JC Virus Leuko-Encephalopathy in Reduced Intensity Conditioning Cord Blood Transplant Recipient with a Review of the Literature
Jean El-Cheikh1,2, Sabine Fürst1,2, Francois Casalonga3, Roberto Crocchiolo1,2, Luca Castagna1,2, Angela Granata1,2, Claire Oudin1,2, Catherine Faucher1,2, Pierre Berger4, Anthony Sarran3 and Didier Blaise1,2
1Unité de Transplantation et de Thérapie Cellulaire (U2T), Institut Paoli-Calmettes, Marseille, France.
2Département d’Onco-Hématologie, Institut Paoli-Calmettes, Marseille, France.
3Département de Radiologie, Institut Paoli-Calmettes, Marseille, France.
4Département de Microbiologie, Institut Paoli-Calmettes, Marseille, France.
2Département d’Onco-Hématologie, Institut Paoli-Calmettes, Marseille, France.
3Département de Radiologie, Institut Paoli-Calmettes, Marseille, France.
4Département de Microbiologie, Institut Paoli-Calmettes, Marseille, France.
Correspondence
to:
Jean El Cheikh, M.D., Unité de Transplantation et de Thérapie
Cellulaire (U2T), Institut Paoli-Calmettes, 232 Boulevard Ste
Marguerite, 13273 Marseille Cedex 09, France. Tel.: +33 491 223823;
Fax: +33 491 223579. E-Mail: elcheikhj@marseille.fnclcc.fr
Published: June 20, 2012
Received: April 30, 2012
Accepted: June 10, 2012
Mediterr J Hematol Infect Dis 2012, 4(1): e2012043, DOI 10.4084/MJHID.2012.043
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
We
report here the case of progressive multifocal leuko-encephalopathy
(PML) related to human polyomavirus JC (JCV) infection after an
allogeneic transplantation with umbilical cord blood cells in
59-year-old woman with follicular Non Hodgkin lymphoma. She presented
with dysphagia and weakness; magnetic resonance imaging demonstrated
marked signal abnormality in the sub-cortical white matter of the left
frontal lobe and in the posterior limb of the right internal capsule.
Polymerase chain reaction (PCR) analysis of the cerebrospinal fluid
(CSF) was positive for John Cunningham (JC) virus. JC viral DNA in the
CSF was positive, establishing the diagnosis of PML. Brain biopsy was
not done. Extensive investigations for other viral infections seen in
immuno-compromised patients were negative. The patient's neurologic
deficits rapidly increased throughout her hospital stay, and she died
one month after the diagnosis. These findings could have practical
implications and demonstrate that in patients presenting neurological
symptoms and radiological signs after UCBT, the JCV encephalitis must
be early suspected.
Introduction.
Progressive multifocal leuko-encephalopathy (PML) is a rapidly progressive demyelinising disorder of the central nervous system almost exclusively encountered in immuno-compromised individuals.[1-4] It is caused by reactivation of the John Cunningham virus (JCV) under conditions of cellular immuno-compromise such as those encountered in patients with acquired immunodeficiency syndrome (AIDS), patients with hematologic and solid organ malignancies receiving chemotherapy, and transplant recipients under immuno-suppression.[1,2] The interest in this disease has recently increased because of its association with natalizumab, a monoclonal antibody directed against α4 integrins that is used to treat Crohn's disease[3,4] and multiple sclerosis.[5-7] Presently, there is no available structured series of JCV encephalitis in literature regarding allogeneic umbilical cord blood transplantation (UCBT), but just anecdotal cases have been reported. Although, there is no universally effective antiviral therapy against JCV and outcome is fatal in the majority of cases.
We hereby describe a rare case of PML, developing after UCBT and provide a comprehensive review of the literature in order to better define the epidemiological, clinical and therapeutic findings of this rare complication.
Case report.
Here, we describe a rare occurrence of PML, with a rapidly fatal outcome, in 59-year-old woman who underwent UCBT for Follicular Non Hodgkin Lymphoma in January 2011; she was in complete remission (CR) confirmed by the PET scan after 5 lines of treatment. At transplantation, no organ dysfunction was present.
The reduced intensity conditioning (RIC) regimen consisted of total body irradiation (2 Gys in 1 fraction), Fludarabine at 40 mg/m2/day for 5 days, and Cyclophosphamide at 50mg/Kg/day. A total nucleated cell dose of 3.3 107/kg body weight was infused on day 0. The cord blood unit and the patient were HLA matched 4/6 with mismatch of locus A and B. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine A at 3 mg/kg/day, and Mycophenolate Mofetyl (MMF) at 500mgx4/day, beginning on day -3. Neutrophil engraftment (>0.5 x 109/L) occurred on day 26.
On day 35, the patient presented a skin grade 2 acute GVHD reaction, without other organ dysfunction, and 2 mg/kg methyl-prednisolone was started; a rapid clinically response was observed. On day 51, the patient developed CMV reactivation detected by quantitative polymerase chain reaction (PCR) in the peripheral blood with 1359 copies; and an antiviral treatment with Gancyclovir (Cymevan) 2.5 mg/kg/day was administrated after adaptation with her renal function.
