Regulatory T-Cells in Chronic Lymphocytic Leukemia and Autoimmune Diseases
Giovanni D’Arena1, Giovanni Rossi2, Barbara Vannata3, Silvia Deaglio4, Giovanna Mansueto1, Fiorella D’Auria5, Teodora Statuto5, Vittorio Simeon6, Laura De Martino7, Aurelio Marandino7, Giovanni Del Poeta8, Vincenzo De Feo7 and Pellegrino Musto1,5,6
1Department of Onco-Hematology, IRCCS “Centro di Riferimento Oncologico della Basilicata”, Rionero in Vulture, Italy
2Hematology and Stem Cell Transplantation Unit, IRCCS “Casa Sollievo della Sofferenza”, San Giovanni Rotondo, Italy
3Hematology Institute, Catholic University of “Sacred Hearth”, Rome, Italy
4Human Genetics Foundation (HuGeF) and Laboratory of Immunogenetics, University of Turin, Italy
5Laboratory of Clinical Research and Advanced Diagnostics, IRCCS "Centro di Riferimento Oncologico della Basilicata, Rionero in Vulture, Italy
6Laboratory of Preclinical and Translational Research, IRCCS “Centro di Riferimento Oncologico della Basilicata”, Rionero in Vulture, Italy
7Department of Pharmaceutical and Biomedical Sciences, University of Salerno, Italy
8Hematology Institute, “Tor Vergata” University, Rome, Italy
2Hematology and Stem Cell Transplantation Unit, IRCCS “Casa Sollievo della Sofferenza”, San Giovanni Rotondo, Italy
3Hematology Institute, Catholic University of “Sacred Hearth”, Rome, Italy
4Human Genetics Foundation (HuGeF) and Laboratory of Immunogenetics, University of Turin, Italy
5Laboratory of Clinical Research and Advanced Diagnostics, IRCCS "Centro di Riferimento Oncologico della Basilicata, Rionero in Vulture, Italy
6Laboratory of Preclinical and Translational Research, IRCCS “Centro di Riferimento Oncologico della Basilicata”, Rionero in Vulture, Italy
7Department of Pharmaceutical and Biomedical Sciences, University of Salerno, Italy
8Hematology Institute, “Tor Vergata” University, Rome, Italy
Correspondence
to:
Dr. Giovanni D’Arena, MD. Department of Onco-Hematology, IRCCS “Centro
di Riferimento Oncologico della Basilicata”, Via Padre Pio n. 1, 85028
Rionero in Vulture (Pz), Italy. Tel: +39.0972.726521 Fax:
+39.0972.726217. E-mail: giovannidarena@libero.it
Published: August 9, 2012
Received: June 2, 2012
Accepted: July 19, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012053, DOI 10.4084/MJHID.2012.053
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Regulatory
T-cells (Tregs) constitute a small subset of cells that are actively
involved in maintaining self-tolerance, in immune homeostasis and in
antitumor immunity. They are thought to play a significant role in the
progression of cancer and are generally increased in patient with
chronic lymphocytic leukemia (CLL). Their number correlates with more
aggressive disease status and is predictive of the time to treatment,
as well. Moreover, it is now clear that dysregulation in Tregs cell
frequency and/or function may result in a plethora of autoimmune
diseases, including multiple sclerosis, type 1 diabetes mellitus,
myasthenia gravis, systemic lupus erythematosus, autoimmune
lymphoproliferative disorders, rheumatoid arthritis, and psoriasis.
Efforts are made aiming to develop approaches to deplete Tregs or
inhibit their function in cancer and autoimmune disorders, as well.
A Brief History
The human immune system is a well-coordinated network of cells, organs and glands acting in harmony to protect the host from a broad range of pathogenic microorganisms and, at the same time, to avoid responsiveness to self-antigens (immunological self-tolerance) and to control the quality and the magnitude of immune responses to non-self-antigens thus avoiding damage to the host (immune homeostasis). Several mechanisms are thought to be involved in this complex control system (Table 1). In this scenario, a distinct small subset of specialized T-lymphocytes, the so-called regulatory T-cells (Tregs), seem to play a pivotal role in maintaining homeostasis and self-tolerance.[1,2] In fact, Tregs act suppressing the function of self-reactive T-cells to protect the host from autoimmune disease. At the same time they seem to be able to prevent antitumor immune responses.[3]
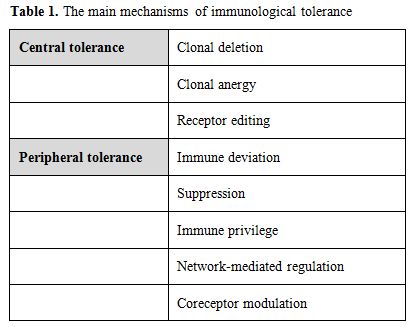
Table 1. The main mechanisms of immunological tolerance
Gershon and Kondo of Yale University firstly proposed the existence of T-cells with suppressive activity more than 40 years ago.[4] However, its better identification lacked for several years and this field of research shrank until to 1995, when Shimon Sakaguchi and coworkers identified a population of CD4+ T-cells expressing surface interleukin-2 (IL)-2 receptor α-chain (recognized by CD25) and termed them ‘regulatory’ T-cells.[5] However, CD25 is not exclusively restricted to Tregs because of its expression on the surface of T effector lymphocytes after activation.[6] Baecher-Allan and co-workers, by means of flow cytometry and in vitro study of sorted cells, identified a very small subset of T cells with high expression of CD25 that exhibited a strong regulatory function in humans.[7-9] CD4+CD25+high cells inhibited proliferation and cytokine secretion by activated CD4+CD25+ responder T-cells in a contact-dependent manner.
In addition, it has been experimentally demonstrated that depleting Tregs produces inflammatory bowel disease, resulting from excessive immune response to intestinal commensal bacteria.[10] Finally, reducing or removing Tregs leads to effective tumor immunity leading in turn to tumor eradication.[11,12]
