Methotrexate Induced Lung Injury in a Patient with Primary CNS Lymphoma: a Case Report
Puneet Chhabra, Arjun Dutt Law, Vikas Suri, Pankaj Malhotra* and Subhash Varma
Department of Internal Medicine, PGIMER Chandigarh 160012, India
Published: April 2, 2012
Received: January, 2012
Accepted: March 26, 2012
Mediterr J Hematol Infect Dis 2012, 4(1): e20120 , DOI 10.4084/MJHID.2012.020
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Methotrexate
is an antimetabolite commonly used in clinical practice for a variety
of indications ranging from rheumatoid arthritis and other connective
tissue disorders to high dose regimens in many malignancies. This
folate antagonist has got a spectrum of toxicities among which
gastrointestinal effects predominate. Lung injury is a well described
but rare event and has been reported most often in patients who have
been on long term oral therapy for rheumatic disorders. Acute lung
injury in a patient receiving a high dose regimen for haematological
malignancies has not been reported previously. We present one
such case of methotrexate related acute lung injury in a patient of
primary CNS lymphoma receiving high dose methotrexate.
Introduction.
Methotrexate has a broad spectrum of side effects ranging from gastrointestinal and haematological dysfunction to the less commonly described lung injury. The most commonly associated lung injury described in association with methotrexate use is pulmonary fibrosis that has been seen in patients who have been receiving long term oral therapy for rheumatoid arthritis. Less well known is the syndrome of acute lung injury that is seen in patients who are treated with high dose regimens as used in the management of osteosarcoma or haematological malignancies. Due to the already immunocompromised nature of these patients, it is important to clinically differentiate this potentially life threatening syndrome from other causes of acute lung injury such as bacterial, viral and fungal infections.
Case Report.
A 58- year- old female was admitted to a tertiary care centre in northern India for the treatment of primary CNS lymphoma. She was diagnosed on the basis of neuroimaging and a stereotactic brain biopsy which showed diffuse large B cell lymphoma. PET CT confirmed isolated CNS involvement. At admission she had an ECOG performance status of 4 because of the disease and neurosurgical intervention. Initially, CSF cytology was negative for malignant cells. Baseline high resolution CT chest was normal.
The patient was started on DeAngelis protocol which included five cycles of high dose intravenous methotrexate and vincristine, oral procarbazine and intrathecal methotrexate preceding whole brain irradiation and post radiation cytarabine. Due to her poor performance status, a lower dose of methotrexate (1gm/m2) was given in the first cycle. Four days after receiving the first cycle she developed fever and shortness of breath. Physical examination revealed presence of bibasilar crepts. Chest roentgenogram revealed bilateral lower zone infiltrates. Blood gases revealed hypoxia at room air. She was given injectable broad spectrum antibiotics and supplemental oxygen. All cultures (bacterial, fungal, viral) were sterile, ß-d glucan was negative and the patient had a gradual improvement in symptoms over the next week. After the first cycle, CSF examination was repeated as per protocol and was positive for malignant cells. Accordingly, a decision was taken to give the usual dose of methotrexate (3.5 gm/m2) in the next cycle. A day after receiving methotrexate administration, the patient again developed breathlessness. Examination revealed diffuse bronchospasm and crepitations in both lung fields. She had no fever throughout this period. High resolution CT of the chest revealed bilateral patchy ground glass opacification as well as basal atelectasis .