On day 68, at the time of her outpatient visit, she developed confusion, short-term memory dysfunction, and altered mental status, with focal signs and abnormal Babinsky reflexes on the left, and lack of the force on the thighs lower limbs. A brain computed tomography (CT) scan demonstrated no specific findings. The patient was described as cachectic, alert, awake, and oriented to person, time, and place. Her speech was fluent; comprehension, naming, and repetition were intact. Examination of the cranial nerves showed mild drooping of the left angle of the mouth; all other cranial nerves were intact. Motor strength was 4+/5 on the left symmetrically in the upper and lower extremities and 5-/5 in the right extremities. Deep tendon reflexes were hyperactive, but no extensor plantar responses were recorded. The complete blood count and chemistry levels were normal, except for positive schizocytes. Blood urea nitrogen and serum creatinine levels were 15.9 mmol/L and 179 µmol/L, respectively. The diagnosis of thrombotic microangiopathy was made in the presence of haemolytic anemia, thrombocytopenia, renal failure and presence of schizocytes in biological assessment. The patient received corticotherapy at 1mg/kg/day, and 5 plasma exchanges were made. The cyclosporine was discontinued and MMF treatment was continued. We assisted rapidly to a response of her haemolytic anemia, improved thrombocytopenia but without improvement of her neurological troubles. With worsening mental status on day 83, a magnetic resonance imaging (MRI) of the brain after gadolinium injection showed a non-enhancing hypo-intensity Image in (T1- sequence) beyond the white matter.
However, after gadolinium injection showed an image in the axial (FLAIR sequence) showing hyper-intensity lesions in the white matter of the frontal lobes. There is no signal abnormality of the cortex. Note that there is no mass effect on the ventricular cavities or midline structures.
Furthermore the MRI in B1000 diffusion sequence showed a restriction of diffusion in the damage areas of the white matter.
On day 84, the patient’s mental status was stable, and a lumbar cerebrospinal fluid (CSF) examination revealed a white blood cell (WBC) count of 1 cells/L, a normal Glucose level of 3.89 mmol/L and a normal protein level of 389mg/L. The mycological (aspergillosis, Cryptococcus), and Gram staining and culture were negative. A specimen was sent for polymerase chain reaction (PCR) analysis for JC virus was positive in the CSF and the serum (negative for CMV, VZV, HHV-6, EBV and herpes simplex virus by PCR). A treatment with 5 mg/kg/week of Vistid (Cidofovir) was initiated on day 86 associated with Mefloquine (Lariam®) and Mirtazapine (Norset®). It was around this time that the patient’s general condition began to worsen. Acute deterioration of cognitive function occurred with confusion and autism. Because of CR of her GVHD; steroids were tapered and ultimately discontinued on day 97. An electroencephalogram demonstrated mild bi-frontal slowing, with diffuse brain damage, more prominent on the left than the right.
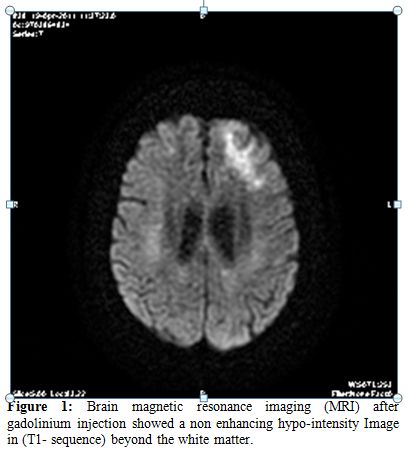
Figure 1. Brain magnetic resonance imaging (MRI) after gadolinium injection showed a non enhancing hypo-intensity Image in (T1- sequence) beyond the white matter.
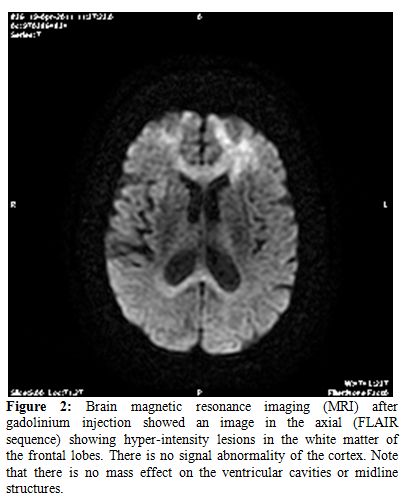
Figure 2.Brain magnetic resonance imaging (MRI) after gadolinium injection showed an image in the axial (FLAIR sequence) showing hyper-intensity lesions in the white matter of the frontal lobes. There is no signal abnormality of the cortex. Note that there is no mass effect on the ventricular cavities or midline structures.
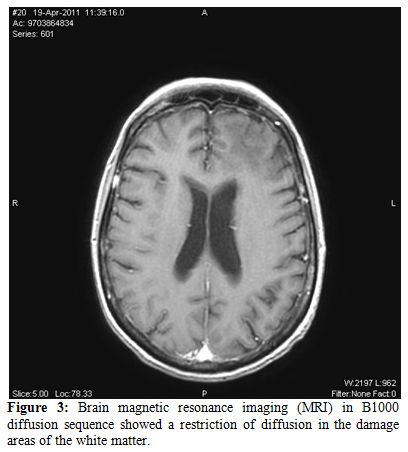
Figure 3.Brain magnetic resonance imaging (MRI) in B1000 diffusion sequence showed a restriction of diffusion in the damage areas of the white matter.
Re-examination of CSF on day 93 revealed a WBC count of 3 cells/mL, a protein level of 323 mg/L, Glucose level of 5.08 mmol/L. JCV was positive in CSF by PCR. HHV- 6, CMV, HSV, VZV, EBV, enterovirus were negatives by qualitative PCR assay. PCR of the CSF for JCV conducted in the same laboratory as previously was positive, establishing the diagnosis.
Throughout her hospital stay, the patient's neurologic condition continued to deteriorate. Her course was marked by an episode of altered mental status, progressive dementia, motor weakness, declining visual acuity and worsening performance status. In the light of clinical deterioration no further exams was performed in respect to the family’s wish for no further intervention. She was continued to a skilled nursing facility in our unit, where she died one month after the diagnosis of PML, on day 110 after UCBT.
Unfortunately we did not perform a neuropathologic examination of the brain by autopsy in respect to the family’s wish.
Discussion.
PML was originally described in 1958 in two patients with chronic lymphocytic leukemia and one with Hodgkin lymphoma.[8] The causative agent, the JC virus, was isolated in 1971 from the brain of a patient with Hodgkin's disease, and the virus was named after him.[9] With the advent of the HIV epidemic, PML was recognized as a major opportunistic infection of AIDS[10,11] but with effective antiretroviral therapy, its incidence and attributable mortality rates have decreased.[12,13]
Most recently, interest in PML has been described by its association with natalizumab (Tysabri®), a promising new drug for the treatment of multiple sclerosis and Crohn's disease.[1,12,14] Other groups in whom PML has been described are patients with chronic lymphoid leukemia treated in particular with fludarabine.[15] The occurrence of PML in non immuno-compromised patients is exceedingly rare. The clinical picture of the disease is known, as well as the microbiological investigations to be performed on CSF. On the contrary we have few experiences about therapy.