More recently, the intracellular transcription factor forkhead/winged helix box P3 (FoxP3), also called scurfin, has been identified as the most accepted marker for Tregs.[13-15] It functions regulating a set of genes involved in the suppression, proliferation and metabolic activities of Tregs. Moreover, CD127, that identified the heterodimeric IL-7 receptor, combined with CD4, CD25 and FoxP3, has been shown to better identify Tregs avoiding the contamination of this small cell population (accounting for 1-4% of circulating CD4+ lymphocytes in humans) with activated T-cells.[16,17]
Tregs and Autoimmunity
It is now clear that dysregulation in Tregs cells may result in a plethora of autoimmune diseases, including multiple sclerosis, type 1 diabetes mellitus, myasthenia gravis, systemic lupus erythematosus, autoimmune lymphoproliferative disorders, rheumatoid arthritis, and psoriasis.[18]
As a matter of the fact, complex genetic disorders typically associated with the MHC chromosomal region as well as the dysregulation of Treg cells frequency and/or function appear to be involved in autoimmune diseases.[19] In particular, FoxP3, IL-2 and relative receptor play a key role in the maintenance of Tregs associated pathological immune responses.[20]
Deficiency in FoxP3 due to genetic mutations results in a lethal X-linked recessive lymphoproliferative disease in mice and human subjects characterized by immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome.[21] This autoimmune disorder is characterized by a severe intestinal pathology, with massive T-cell infiltration, type 1 diabetes mellitus, eczema, anemia, liver infiltration, thrombocytopenia, hypothyroidism, and the presence of various autoantibodies. FoxP3 deficiency was also found in the multiple sclerosis although Treg cells frequency was comparable with healthy individuals.[22,23] Similar results emerged in type 1 autoimmune diabetes, psoriasis, myasthenia gravis and autoimmune polyglandular syndromes (APS).[24-26] The degree of deficiency of functional anomaly of FoxP3+ natural Tregs is able to alter the manifestation of autoimmunity. Alterations of Tregs were also reported in rheumatoid arthritis and in idiopathic juvenile arthritis. Results obtained may suggest a possible role of Tregs in the downregulation of the joint inflammation.[27]
Defining Tregs
Taken all above into account, Tregs may be defined as a small population of T-cells with a relevant role in the immune homeostasis. For this reason, they are actively involved in the immunosurveillance against autoimmune disorders and cancer, as well. Tregs may be defined as CD4+ T-cells expressing CD25 at high levels, cytoplasmic FoxP3, and very low to undetectable CD127 on their surface (Figure 1). However, several other markers have been associated to Tregs, but none of them may be considered as a unique marker (Table 2).
Two main subsets of Tregs have been described according to their origin. Innate (or naturally occurring) Tregs originate in the thymus as a consequence of the interaction with high-affinity antigens expressed in thymic stroma and constitutively expressing FoxP3.[28] They are involved in immune homeostatis, thus suppressing the response against self antigens. Such cells persist throughout life despite thymic involution after puberty. Adaptative Tregs emerges also from the thymus but acquire its suppressive activity in periphery regulating the response against self and non-self-antigens.[29] Figure 2 summarizes the generation and subpopulations of Tregs.
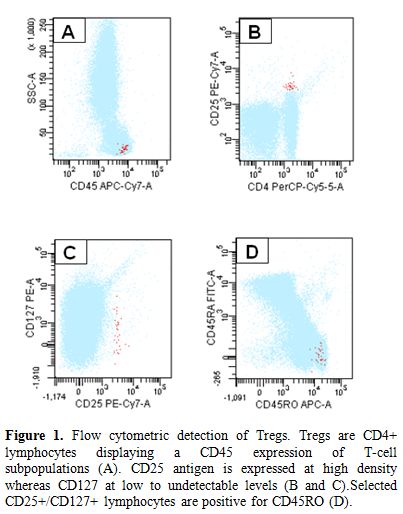
Figure 1. Flow cytometric detection of Tregs. Tregs are CD4+ lymphocytes displaying a CD45 expression of T-cell subpopulations (A). CD25 antigen is expressed at high density whereas CD127 at low to undetectable levels (B and C). Selected CD25+/CD127+ lymphocytes are positive for CD45RO (D).
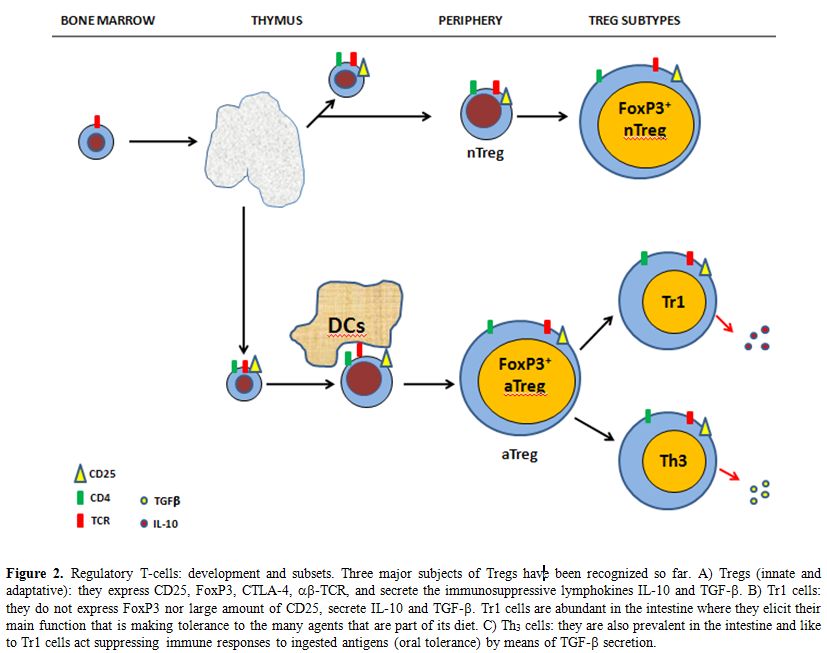
Figure 2. Regulatory T-cells: development and subsets. Three major subjects of Tregs have been recognized so far. A) Tregs (innate and adaptative): they express CD25, FoxP3, CTLA-4, α▀-TCR, and secrete the immunosuppressive lymphokines IL-10 and TGF-▀. B) Tr1 cells: they do not express FoxP3 nor large amount of CD25, secrete IL-10 and TGF-▀. Tr1 cells are abundant in the intestine where they elicit their main function that is making tolerance to the many agents that are part of its diet. C) Th3 cells: they are also prevalent in the intestine and like to Tr1 cells act suppressing immune responses to ingested antigens (oral tolerance) by means of TGF-▀ secretion.
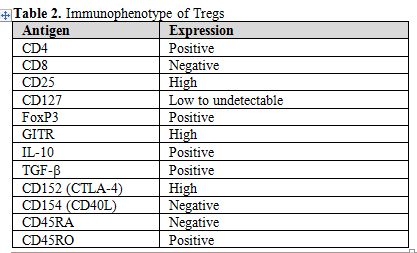
Table 2. Immunophenotype of Tregs
Tregs have been shown to suppress the proliferation of antigen-stimulated na´ve T-cells and several mechanisms have been suggested by means of which they elicit their suppressive activity.[30,31] Either natural and adaptative Tregs are antigen-specific and are seen to need T-cell receptor (TCR) triggering to become suppressive[31,32] despite this latter point is still controversial.[33] In vitro studies suggested that activated Tregs suppress activated CD4+ or CD8+ effector T-cells by means of cell-to-cell contact. In this mechanism a crucial role is played by the ligation of CD80/CD86 complex on effector cells by cytotoxic T-lymphocytes antigen-4 (CTLA-4) on Tregs surface resulting in the transmission of inhibitory signals of T-cell function.[34,35] In a similar fashion, Tregs seem to modulate dendritic cells (DCs) function resulting in the expression and activation of indoleamine 2,3-dioxygenase degradation.[36] DCs may be blocked in maturation and/or activation by release of IL-10 and TGF-▀ that resulting in antigen-presenting capacity impairment due to down-regulation of major histocompatibility complex (MHC) class II and in interfering in costimulatory molecules expression.[37,38] Other in vitro studies suggest Tregs inhibition by means of the release of suppressive cytokines, such as IL-10 and TGF-▀.[39-41] Activated Tregs are capable to express granzyme A or perforin and kill activated CD4+ or CD8+ T-cells, through the perforin-dependent way.[42,43]
