx.doi.org/10.1016/j.hepres.2005.06.005 PMid:16112900

- A. Attilakos, E. Lagona, F. Sharifi, A.
Voutsioti, A. Mavri, M. Markouri, Epstein-Barr Virus Infectious
Mononucleosis Associated with Acute Acalculous Cholecystitis. Infection
2007; 35: 118–119

- Rouphael NG, Talati NJ, Vaughan C,
Cunningham K, Moreira R, Gould C. Infections associated with
haemophagocytic syndrome. Lancet Infect Dis. 2007 Dec;7(12):814-22. http://dx.doi.org/10.1016/S1473-3099(07)70290-6

- Imashuku S, Kuriyama K, Teramura T, Ishii
E, Kinugawa N, Kato M, Sako M, Hibi S. Requirement for etoposide in the
treatment of Epstein-Barr virus-associated hemophagocytic
lymphohistiocytosis J Clin Oncol. 2001 May 15;19(10):2665-73.
PMid:11352958

- Mahlaoui N, Ouachee-Chardin M, de Saint BG
et al. Immunotherapy of familial hemophagocytic lymphohistiocytosis
with antithymocyte globulins: a single-center retrospective report of
38 patients. Pediatrics 2007;120(3):e622-e628 http://dx.doi.org/10.1542/peds.2006-3164 PMid:17698967

- Jordan MB, Allen CE, Weitzman S,
Filipovich AH, McClain KL How I treat hemophagocytic
lymphohistocytosis. Blood 2011; 118:4041-52. http://dx.doi.org/10.1182/blood-2011-03-278127 PMid:21828139

- Milone MC, Tsai DE, Hodinka RL et al.
Treatment of primary Epstein-Barr virus infection in patients with
X-linked lymphoproliferative disease using B-cell-directed therapy.
Blood 2005;105(3):994-996. http://dx.doi.org/10.1182/blood-2004-07- 2965 PMid:15494422

- Awaya N, Adachi A, Mori T, Kamata H,
Nakahara J, Yokoyama K, Yamada T, Kizaki M, Sakamoto M, Ikeda Y,
Okamoto S. Fulminant Epstein-Barr virus (EBV)-associated T-cell
lymphoproliferative disorder with hemophagocytosis following autologous
peripheral blood stem cell transplantation for relapsed
angioimmunoblastic T-cell lymphoma. Leuk Res. 2006 Aug;30(8):1059-62. http://dx.doi.org/10.1016/j.leukres.2005.10.022 PMid:16330097