However, to our knowledge, the number of reports of JCV encephalitis on this issue is not numerous and there exist only a few histopathologic descriptions. Although older age has been associated with idiopathic CD4+ lymphocytopenia,[16] and the latter has been associated with PML,[17] our patient did not have a control for CD4+ lymphocytopenia. With her lymphocyte count ranging between 200 and 1300 lymphocytes per microliter, however, it is possible that she had transient CD4+ lymphocytopenia. The association of aging with CD4+ lymphocytopenia[18,19] and the high JCV sero prevalence in adults[20] should increase awareness about the possible diagnosis of PML in the appropriate clinical and radiographic setting.
JC virus disease is a rare infectious complication after allo-SCT.[21] Studies have shown that HHV-6 infection occurs frequently in adult patients after UCBT,[22] and that UCBT is a risk factor for HHV-6 encephalitis.[22] There are no data in the literature on the frequency of JCV after UCBT.
Several reports have indicated that the CNS CMV infection in stem cell transplant patients was associated with HHV-6 virus encephalitis, in that there is evidence of high-level viremia, retinitis, or extra-neural involvement.[22-23]
Negative PCR results for JCV-DNA in the CSF have been described in patients with AIDS,[20,22] and a correlation with active antiretroviral treatment has been hypothesized.[22] The fact that a negative PCR result for JCV-DNA cannot completely exclude PML has raised the need for a new consensus terminology, in which immuno-suppressed patients with clinical and radiographic features consistent with PML and no other etiology should be considered as "possible PML".[22]
In contrast, our patient and 2 others described in the literature[24] had relatively low levels of virus loads in the peripheral blood and no clinical evidence of invasive JC virus disease outside the brain. While strategies to reverse immunodeficiency, such as discontinuation of immunosuppressive therapy, institution of antiretroviral therapy in HIV-positive patients,[22] plasma exchange and immuno-adsorption in natalizumab treated patients,[26,27] work well in certain groups. Cytarabine,[22,28,29] Cidofovir,[22,30,31] Topotecan[32] and Mirtazapine[33] have been investigated as therapeutic agents, mostly in patients with AIDS, with variable results and toxicities. A study to investigate the effects of Mefloquine in PML is currently ongoing.[34]
Recently, Fianchi L. et al. described an atypical presentation of PML in a multiple myeloma patient after auto-SCT successfully treated with i.v. immunoglobulin in addition to the combination therapy.[35] A synergistic effect in this case could be hypothesized; with the immunomodulation and perhaps re-myelination by i.v. Immunoglobulin, block of viral cell entry by 5-HT2a receptor antagonist and inhibition of viral replication in cells by Mefloquine. This seemed to be an attractive option; however, the value of this approach remains to be determined in clinical trials.
In addition to the significant immune dysfunction associated with UCBT and high-dose corticosteroid treatment poor drug penetration to the CNS and low dosage of Gancyclovir and Cidofovir likely contributed to treatment failure in this case. Although no resistance data are available on this virus. Patients with delayed immune reconstitution and persistently low CD4 count may be at high risk for JCV encephalitis, in particular following augmentation of immuno-suppression for treatment of GVHD or in patients treated with rituximab (anti CD20).[36,37] The early occurrence of encephalitis after UCBT may have an impact in association with microbiological and MRI findings. Interestingly, our patient developed JCV encephalitis following an episode of CMV reactivation and thrombotic microangiopathy syndrome.[38,39] Several investigators have reported that JCV is associated with increased risk for invasive and symptomatic CMV disease.[40,41] Although the most important risk factor of JCV encephalitis in our patient was her severely immuno-compromised status it is possible that the antecedent episode of CMV infection, the previous acute GVHD and the thrombotic microangiopathy, contributed to the fatal outcome of this patient. Early diagnosis of PML is, however, equally crucial to start adequate therapy before irreversible neurological damage has occurred.
In conclusion, these findings of JC virus encephalitis following RIC UCBT could have practical implications in patients presenting neurological symptoms and radiological signs after UCBT, the JCV encephalitis must be early suspected. Unfortunately inadequate drug penetration and the multi antiviral resistance as well as the severely immuno-suppressed state of this patient may have contributed to treatment failure.
Funding.
We would like to thank the Association pour la recherche sur le Cancer (ARC) (Pole ARECA) for their generous support of our research. Our group is supported by several grants from the French Ministry of Health as part of the Programme Hospitalier de Recherche Clinique (PHRC).
Acknowledgments.
We thank the nursing staff for providing excellent care for our patients and the physicians of the Hematology Department at the Institut Paoli-Calmettes for their important study contributions and dedicated patient care.