Tregs and Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL), the most common form of leukemia in Western countries, is characterized by the accumulation of monoclonal B-lymphocytes in bone marrow, lymphoid organs and peripheral blood.[44] Moreover, there is increasing evidence of T cell dysfunction in CLL and this may probably contribute to the etiology and the progression of the disease.[45,46] Several authors reported that Tregs are increased in CLL patients.[47-51] Using multicolor flow cytometry, we showed that CLL patients had a higher absolute number of circulating Tregs compared to age and sex-matched controls.[51] In addition, Tregs cell number was significantly correlated to more advanced Rai clinical stages, peripheral blood B-lymphocytosis, more elevated LDH levels, and absolute number of CD38+ neoplastic B-cells.
The evidence that Tregs are reduced after therapy with fludarabine, agrees with the hypothesis that these cells play a critical role in protecting CLL cells from getting killed by the immune system.[47] The same happens when patients with CLL were treated with thalidomide.[52] This drug and its analogues, such as lenalidomide, acts as immunomodulatory agents targeting the microenvironment and both are shown to be effective in the treatment of CLL patients, probably by means of TNF modulation.[53-55]
The prognostic role of Tregs have been poorly investigated. Only two paper reported that a shorter time to first treatment may be predicted by the circulating number of Tregs.[56,57] As showed in Figure 3, we found a best predictive cut-off of absolute circulating Tregs able to identify patients with early stage CLL at higher risk of requiring therapy.[57]
Finally, we have studied Tregs in ‘clinical’ monoclonal B-cell lymphocytosis (MBL), a condition in which less than 5000/ÁL circulating monoclonal B-cells, in absence of other features of lymphoproliferative disorders, is found.[58] We showed that MBL patients had a lower absolute number of Tregs, compared to CLL patients, but higher than controls (Figure 4).[59] Taken together, these data show that the tumor mass (from MBL low to intermediate to high-risk CLL) and the circulating Tregs increase simultaneously, thus suggesting that the expected result is a more robust inhibition of tumor inhibiting cells and, ultimately, a greater expansion of neoplastic B cells.
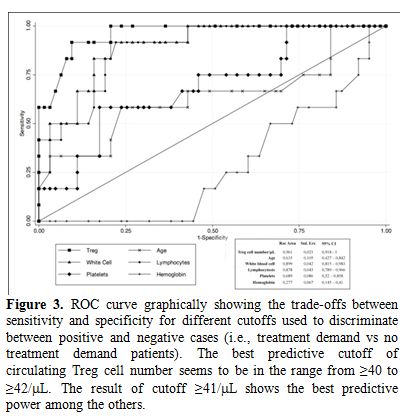
Figure 3. ROC curve graphically showing the trade-offs between sensitivity and specificity for different cutoffs used to discriminate between positive and negative cases (i.e., treatment demand vs no treatment demand patients). The best predictive cutoff of circulating Treg cell number seems to be in the range from ≥40 to ≥42/ÁL. The result of cutoff ≥41/ÁL shows the best predictive power among the others.
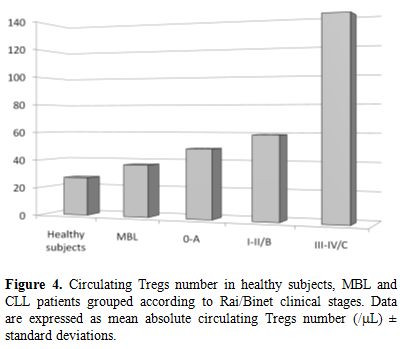
Figure 4. Circulating Tregs number in healthy subjects, MBL and CLL patients grouped according to Rai/Binet clinical stages. Data are expressed as mean absolute circulating Tregs number (/ÁL) ▒ standard deviations
Conclusions
Tregs play a critical role in immune tolerance (maintaining peripheral tolerance to self-antigens) and in immune homeostasis (regulating the immune response to non self-antigens). Moreover, it is now clear that Tregs have a role in suppressing tumor-specific immunity and for that reason are actively involved in the etiology and in progression of cancer, such as CLL, the most frequent form of leukemia in Western countries. Tregs disregulation is thought to be also involved in the pathogenesis of autoimmune disorders. In light of this, Tregs appear as having a great potential in treating autoimmunity and cancer. There is now considerable evidence in preclinical models to suggest that adoptive Tregs therapy will be highly efficacious. For that reason, clinical strategies are developing to target such cells aiming to modulate their suppressive function.[60-65]
The human immune system is a well-coordinated network of cells, organs and glands acting in harmony to protect the host from a broad range of pathogenic microorganisms and, at the same time, to avoid responsiveness to self-antigens (immunological self-tolerance) and to control the quality and the magnitude of immune responses to non-self-antigens thus avoiding damage to the host (immune homeostasis). Several mechanisms are thought to be involved in this complex control system (Table 1). In this scenario, a distinct small subset of specialized T-lymphocytes, the so-called regulatory T-cells (Tregs), seem to play a pivotal role in maintaining homeostasis and self-tolerance.[1,2] In fact, Tregs act suppressing the function of self-reactive T-cells to protect the host from autoimmune disease. At the same time they seem to be able to prevent antitumor immune responses.[3]