Blood gases revealed severe hypoxemia, cultures were sterile, fungal and viral serologies were negative and patient had remained afebrile throughout this episode. A possibility of drug induced lung injury was kept. On the basis of clinical criteria devised by Searles and Mckendry the patient was diagnosed with methotrexate induced lung injury.[1] She was managed with intravenous and inhaled corticosteroids in addition to inhaled bronchodilators. She improved with this management and was taken off oxygen after two weeks. Due to methotrexate toxicity further high dose chemotherapy was deferred and she was advised to proceed with radiation therapy. She was put on temozolomide therapy post whole brain radiotherapy. She remains on close follow- up one year later. Serial PET CT scans have shown remission of lymphoma and no recurrence after one year. No progression of lung injury or any fibrotic changes have been seen.
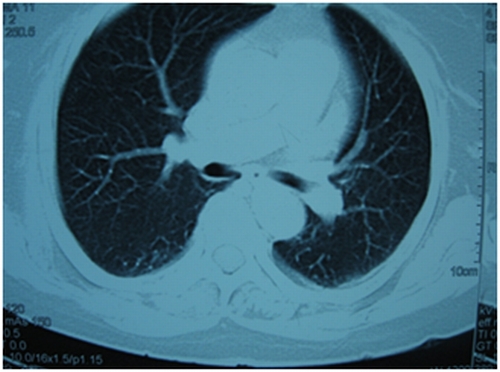
Figure 1. Figure showing normal tracheobronchial tree, vessels and normal lung parenchyma.
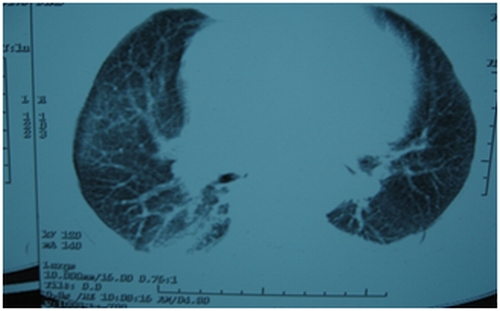
Figure 2.
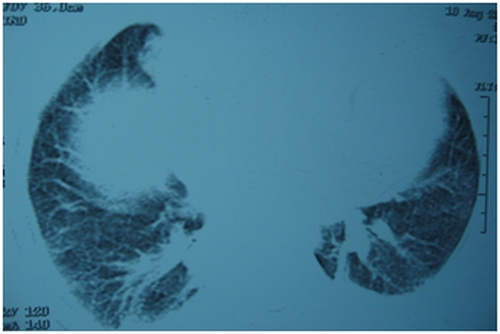
Figure 3.
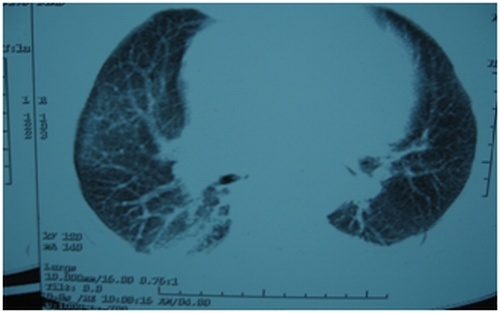
Figure 4.
Figure 2,3,4. HRCT chest [after giving methotrexate], showing normal tracheobronchial tree, vessels but lung parenchymal changes in the form of b/l patchy ground glass opacities with b/l basal atelectasis.
Discussion.
Methotrexate is an antimetabolite acting via folate inhibition. Therefore, it acts on tissues which have high rates of cellular division such as skin, gastrointestinal tract, liver and bone marrow. It is highly teratogenic and is classified as a category X drug in pregnancy. The effect of methotrexate on the lungs is less well studied. The spectrum of pulmonary toxicities involves hypersensitivity pneumonitis,[2] interstitial fibrosis, pulmonary edema,[3] bronchitis with airway hyperreactivity, bronchiolitis obliterans with organizing pneumonia (BOOP) and pulmonary nodules.[4] The symptoms are nonspecific and include nonproductive cough, shortness of breath, chest pain and vague constitutional symptoms. The time of onset of symptoms after administration of methotrexate is highly variable.The injury does not appear to be dependent upon the dose. Cases have been reported where injury has occurred with doses as low as 7.5 mg to as high as 3600 mg. Even a single dose of methotrexate has been known to cause lung injury.The mechanism of lung injury has not been studied in detail however few studies have shown that cytokine modulation via P38-MAP kinase pathway may play an important role.[5] It was also thought that polymorphism in the methylenetetrahydrofolate genes may be linked with methotrexate toxicity. Some studies show that polymorphism of the C677T methylenetetrahydrofolate gene is associated with increased toxicity of methotrexate while others do not.[6,7] However a few more studies are required to prove the association between methylenetetrahydrofolate polymorphism and methotrexate toxicity. As most patients receiving methotrexate are also susceptible to infections due to their underlying disease, investigation for infectious causes of lung injury should be done in all patients. Bronchoalveolar lavage is important to rule out other infective etiologies however the findings in methotrexate induced lung injury are nonspecific and reveal a lymphocytic infiltrate. Radiographic findings reveal bilateral alveolar and interstitial infiltrate which are common in bibasilar regions but may be unremarkable.[8,9,10] Lung biopsy reveals the presence of lymphocytes although occasional neutrophilic infiltrates may also be present.[11] Eosinophilia, hyperplasia of Type 2 pneumocytes and interstitial fibrosis have also been described. Due to the absence of specific clinical, radiological and pathological findings, it is important to have a high index of suspicion and rule out other more common causes. Several criteria have been devised by Carson, Searle and Kremer (Table 1). Treatment involves withdrawal of methotrexate and administration of steroids. Spontaneous resolution despite continuation of the drug has also been described.[12] Restarting methotrexate may lead to recurrence of lung injury although successful reintroduction of oral methotrexate has been described in two cases.[13] Leucovorin rescue does not protect against methotrexate induced lung damage. Most patients have an uneventful recovery following lung injury,[14] however, some may have persistent lung damage causing fibrosis and restrictive lung disease.