Progressive multifocal leuko-encephalopathy (PML) is a rapidly progressive demyelinising disorder of the central nervous system almost exclusively encountered in immuno-compromised individuals.[1-4] It is caused by reactivation of the John Cunningham virus (JCV) under conditions of cellular immuno-compromise such as those encountered in patients with acquired immunodeficiency syndrome (AIDS), patients with hematologic and solid organ malignancies receiving chemotherapy, and transplant recipients under immuno-suppression.[1,2] The interest in this disease has recently increased because of its association with natalizumab, a monoclonal antibody directed against α4 integrins that is used to treat Crohn's disease[3,4] and multiple sclerosis.[5-7] Presently, there is no available structured series of JCV encephalitis in literature regarding allogeneic umbilical cord blood transplantation (UCBT), but just anecdotal cases have been reported. Although, there is no universally effective antiviral therapy against JCV and outcome is fatal in the majority of cases.
We hereby describe a rare case of PML, developing after UCBT and provide a comprehensive review of the literature in order to better define the epidemiological, clinical and therapeutic findings of this rare complication.
Case report.
Here, we describe a rare occurrence of PML, with a rapidly fatal outcome, in 59-year-old woman who underwent UCBT for Follicular Non Hodgkin Lymphoma in January 2011; she was in complete remission (CR) confirmed by the PET scan after 5 lines of treatment. At transplantation, no organ dysfunction was present.
The reduced intensity conditioning (RIC) regimen consisted of total body irradiation (2 Gys in 1 fraction), Fludarabine at 40 mg/m2/day for 5 days, and Cyclophosphamide at 50mg/Kg/day. A total nucleated cell dose of 3.3 107/kg body weight was infused on day 0. The cord blood unit and the patient were HLA matched 4/6 with mismatch of locus A and B. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine A at 3 mg/kg/day, and Mycophenolate Mofetyl (MMF) at 500mgx4/day, beginning on day -3. Neutrophil engraftment (>0.5 x 109/L) occurred on day 26.
On day 35, the patient presented a skin grade 2 acute GVHD reaction, without other organ dysfunction, and 2 mg/kg methyl-prednisolone was started; a rapid clinically response was observed. On day 51, the patient developed CMV reactivation detected by quantitative polymerase chain reaction (PCR) in the peripheral blood with 1359 copies; and an antiviral treatment with Gancyclovir (Cymevan) 2.5 mg/kg/day was administrated after adaptation with her renal function.
On day 68, at the time of her outpatient visit, she developed confusion, short-term memory dysfunction, and altered mental status, with focal signs and abnormal Babinsky reflexes on the left, and lack of the force on the thighs lower limbs. A brain computed tomography (CT) scan demonstrated no specific findings. The patient was described as cachectic, alert, awake, and oriented to person, time, and place. Her speech was fluent; comprehension, naming, and repetition were intact. Examination of the cranial nerves showed mild drooping of the left angle of the mouth; all other cranial nerves were intact. Motor strength was 4+/5 on the left symmetrically in the upper and lower extremities and 5-/5 in the right extremities. Deep tendon reflexes were hyperactive, but no extensor plantar responses were recorded. The complete blood count and chemistry levels were normal, except for positive schizocytes. Blood urea nitrogen and serum creatinine levels were 15.9 mmol/L and 179 µmol/L, respectively. The diagnosis of thrombotic microangiopathy was made in the presence of haemolytic anemia, thrombocytopenia, renal failure and presence of schizocytes in biological assessment. The patient received corticotherapy at 1mg/kg/day, and 5 plasma exchanges were made. The cyclosporine was discontinued and MMF treatment was continued. We assisted rapidly to a response of her haemolytic anemia, improved thrombocytopenia but without improvement of her neurological troubles. With worsening mental status on day 83, a magnetic resonance imaging (MRI) of the brain after gadolinium injection showed a non-enhancing hypo-intensity Image in (T1- sequence) beyond the white matter.
However, after gadolinium injection showed an image in the axial (FLAIR sequence) showing hyper-intensity lesions in the white matter of the frontal lobes. There is no signal abnormality of the cortex. Note that there is no mass effect on the ventricular cavities or midline structures.
Furthermore the MRI in B1000 diffusion sequence showed a restriction of diffusion in the damage areas of the white matter.
On day 84, the patient’s mental status was stable, and a lumbar cerebrospinal fluid (CSF) examination revealed a white blood cell (WBC) count of 1 cells/L, a normal Glucose level of 3.89 mmol/L and a normal protein level of 389mg/L. The mycological (aspergillosis, Cryptococcus), and Gram staining and culture were negative. A specimen was sent for polymerase chain reaction (PCR) analysis for JC virus was positive in the CSF and the serum (negative for CMV, VZV, HHV-6, EBV and herpes simplex virus by PCR). A treatment with 5 mg/kg/week of Vistid (Cidofovir) was initiated on day 86 associated with Mefloquine (Lariam®) and Mirtazapine (Norset®). It was around this time that the patient’s general condition began to worsen. Acute deterioration of cognitive function occurred with confusion and autism. Because of CR of her GVHD; steroids were tapered and ultimately discontinued on day 97. An electroencephalogram demonstrated mild bi-frontal slowing, with diffuse brain damage, more prominent on the left than the right.