Table 1. The main mechanisms of immunological tolerance
Gershon and Kondo of Yale University firstly proposed the existence of T-cells with suppressive activity more than 40 years ago.[4] However, its better identification lacked for several years and this field of research shrank until to 1995, when Shimon Sakaguchi and coworkers identified a population of CD4+ T-cells expressing surface interleukin-2 (IL)-2 receptor α-chain (recognized by CD25) and termed them ‘regulatory’ T-cells.[5] However, CD25 is not exclusively restricted to Tregs because of its expression on the surface of T effector lymphocytes after activation.[6] Baecher-Allan and co-workers, by means of flow cytometry and in vitro study of sorted cells, identified a very small subset of T cells with high expression of CD25 that exhibited a strong regulatory function in humans.[7-9] CD4+CD25+high cells inhibited proliferation and cytokine secretion by activated CD4+CD25+ responder T-cells in a contact-dependent manner.
In addition, it has been experimentally demonstrated that depleting Tregs produces inflammatory bowel disease, resulting from excessive immune response to intestinal commensal bacteria.[10] Finally, reducing or removing Tregs leads to effective tumor immunity leading in turn to tumor eradication.[11,12]
More recently, the intracellular transcription factor forkhead/winged helix box P3 (FoxP3), also called scurfin, has been identified as the most accepted marker for Tregs.[13-15] It functions regulating a set of genes involved in the suppression, proliferation and metabolic activities of Tregs. Moreover, CD127, that identified the heterodimeric IL-7 receptor, combined with CD4, CD25 and FoxP3, has been shown to better identify Tregs avoiding the contamination of this small cell population (accounting for 1-4% of circulating CD4+ lymphocytes in humans) with activated T-cells.[16,17]
Tregs and Autoimmunity
It is now clear that dysregulation in Tregs cells may result in a plethora of autoimmune diseases, including multiple sclerosis, type 1 diabetes mellitus, myasthenia gravis, systemic lupus erythematosus, autoimmune lymphoproliferative disorders, rheumatoid arthritis, and psoriasis.[18]
As a matter of the fact, complex genetic disorders typically associated with the MHC chromosomal region as well as the dysregulation of Treg cells frequency and/or function appear to be involved in autoimmune diseases.[19] In particular, FoxP3, IL-2 and relative receptor play a key role in the maintenance of Tregs associated pathological immune responses.[20]
Deficiency in FoxP3 due to genetic mutations results in a lethal X-linked recessive lymphoproliferative disease in mice and human subjects characterized by immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome.[21] This autoimmune disorder is characterized by a severe intestinal pathology, with massive T-cell infiltration, type 1 diabetes mellitus, eczema, anemia, liver infiltration, thrombocytopenia, hypothyroidism, and the presence of various autoantibodies. FoxP3 deficiency was also found in the multiple sclerosis although Treg cells frequency was comparable with healthy individuals.[22,23] Similar results emerged in type 1 autoimmune diabetes, psoriasis, myasthenia gravis and autoimmune polyglandular syndromes (APS).[24-26] The degree of deficiency of functional anomaly of FoxP3+ natural Tregs is able to alter the manifestation of autoimmunity. Alterations of Tregs were also reported in rheumatoid arthritis and in idiopathic juvenile arthritis. Results obtained may suggest a possible role of Tregs in the downregulation of the joint inflammation.[27]
Defining Tregs
Taken all above into account, Tregs may be defined as a small population of T-cells with a relevant role in the immune homeostasis. For this reason, they are actively involved in the immunosurveillance against autoimmune disorders and cancer, as well. Tregs may be defined as CD4+ T-cells expressing CD25 at high levels, cytoplasmic FoxP3, and very low to undetectable CD127 on their surface (Figure 1). However, several other markers have been associated to Tregs, but none of them may be considered as a unique marker (Table 2).
Two main subsets of Tregs have been described according to their origin. Innate (or naturally occurring) Tregs originate in the thymus as a consequence of the interaction with high-affinity antigens expressed in thymic stroma and constitutively expressing FoxP3.[28] They are involved in immune homeostatis, thus suppressing the response against self antigens. Such cells persist throughout life despite thymic involution after puberty. Adaptative Tregs emerges also from the thymus but acquire its suppressive activity in periphery regulating the response against self and non-self-antigens.[29] Figure 2 summarizes the generation and subpopulations of Tregs.