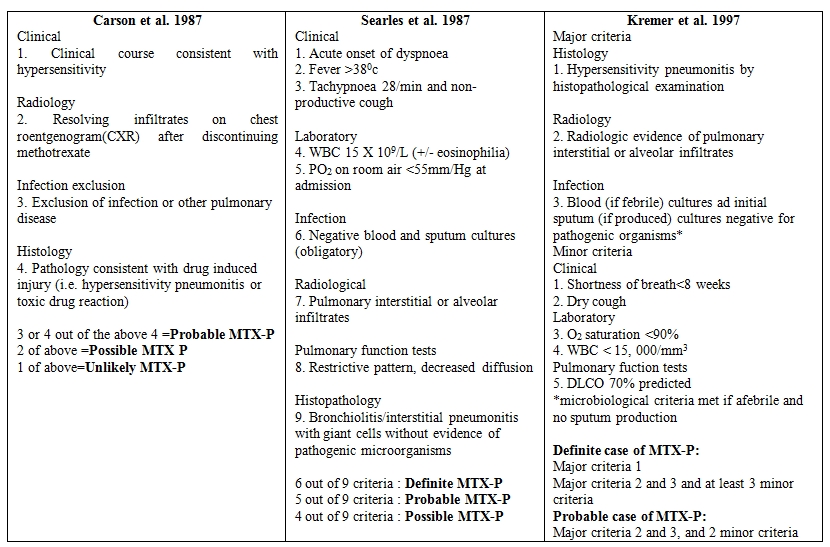
Table 1.
Methotrexate has a broad spectrum of side effects ranging from gastrointestinal and haematological dysfunction to the less commonly described lung injury. The most commonly associated lung injury described in association with methotrexate use is pulmonary fibrosis that has been seen in patients who have been receiving long term oral therapy for rheumatoid arthritis. Less well known is the syndrome of acute lung injury that is seen in patients who are treated with high dose regimens as used in the management of osteosarcoma or haematological malignancies. Due to the already immunocompromised nature of these patients, it is important to clinically differentiate this potentially life threatening syndrome from other causes of acute lung injury such as bacterial, viral and fungal infections.
Case Report.
A 58- year- old female was admitted to a tertiary care centre in northern India for the treatment of primary CNS lymphoma. She was diagnosed on the basis of neuroimaging and a stereotactic brain biopsy which showed diffuse large B cell lymphoma. PET CT confirmed isolated CNS involvement. At admission she had an ECOG performance status of 4 because of the disease and neurosurgical intervention. Initially, CSF cytology was negative for malignant cells. Baseline high resolution CT chest was normal.
The patient was started on DeAngelis protocol which included five cycles of high dose intravenous methotrexate and vincristine, oral procarbazine and intrathecal methotrexate preceding whole brain irradiation and post radiation cytarabine. Due to her poor performance status, a lower dose of methotrexate (1gm/m2) was given in the first cycle. Four days after receiving the first cycle she developed fever and shortness of breath. Physical examination revealed presence of bibasilar crepts. Chest roentgenogram revealed bilateral lower zone infiltrates. Blood gases revealed hypoxia at room air. She was given injectable broad spectrum antibiotics and supplemental oxygen. All cultures (bacterial, fungal, viral) were sterile, ß-d glucan was negative and the patient had a gradual improvement in symptoms over the next week. After the first cycle, CSF examination was repeated as per protocol and was positive for malignant cells. Accordingly, a decision was taken to give the usual dose of methotrexate (3.5 gm/m2) in the next cycle. A day after receiving methotrexate administration, the patient again developed breathlessness. Examination revealed diffuse bronchospasm and crepitations in both lung fields. She had no fever throughout this period. High resolution CT of the chest revealed bilateral patchy ground glass opacification as well as basal atelectasis .
Blood gases revealed severe hypoxemia, cultures were sterile, fungal and viral serologies were negative and patient had remained afebrile throughout this episode. A possibility of drug induced lung injury was kept. On the basis of clinical criteria devised by Searles and Mckendry the patient was diagnosed with methotrexate induced lung injury.[1] She was managed with intravenous and inhaled corticosteroids in addition to inhaled bronchodilators. She improved with this management and was taken off oxygen after two weeks. Due to methotrexate toxicity further high dose chemotherapy was deferred and she was advised to proceed with radiation therapy. She was put on temozolomide therapy post whole brain radiotherapy. She remains on close follow- up one year later. Serial PET CT scans have shown remission of lymphoma and no recurrence after one year. No progression of lung injury or any fibrotic changes have been seen.