Figure 1. Brain magnetic resonance imaging (MRI) after gadolinium injection showed a non enhancing hypo-intensity Image in (T1- sequence) beyond the white matter.

Figure 2.Brain magnetic resonance imaging (MRI) after gadolinium injection showed an image in the axial (FLAIR sequence) showing hyper-intensity lesions in the white matter of the frontal lobes. There is no signal abnormality of the cortex. Note that there is no mass effect on the ventricular cavities or midline structures.

Figure 3.Brain magnetic resonance imaging (MRI) in B1000 diffusion sequence showed a restriction of diffusion in the damage areas of the white matter.
Re-examination of CSF on day 93 revealed a WBC count of 3 cells/mL, a protein level of 323 mg/L, Glucose level of 5.08 mmol/L. JCV was positive in CSF by PCR. HHV- 6, CMV, HSV, VZV, EBV, enterovirus were negatives by qualitative PCR assay. PCR of the CSF for JCV conducted in the same laboratory as previously was positive, establishing the diagnosis.
Throughout her hospital stay, the patient's neurologic condition continued to deteriorate. Her course was marked by an episode of altered mental status, progressive dementia, motor weakness, declining visual acuity and worsening performance status. In the light of clinical deterioration no further exams was performed in respect to the family’s wish for no further intervention. She was continued to a skilled nursing facility in our unit, where she died one month after the diagnosis of PML, on day 110 after UCBT.
Unfortunately we did not perform a neuropathologic examination of the brain by autopsy in respect to the family’s wish.
Discussion.
PML was originally described in 1958 in two patients with chronic lymphocytic leukemia and one with Hodgkin lymphoma.[8] The causative agent, the JC virus, was isolated in 1971 from the brain of a patient with Hodgkin's disease, and the virus was named after him.[9] With the advent of the HIV epidemic, PML was recognized as a major opportunistic infection of AIDS[10,11] but with effective antiretroviral therapy, its incidence and attributable mortality rates have decreased.[12,13]
Most recently, interest in PML has been described by its association with natalizumab (Tysabri®), a promising new drug for the treatment of multiple sclerosis and Crohn's disease.[1,12,14] Other groups in whom PML has been described are patients with chronic lymphoid leukemia treated in particular with fludarabine.[15] The occurrence of PML in non immuno-compromised patients is exceedingly rare. The clinical picture of the disease is known, as well as the microbiological investigations to be performed on CSF. On the contrary we have few experiences about therapy.
However, to our knowledge, the number of reports of JCV encephalitis on this issue is not numerous and there exist only a few histopathologic descriptions. Although older age has been associated with idiopathic CD4+ lymphocytopenia,[16] and the latter has been associated with PML,[17] our patient did not have a control for CD4+ lymphocytopenia. With her lymphocyte count ranging between 200 and 1300 lymphocytes per microliter, however, it is possible that she had transient CD4+ lymphocytopenia. The association of aging with CD4+ lymphocytopenia[18,19] and the high JCV sero prevalence in adults[20] should increase awareness about the possible diagnosis of PML in the appropriate clinical and radiographic setting.
JC virus disease is a rare infectious complication after allo-SCT.[21] Studies have shown that HHV-6 infection occurs frequently in adult patients after UCBT,[22] and that UCBT is a risk factor for HHV-6 encephalitis.[22] There are no data in the literature on the frequency of JCV after UCBT.
Several reports have indicated that the CNS CMV infection in stem cell transplant patients was associated with HHV-6 virus encephalitis, in that there is evidence of high-level viremia, retinitis, or extra-neural involvement.[22-23]
Negative PCR results for JCV-DNA in the CSF have been described in patients with AIDS,[20,22] and a correlation with active antiretroviral treatment has been hypothesized.[22] The fact that a negative PCR result for JCV-DNA cannot completely exclude PML has raised the need for a new consensus terminology, in which immuno-suppressed patients with clinical and radiographic features consistent with PML and no other etiology should be considered as "possible PML".[22]
In contrast, our patient and 2 others described in the literature[24] had relatively low levels of virus loads in the peripheral blood and no clinical evidence of invasive JC virus disease outside the brain. While strategies to reverse immunodeficiency, such as discontinuation of immunosuppressive therapy, institution of antiretroviral therapy in HIV-positive patients,[22] plasma exchange and immuno-adsorption in natalizumab treated patients,[26,27] work well in certain groups. Cytarabine,[22,28,29] Cidofovir,[22,30,31] Topotecan[32] and Mirtazapine[33] have been investigated as therapeutic agents, mostly in patients with AIDS, with variable results and toxicities. A study to investigate the effects of Mefloquine in PML is currently ongoing.[34]
Recently, Fianchi L. et al. described an atypical presentation of PML in a multiple myeloma patient after auto-SCT successfully treated with i.v. immunoglobulin in addition to the combination therapy.[35] A synergistic effect in this case could be hypothesized; with the immunomodulation and perhaps re-myelination by i.v. Immunoglobulin, block of viral cell entry by 5-HT2a receptor antagonist and inhibition of viral replication in cells by Mefloquine. This seemed to be an attractive option; however, the value of this approach remains to be determined in clinical trials.
In addition to the significant immune dysfunction associated with UCBT and high-dose corticosteroid treatment poor drug penetration to the CNS and low dosage of Gancyclovir and Cidofovir likely contributed to treatment failure in this case. Although no resistance data are available on this virus. Patients with delayed immune reconstitution and persistently low CD4 count may be at high risk for JCV encephalitis, in particular following augmentation of immuno-suppression for treatment of GVHD or in patients treated with rituximab (anti CD20).[36,37] The early occurrence of encephalitis after UCBT may have an impact in association with microbiological and MRI findings. Interestingly, our patient developed JCV encephalitis following an episode of CMV reactivation and thrombotic microangiopathy syndrome.[38,39] Several investigators have reported that JCV is associated with increased risk for invasive and symptomatic CMV disease.[40,41] Although the most important risk factor of JCV encephalitis in our patient was her severely immuno-compromised status it is possible that the antecedent episode of CMV infection, the previous acute GVHD and the thrombotic microangiopathy, contributed to the fatal outcome of this patient. Early diagnosis of PML is, however, equally crucial to start adequate therapy before irreversible neurological damage has occurred.
In conclusion, these findings of JC virus encephalitis following RIC UCBT could have practical implications in patients presenting neurological symptoms and radiological signs after UCBT, the JCV encephalitis must be early suspected. Unfortunately inadequate drug penetration and the multi antiviral resistance as well as the severely immuno-suppressed state of this patient may have contributed to treatment failure.
Funding.
We would like to thank the Association pour la recherche sur le Cancer (ARC) (Pole ARECA) for their generous support of our research. Our group is supported by several grants from the French Ministry of Health as part of the Programme Hospitalier de Recherche Clinique (PHRC).
Acknowledgments.
We thank the nursing staff for providing excellent care for our patients and the physicians of the Hematology Department at the Institut Paoli-Calmettes for their important study contributions and dedicated patient care.
References
- Koralnik IJ. New insights into progressive multifocal leukoencephalopathy. Curr Opin. Neurol. 2004; 17: 365-370. http://dx.doi.org/10.1097/00019052-200406000-00019 PMid:15167073