Figure 1. Flow cytometric detection of Tregs. Tregs are CD4+ lymphocytes displaying a CD45 expression of T-cell subpopulations (A). CD25 antigen is expressed at high density whereas CD127 at low to undetectable levels (B and C). Selected CD25+/CD127+ lymphocytes are positive for CD45RO (D).

Figure 2. Regulatory T-cells: development and subsets. Three major subjects of Tregs have been recognized so far. A) Tregs (innate and adaptative): they express CD25, FoxP3, CTLA-4, α▀-TCR, and secrete the immunosuppressive lymphokines IL-10 and TGF-▀. B) Tr1 cells: they do not express FoxP3 nor large amount of CD25, secrete IL-10 and TGF-▀. Tr1 cells are abundant in the intestine where they elicit their main function that is making tolerance to the many agents that are part of its diet. C) Th3 cells: they are also prevalent in the intestine and like to Tr1 cells act suppressing immune responses to ingested antigens (oral tolerance) by means of TGF-▀ secretion.

Table 2. Immunophenotype of Tregs
Tregs have been shown to suppress the proliferation of antigen-stimulated na´ve T-cells and several mechanisms have been suggested by means of which they elicit their suppressive activity.[30,31] Either natural and adaptative Tregs are antigen-specific and are seen to need T-cell receptor (TCR) triggering to become suppressive[31,32] despite this latter point is still controversial.[33] In vitro studies suggested that activated Tregs suppress activated CD4+ or CD8+ effector T-cells by means of cell-to-cell contact. In this mechanism a crucial role is played by the ligation of CD80/CD86 complex on effector cells by cytotoxic T-lymphocytes antigen-4 (CTLA-4) on Tregs surface resulting in the transmission of inhibitory signals of T-cell function.[34,35] In a similar fashion, Tregs seem to modulate dendritic cells (DCs) function resulting in the expression and activation of indoleamine 2,3-dioxygenase degradation.[36] DCs may be blocked in maturation and/or activation by release of IL-10 and TGF-▀ that resulting in antigen-presenting capacity impairment due to down-regulation of major histocompatibility complex (MHC) class II and in interfering in costimulatory molecules expression.[37,38] Other in vitro studies suggest Tregs inhibition by means of the release of suppressive cytokines, such as IL-10 and TGF-▀.[39-41] Activated Tregs are capable to express granzyme A or perforin and kill activated CD4+ or CD8+ T-cells, through the perforin-dependent way.[42,43]
Tregs and Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL), the most common form of leukemia in Western countries, is characterized by the accumulation of monoclonal B-lymphocytes in bone marrow, lymphoid organs and peripheral blood.[44] Moreover, there is increasing evidence of T cell dysfunction in CLL and this may probably contribute to the etiology and the progression of the disease.[45,46] Several authors reported that Tregs are increased in CLL patients.[47-51] Using multicolor flow cytometry, we showed that CLL patients had a higher absolute number of circulating Tregs compared to age and sex-matched controls.[51] In addition, Tregs cell number was significantly correlated to more advanced Rai clinical stages, peripheral blood B-lymphocytosis, more elevated LDH levels, and absolute number of CD38+ neoplastic B-cells.
The evidence that Tregs are reduced after therapy with fludarabine, agrees with the hypothesis that these cells play a critical role in protecting CLL cells from getting killed by the immune system.[47] The same happens when patients with CLL were treated with thalidomide.[52] This drug and its analogues, such as lenalidomide, acts as immunomodulatory agents targeting the microenvironment and both are shown to be effective in the treatment of CLL patients, probably by means of TNF modulation.[53-55]
The prognostic role of Tregs have been poorly investigated. Only two paper reported that a shorter time to first treatment may be predicted by the circulating number of Tregs.[56,57] As showed in Figure 3, we found a best predictive cut-off of absolute circulating Tregs able to identify patients with early stage CLL at higher risk of requiring therapy.[57]
Finally, we have studied Tregs in ‘clinical’ monoclonal B-cell lymphocytosis (MBL), a condition in which less than 5000/ÁL circulating monoclonal B-cells, in absence of other features of lymphoproliferative disorders, is found.[58] We showed that MBL patients had a lower absolute number of Tregs, compared to CLL patients, but higher than controls (Figure 4).[59] Taken together, these data show that the tumor mass (from MBL low to intermediate to high-risk CLL) and the circulating Tregs increase simultaneously, thus suggesting that the expected result is a more robust inhibition of tumor inhibiting cells and, ultimately, a greater expansion of neoplastic B cells.