Figure 1. Figure showing normal tracheobronchial tree, vessels and normal lung parenchyma.

Figure 2.

Figure 3.

Figure 4.
Figure 2,3,4. HRCT chest [after giving methotrexate], showing normal tracheobronchial tree, vessels but lung parenchymal changes in the form of b/l patchy ground glass opacities with b/l basal atelectasis.
Discussion.
Methotrexate is an antimetabolite acting via folate inhibition. Therefore, it acts on tissues which have high rates of cellular division such as skin, gastrointestinal tract, liver and bone marrow. It is highly teratogenic and is classified as a category X drug in pregnancy. The effect of methotrexate on the lungs is less well studied. The spectrum of pulmonary toxicities involves hypersensitivity pneumonitis,[2] interstitial fibrosis, pulmonary edema,[3] bronchitis with airway hyperreactivity, bronchiolitis obliterans with organizing pneumonia (BOOP) and pulmonary nodules.[4] The symptoms are nonspecific and include nonproductive cough, shortness of breath, chest pain and vague constitutional symptoms. The time of onset of symptoms after administration of methotrexate is highly variable.The injury does not appear to be dependent upon the dose. Cases have been reported where injury has occurred with doses as low as 7.5 mg to as high as 3600 mg. Even a single dose of methotrexate has been known to cause lung injury.The mechanism of lung injury has not been studied in detail however few studies have shown that cytokine modulation via P38-MAP kinase pathway may play an important role.[5] It was also thought that polymorphism in the methylenetetrahydrofolate genes may be linked with methotrexate toxicity. Some studies show that polymorphism of the C677T methylenetetrahydrofolate gene is associated with increased toxicity of methotrexate while others do not.[6,7] However a few more studies are required to prove the association between methylenetetrahydrofolate polymorphism and methotrexate toxicity. As most patients receiving methotrexate are also susceptible to infections due to their underlying disease, investigation for infectious causes of lung injury should be done in all patients. Bronchoalveolar lavage is important to rule out other infective etiologies however the findings in methotrexate induced lung injury are nonspecific and reveal a lymphocytic infiltrate. Radiographic findings reveal bilateral alveolar and interstitial infiltrate which are common in bibasilar regions but may be unremarkable.[8,9,10] Lung biopsy reveals the presence of lymphocytes although occasional neutrophilic infiltrates may also be present.[11] Eosinophilia, hyperplasia of Type 2 pneumocytes and interstitial fibrosis have also been described. Due to the absence of specific clinical, radiological and pathological findings, it is important to have a high index of suspicion and rule out other more common causes. Several criteria have been devised by Carson, Searle and Kremer (Table 1). Treatment involves withdrawal of methotrexate and administration of steroids. Spontaneous resolution despite continuation of the drug has also been described.[12] Restarting methotrexate may lead to recurrence of lung injury although successful reintroduction of oral methotrexate has been described in two cases.[13] Leucovorin rescue does not protect against methotrexate induced lung damage. Most patients have an uneventful recovery following lung injury,[14] however, some may have persistent lung damage causing fibrosis and restrictive lung disease.