- Vaklavas C, Sotelo-Rafiq EP, Lovy J,
Escobar MA, Tsimberidou AM. Progressive multifocal leukoencephalopathy
in a patient without apparent immunosuppression. Virol. J 2010; 7: 256.
http://dx.doi.org/10.1186/1743-422X-7-256 PMid:20920200 PMCid:2954859

- Bellizzi A, Barucca V, Fioriti D, Colosimo
MT, Mischitelli M, Anzivino E et al. Early years of biological agents
therapy in Crohn's disease and risk of the human polyomavirus JC
reactivation. J Cell Physiol 2010; 224: 316-326. http://dx.doi.org/10.1002/jcp.22146 PMid:20432445

- Bellizzi A, Barucca V, Di NG, Fioriti F,
Iebba V, Schippa S et al. JC Viral reactivation in a pediatric patient
with Crohn's disease. Int J Immunopathol. Pharmacol. 2010; 23: 955-959.
PMid:20943069

- Achiron A, Miron S, Shoenfeld Y. Does the
flap of a butterfly's wings in Brazil set off a tornado in Texas?--The
JC Virus Story in multiple sclerosis. Isr. Med Assoc. J 2005; 7:
283-285. PMid:15909458

- Agostini HT, Ryschkewitsch CF, Singer EJ,
Stoner GL. Co-infection with two JC virus genotypes in brain,
cerebrospinal fluid or urinary tract detected by direct cycle
sequencing of PCR products. J Neurovirol. 1996; 2: 259-267. http://dx.doi.org/10.3109/13550289609146889 PMid:8799217

- Alvarez-Lafuente R, Garcia-Montojo M, de
LH, V, Bartolome M, Arroyo R. JC virus in cerebrospinal fluid samples
of multiple sclerosis patients at the first demyelinating event. Mult.
Scler. 2007; 13: 590-595. http://dx.doi.org/10.1177/1352458506073116 PMid:17548437

- ASTROM KE, MANCALL EL, RICHARDSON EP, Jr.
Progressive multifocal leuko-encephalopathy; a hitherto unrecognized
complication of chronic lymphatic leukaemia and Hodgkin's disease.
Brain 1958; 81: 93-111. PMid:13523006