Figure 3. ROC curve graphically showing the trade-offs between sensitivity and specificity for different cutoffs used to discriminate between positive and negative cases (i.e., treatment demand vs no treatment demand patients). The best predictive cutoff of circulating Treg cell number seems to be in the range from ≥40 to ≥42/ÁL. The result of cutoff ≥41/ÁL shows the best predictive power among the others.

Figure 4. Circulating Tregs number in healthy subjects, MBL and CLL patients grouped according to Rai/Binet clinical stages. Data are expressed as mean absolute circulating Tregs number (/ÁL) ▒ standard deviations
Conclusions
Tregs play a critical role in immune tolerance (maintaining peripheral tolerance to self-antigens) and in immune homeostasis (regulating the immune response to non self-antigens). Moreover, it is now clear that Tregs have a role in suppressing tumor-specific immunity and for that reason are actively involved in the etiology and in progression of cancer, such as CLL, the most frequent form of leukemia in Western countries. Tregs disregulation is thought to be also involved in the pathogenesis of autoimmune disorders. In light of this, Tregs appear as having a great potential in treating autoimmunity and cancer. There is now considerable evidence in preclinical models to suggest that adoptive Tregs therapy will be highly efficacious. For that reason, clinical strategies are developing to target such cells aiming to modulate their suppressive function.[60-65]
References
- Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med 2006; 354: 1166-1176. http://dx.doi.org/10.1056/NEJMra055446 PMid:16540617

- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133: 775-787. http://dx.doi.org/10.1016/j.cell.2008.05.009 PMid:18510923

- Beyer M, Schultze JL. Regulatory T cells in cancer. Blood 2006; 108: 804-811. http://dx.doi.org/10.1182/blood-2006-02-002774 PMid:16861339