Table 1.
References
- Searle
G, McKendry RJR: Methotrexate pneumonitis in rheumatoid arthritis:
Potential risk factors. Four case reports and a review of the
literature. J Rheumatol 1987, 14:1164-1171 PMid:3325643

- Katzenstein AA, Askin FB: Immunologic lung disease. In Surgical Pathology of Non-Neoplastic Lung Disease. Philadelphia, WB Saunders, 1982: 108-138
- Bernstein ML, Sobel DB, Wimmer RS:
Noncardiogenic pulmonary edema following injection of methotrexate into
the cerebrospinal fluid. Cancer 1982, 50:866-868 http://dx.doi.org/10.1002/1097-0142(19820901)50:5<866::AID-CNCR2820500510>3.0.CO;2-6

- Alarcon GS, Koopman WJ, McCarty MJ:
Nonperipheral accelerated nodulosis in a methotrexate-treated
rheumatoid arthritis patient. Arthritis Rheum1993, 36:132-133 http://dx.doi.org/10.1002/art.1780360121

- Kim YJ, Song M, Ryu JC:Inflammation in
methotrexate-induced pulmonary toxicity occurs via the p38 MAPK
pathway. Toxicology. 2009 256(3):183-190 http://dx.doi.org/10.1016/j.tox.2008.11.016 PMid:19100307

- Ongaro A, De Mattei M, Della Porta MG,
Rigolin G, Ambrosio C, Di Raimondo F, Pellati A, Masieri FF, Caruso A,
Catozzi L, Gemmati D: Gene polymorphisms in folate metabolizing enzymes
in adult acute lymphoblastic leukemia: effects on methotrexate-related
toxicity and survival Haematologica. 2009;94(10):1391-1398. http://dx.doi.org/10.3324/haematol.2009.008326 PMid:19648163 PMCid:2754955

- Taşbaş O, Borman P, Gürhan Karabulut H,
Tükün A, Yorgancıoğlu R: The Frequency of A1298C and C677T
Polymorphisms of the Methylentetrahydrofolate Gene in Turkish Patients
with Rheumatoid Arthritis: Relationship with Methotrexate Toxicity Open
Rheumatol J. 2011;5:30-35

- Cannon GW, Ward JR, Clegg DO, Samuelson CO
Jr, Abbott TM: Acute lung disease associated with low-dose pulse
methotrexate therapy in patients with rheumatoid arthritis. Arthritis
Rheum1983, 26:1269-1274. http://dx.doi.org/10.1002/art.1780261015

- Carson CW, Cannon GW, Egger MJ,Ward JR,
Clegg DO: Pulmonary disease during the treatment of rheumatoid
arthritis with low dose pulse methotrexate. Semin Arthritis Rheum, 1987
16:186-195. http://dx.doi.org/10.1016/0049-0172(87)90021-7

- St. Clair EW, Rice JR, Snyderman R:
Pneumonitis complicating low-dose methotrexate therapy in rheumatoid
arthritis. Arch Intern Med 145:2035-2038, 1985 http://dx.doi.org/10.1001/archinte.1985.00360110105023 PMid:4062454

- Katzenstein AA, Askin FB: Immunologic lung disease. In Surgical Pathology of Non-Neoplastic Lung Disease. Philadelphia, WB Saunders, 1982, pp 108-138
- Clarysse AM, Cathey WJ, Cartwright
GE,Wintrobe MM: Pulmonary disease complicating intermittent therapy
with methotrexate. JAMA 1969, 209:1861-1864 http://dx.doi.org/10.1001/jama.1969.03160250017003

- Cook NJ, Carroll GJ: Successful
reintroduction of methotrexate after pneumonitis in two patients with
rheumatoid arthritis. Ann Rheum Dis 1992, 51:272-274 http://dx.doi.org/10.1136/ard.51.2.272 PMid:1290480 PMCid:1005673

- Hargreaves MR, Mowat AG, Benson MK: Acute
pneumonitis associated with low dose methotrexate treatment for
rheumatoid arthritis: Report of five cases and review of published
reports. Thorax 1992, 47:628-633 http://dx.doi.org/10.1136/thx.47.8.628 PMid:1412121 PMCid:463926