- Padgett BL, Walker DL, ZuRhein GM, Eckroade
RJ, Dessel BH. Cultivation of papova-like virus from human brain with
progressive multifocal leucoencephalopathy. Lancet 1971; 1: 1257-1260. http://dx.doi.org/10.1016/S0140-6736(71)91777-6

- Berger JR, Chauhan A, Galey D, Nath A.
Epidemiological evidence and molecular basis of interactions between
HIV and JC virus. J Neurovirol. 2001; 7: 329-338. http://dx.doi.org/10.1080/13550280152537193 PMid:11517412

- Berger JR, Houff S. Progressive multifocal
leukoencephalopathy: lessons from AIDS and natalizumab. Neurol. Res
2006; 28: 299-305. http://dx.doi.org/10.1179/016164106X98198 PMid:16687057

- Khanna N, Wolbers M, Mueller NJ, Garzoni
C, Du Pasquier RA, Fux CA et al. JC virus-specific immune responses in
human immunodeficiency virus type 1 patients with progressive
multifocal leukoencephalopathy. J Virol. 2009; 83: 4404-4411. http://dx.doi.org/10.1128/JVI.02657-08 PMid:19211737 PMCid:2668450

- Padgett BL, Walker DL, ZuRhein GM,
Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human
brain with progressive multifocal leucoencephalopathy. Lancet 1971; 1:
1257-1260. http://dx.doi.org/10.1016/S0140-6736(71)91777-6

- Koralnik IJ. Progressive multifocal
leukoencephalopathy revisited: Has the disease outgrown its name? Ann
Neurol. 2006; 60: 162-173. http://dx.doi.org/10.1002/ana.20933 PMid:16862584

- D'Souza A, Wilson J, Mukherjee S,
Jaiyesimi I. Progressive multifocal leukoencephalopathy in chronic
lymphocytic leukemia: a report of three cases and review of the
literature. Clin Lymphoma Myeloma Leuk 2010; 10: E1-E9. http://dx.doi.org/10.3816/CLML.2010.n.009

- Rea IM, Alexander HD, Crockard AD, Morris TC. CD4 lymphopenia in very elderly people. Lancet 1996; 347: 328-329. http://dx.doi.org/10.1016/S0140-6736(96)90504-8

- Zonios DI, Falloon J, Bennett JE, Shaw PA,
Chaitt D, Baseler MW et al. Idiopathic CD4+ lymphocytopenia: natural
history and prognostic factors. Blood 2008; 112: 287-294. http://dx.doi.org/10.1182/blood-2007-12-127878 PMid:18456875 PMCid:2442741

- Smith DK, Neal JJ, Holmberg SD.
Unexplained opportunistic infections and CD4+ T-lymphocytopenia without
HIV infection. An investigation of cases in the United States. The
Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task
Force. N. Engl. J Med 1993; 328: 373-379. http://dx.doi.org/10.1056/NEJM199302113280601 PMid:8093633

- Stolt A, Sasnauskas K, Koskela P, Lehtinen
M, Dillner J. Seroepidemiology of the human polyomaviruses. J Gen.
Virol. 2003; 84: 1499-1504. http://dx.doi.org/10.1099/vir.0.18842-0 PMid:12771419

- Khorshid O, de ME, Martin T, Jones RB,
Shpall EJ, Nieto Y et al. Unrelated umbilical cord blood stem cell
transplant after failure of haploidentical or matched unrelated donor
hematopoietic stem cell transplant. Leukemia 2003; 17: 2538-2540. http://dx.doi.org/10.1038/sj.leu.2403163 PMid:14523458

- Weber T, Trebst C, Frye S, Cinque P, Vago
L, Sindic CJ et al. Analysis of the systemic and intrathecal humoral
immune response in progressive multifocal leukoencephalopathy. J
Infect. Dis. 1997; 176: 250-254. http://dx.doi.org/10.1086/514032 PMid:9207375

- Manz HJ, Dinsdale HB, Morrin PA.
Progressive multifocal leukoencephalopathy after renal transplantation.
Demonstration of Papova-like virions. Ann Intern. Med 1971; 75: 77-81.
PMid:4933296

- Van AG, Van RM, Sciot R, Dubois B,
Vermeire S, Noman M et al. Progressive multifocal leukoencephalopathy
after natalizumab therapy for Crohn's disease. N. Engl. J Med 2005;
353: 362-368. PMid:15947080

- Kharfan-Dabaja MA, Ayala E, Greene J,
Rojiani A, Murtagh FR, Anasetti C. Two cases of progressive multifocal
leukoencephalopathy after allogeneic hematopoietic cell transplantation
and a review of the literature. Bone Marrow Transplant. 2007; 39:
101-107. http://dx.doi.org/10.1038/sj.bmt.1705548 PMid:17143300

- Yousry TA, Major EO, Ryschkewitsch C,
Fahle G, Fischer S, Hou J et al. Evaluation of patients treated with
natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J
Med 2006; 354: 924-933. http://dx.doi.org/10.1056/NEJMoa054693 PMid:16510746 PMCid:1934511