- Gershon R, Kondo K. Cell interactions in
the induction of tolerance: the role of thymic lymphocytes. Immunology
1970; 18: 723-737. PMid:4911896 PMCid:1455602

- Sakaguchi S, Sakaguchi N, Asano M, Itoh M,
Toda M. Immunologic tolerance maintained by activated T cells
expressing IL-2 receptor α-chain (CD25): breakdown of a single
mechanism of self-tolerance causes various autoimmune diseases. J
Immunol 1995; 155: 1151-1164. PMid:7636184

- Robb RJ, Munck A, Smith KA. T cell growth
factor receptors. Quantitation, specificity, and biological relevance.
J Exp Med 1981; 154: 1455-1474. http://dx.doi.org/10.1084/jem.154.5.1455 PMid:6975347 PMCid:2186509

- Baecher-Allan C, Brown JA, Freeman GJ,
Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J
Immunol 2001; 167: 1245-1253. PMid:11466340

- Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol 2004; 16: 89-97. http://dx.doi.org/10.1016/j.smim.2003.12.005 PMid:15036232

- Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev 2006; 212: 203-216. http://dx.doi.org/10.1111/j.0105-2896.2006.00417.x PMid:16903916

- Singh B, Read S, Asseman C, Malstrom V,
Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of
intestinal inflammation by regulatory T cells. Immunol Rev 2001; 182:
190-200. http://dx.doi.org/10.1034/j.1600-065X.2001.1820115.x PMid:11722634

- Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol 2007; 19: 217-223. http://dx.doi.org/10.1016/j.coi.2007.02.004 PMid:17306521

- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol 2005; 6: 353-360. http://dx.doi.org/10.1038/ni1181 PMid:15785761

- Hori S, Nomura T, Sakaguchi S. Control of
regulatory T cell development by the transcription factor FoxP3.
Science 2003; 299: 1057-1061. http://dx.doi.org/10.1126/science.1079490 PMid:12522256

- Fontenot JD, Gavin MA, Rudensky AY. FOXP3
programs the development and function of CD4+CD25+ regulatory T-cells.
Nat Immunol 2003; 4: 330-336. http://dx.doi.org/10.1038/ni904 PMid:12612578

- Rudensky AY, Fontenot JD. A well adapted
regulatory contrivance: regulatory T cell development and the forkhead
family transcription factor FoxP3. Nature Immunol 2005; 6: 331-337. http://dx.doi.org/10.1038/ni1179 PMid:15785758

- Seddiki N, Santner-Nanan B, Martinson J,
Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan
R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2
and IL-7 receptors discriminate between human regulatory and activated
T cells. J Exp Med 2006; 203: 1693-1700. http://dx.doi.org/10.1084/jem.20060468 PMid:16818676 PMCid:2118333

- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee
MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B,
Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression
inversely correlates with FoxP3 and suppressive function of human CD4+
Treg cells. J Exp Med 2006; 203: 1700-1711. http://dx.doi.org/10.1084/jem.20060772 PMid:16818678 PMCid:2118339

- Dejaco C, Duftner C, Grubeck-Loebenstein
B, Schirmer M. Imbalance of regulatory cells in human autoimmune
diseases. Immunology 2005; 117: 289-300. http://dx.doi.org/10.1111/j.1365-2567.2005.02317.x PMid:16476048 PMCid:1782226

- Hafler DA, de Jager PL. Applying a new
generation of genetic maps to understand human inflammatory disease.
Nat Rev Immunol 2005; 5: 83-91. http://dx.doi.org/10.1038/nri1532 PMid:15630431

- Sakaguchi S, Ono M, Setoguchi R, Yagi H,
Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+
CD4+ natural regulatory T cells in dominant self-tolerance and
autoimmune disease. Immunol Rev 2006; 212: 8-27. http://dx.doi.org/10.1111/j.0105-2896.2006.00427.x PMid:16903903

- Godfrey VL, Wilkinson JE, Russell LB.
X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J
Pathol 1991; 138: 1379-1387. PMid:2053595 PMCid:1886400

- Viglietta V, Baecher-Allan C, Weiner HL,
Hfler DA. Loss of cunctional suppression by CD4+CD25+ regulatory T
cells in patients with multiple sclerosis. J Exp Med 2004; 199:
971-979. http://dx.doi.org/10.1084/jem.20031579 PMid:15067033 PMCid:2211881

- Huan J, Culbertson N, Spencer L,
Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H,
Vandenbark AA. Decreased FOXP3 in multiple sclerosis patients. J
Neurosci Res 2005; 81: 45-52. http://dx.doi.org/10.1002/jnr.20522 PMid:15952173

- Kriegel MA, Lohamann T, Gabler C, Blank N,
Kalden JR, Lorenz HM. Defective suppressor function of human CD4+CD25+
regulatory T cells in autoimmune polygranular T cells in
autoimmunepolygrandular syndrome type II. J Exp Med 2004; 199:
1285-1891. http://dx.doi.org/10.1084/jem.20032158 PMid:15117972 PMCid:2211900