- Wenning W, Haghikia A, Laubenberger J,
Clifford DB, Behrens PF, Chan A et al. Treatment of progressive
multifocal leukoencephalopathy associated with natalizumab. N. Engl. J
Med 2009; 361: 1075-1080. http://dx.doi.org/10.1056/NEJMoa0810257 PMid:19741228

- Hall CD, Dafni U, Simpson D, Clifford D,
Wetherill PE, Cohen B et al. Failure of cytarabine in progressive
multifocal leukoencephalopathy associated with human immunodeficiency
virus infection. AIDS Clinical Trials Group 243 Team. N. Engl. J Med
1998; 338: 1345-1351. http://dx.doi.org/10.1056/NEJM199805073381903 PMid:9571254

- De LA, Giancola ML, Cingolani A, Ammassari
A, Gillini L, Murri R et al. Clinical and virological monitoring during
treatment with intrathecal cytarabine in patients with AIDS-associated
progressive multifocal leukoencephalopathy. Clin Infect. Dis. 1999; 28:
624-628. http://dx.doi.org/10.1086/515153 PMid:10194089

- De LA, Giancola ML, Ammassari A, Grisetti
S, Cingolani A, Paglia MG et al. Cidofovir added to HAART improves
virological and clinical outcome in AIDS-associated progressive
multifocal leukoencephalopathy. AIDS 2000; 14: F117-F121. http://dx.doi.org/10.1097/00002030-200009290-00001 PMid:11061646

- De LA, Ammassari A, Pezzotti P, Cinque P,
Gasnault J, Berenguer J et al. Cidofovir in addition to antiretroviral
treatment is not effective for AIDS-associated progressive multifocal
leukoencephalopathy: a multicohort analysis. AIDS 2008; 22: 1759-1767. http://dx.doi.org/10.1097/QAD.0b013e32830a5043 PMid:18753934

- Royal W, III, Dupont B, McGuire D, Chang
L, Goodkin K, Ernst T et al. Topotecan in the treatment of acquired
immunodeficiency syndrome-related progressive multifocal
leukoencephalopathy. J Neurovirol. 2003; 9: 411-419. http://dx.doi.org/10.1080/713831540 PMid:12775425

- Cettomai D, McArthur JC. Mirtazapine use
in human immunodeficiency virus-infected patients with progressive
multifocal leukoencephalopathy. Arch. Neurol. 2009; 66: 255-258. http://dx.doi.org/10.1001/archneurol.2008.557 PMid:19204164

- Study to Explore the Effect of Mefloquine
in Subjects With Progressive Multifocal Leukoencephalopathy (PML).
Pathologe 2011. PMid:12883977

- Zhang DL, Lapeyraque AL, Popon M, Loirat
C, Jacqz-Aigrain E. Pharmacokinetics of ganciclovir in pediatric renal
transplant recipients. Pediatric Nephrology 2003; 18: 943-948. http://dx.doi.org/10.1007/s00467-003-1226-x PMid:20190843

- Fianchi L, Colosimo C, De Luca A, Pompucci
A, Cattani P, Voso MT et al. Atypical presentation of progressive
multifocal leukoencephalopathy in a multiple myeloma patient after
auto-SCT successfully treated with combination therapy. Bone Marrow
Transplant. 2010 Nov;45(11):1668-70. http://dx.doi.org/10.1038/bmt.2010.33 PMid:21225213

- Silva CA, de Oliveira ACP, Vilas-Boas L,
Fink MCDS, Pannuti CS, Vidal JE. Neurologic Cytomegalovirus
Complications in Patients with Aids: Retrospective Review of 13 Cases
and Review of the Literature. Revista do Instituto de Medicina Tropical
de Sao Paulo 2010; 52: 305-310. PMid:21054716

- da Silva RL, Ferreira I, Teixeira G,
Cordeiro D, Mafra M, Costa I et al. BK virus encephalitis with
thrombotic microangiopathy in an allogeneic hematopoietic stem cell
transplant recipient. Transplant Infectious Disease 2011; 13: 161-167. http://dx.doi.org/10.1111/j.1399-3062.2010.00581.x PMid:18090919

- Zu-Rhein GM, Lo SC, Hulette CM, Powers JM.
A novel cerebral microangiopathy with endothelial cell atypia and
multifocal white matter lesions: A direct Mycoplasmal infection?
Journal of Neuropathology and Experimental Neurology 2007; 66:
1100-1117. http://dx.doi.org/10.1097/NEN.0b013e31815c1e09 PMid:15804960

- Eberwein P, Hansen LL, Agostini HT.
Genotypes of JC virus, DNA of cytomegalovirus, and proviral DNA of
human immunodeficiency virus in eyes of acquired immunodeficiency
syndrome patients. Journal of Neurovirology 2005; 11: 58-65. http://dx.doi.org/10.1080/13550280590900391 PMid:12721937

- Ling PD, Lednicky JA, Keitel WA, Poston
DG, White ZS, Peng RS et al. The dynamics of herpesvirus and
polyomavirus reactivation and shedding in healthy adults: A 14-month
longitudinal study. Journal of Infectious Diseases 2003; 187:
1571-1580. http://dx.doi.org/10.1086/374739 PMid:20630132

- Helantera I, Koskinen P. Association of
immune cell function assay with protocol biopsy findings and viral
infections in well matched kidney transplant recipients. Clinical
Nephrology 2010; 74: 123-131.