- Balandina A, Lecart S, Dartevelle P,
Saoudi A, Berrih-Aknin S. Functional defect of regulator CD4(+)CD25+ T
cells in the thymus of patients with autoimmune myasthenia gravis.
Blood 2005; 105: 735-41. http://dx.doi.org/10.1182/blood-2003-11-3900 PMid:15454488 PMCid:1847365

- Ono M, Shimizu J, Miyachi Y, Sakaguchi S.
Control of autoimmune myocarditis and multi-organ inflammation by
GITRhigh FoxP3- expressing CD25+ and CD25- regulatory T cells. J
Immunol 2006; 176: 4748-4756. PMid:16585568

- Horak I, Lohler J, Ma A, Smith KA.
Interleukin-2 deficient mice: a new model to study autoimmunity and
self-tolerance. Immunol Rev 1995; 148: 35-44. http://dx.doi.org/10.1111/j.1600-065X.1995.tb00092.x PMid:8825281

- Aschenbrenner K, D’Cruz LH, Vollmann EH,
Hintenberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of
FoxP3 regulatory T cells specific for self antigen expressed and
presented by Aire+ medullary thymic epithelial cells. Nature Immunol
2007; 8: 351-358. http://dx.doi.org/10.1038/ni1444 PMid:17322887

- Piccirillo CA, Thornton AM. Cornerstone of
peripheral tolerance: naturally occurring CD4+CD24+ regulatory T cells.
Trends Immunol 2004; 25: 374-380. http://dx.doi.org/10.1016/j.it.2004.04.009 PMid:15207505

- Corthay A. How do regulatory T cells work? Scand J Immunol 2009; 70: 326-336. http://dx.doi.org/10.1111/j.1365-3083.2009.02308.x PMid:19751267 PMCid:2784904

- Sakaguchi S, Wing K, Onishi Y,
Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress
immune responses? Int Immunol 2009; 21: 1105-1111. http://dx.doi.org/10.1093/intimm/dxp095 PMid:19737784

- Thornton AM, Shevac EM. CD4+CD25+
immunoregulatory T cells suppress polyclonal T cell activation in vitro
by inhibiting interleukin 2 production. J Exp Med 1998; 188: 287-296. http://dx.doi.org/10.1084/jem.188.2.287 PMid:9670041 PMCid:2212461

- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi
N, Itoh M, Iwata M, Shimizu J, Jakaguchi J. Immunologic self-tolerance
maintained by CD25+CD4+ naturally anergic and suppressive T cells:
induction of autoimmune disease by breaking their anergic/suppressive
state. Int Immunol 1998; 10: 1969-1980. http://dx.doi.org/10.1093/intimm/10.12.1969 PMid:9885918

- Walunas TL, Bluestone JA. CTLA-4 regulates
tolerance induction and T cell differentiation in vivo. J Immunol 1998;
160: 3855-3860. PMid:9558090

- Shimuzu J, Yamazaki S, Takahashi T, Ishida
Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through
GITR breaks immunological self-tolerance. Nat Immunol 2002; 3: 135-142.
http://dx.doi.org/10.1038/ni759 PMid:11812990

- Deaglio S, Dwyer KM, Gao W, Friedman D,
Usheva A, Erat A, Chen JF, Enjyoji K, Linden K, Oukka M, Kuchroo VK,
Strom TB, Robson SC. Adenosine generations catalyzed by CD39 and CD73
expressed on regulatory T cells mediates immune suppression. J Exp Med
2007; 204: 1257-1265. http://dx.doi.org/10.1084/jem.20062512 PMid:17502665 PMCid:2118603

- Geissmann F, Revy P, Regnault A,
Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A.
TGF-beta 1 prevents the noncognate maturation of human dendritic
Langherans cells. J Immunol 1999; 162: 4567-4575. PMid:10201996

- Strobl H, Knapp W. TGF-β1 regulation of dendritic cells. Microbes Infection 1999; 1: 1283-1290. http://dx.doi.org/10.1016/S1286-4579(99)00256-7

- Shevac EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2002; 2: 389-400. PMid:12093005

- Taylor A, Vehagen J, Blaser K, Akdis M,
Akdis CA. Mechanisms of immune suppression by interleukin-10 and
transforming growth factor-β: the role of T regulatory cells. Immunol
2006; 117: 443-442.

- Levings MK, Bacchetta R, Schulz U,
Roncarolo MG. The role of IL-10 and TGF-β in the differentiation and
effector function of T regulatory cells. Int Arch Allergy Immunol 2002;
129: 263-276. http://dx.doi.org/10.1159/000067596 PMid:12483031

- Grossman WJ, Verbsky JW, Barchet W,
Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the
perforin pathway to cause autologous target cell death. Immunity 2004;
21: 589-601. http://dx.doi.org/10.1016/j.immuni.2004.09.002 PMid:15485635

- Grossman WJ, Verbsky JW, Tollefsen BL,
Kemper C, Atkinson JP, Levy TJ. Differential expression of granzymes A
and B in human cytotoxic lymphocyte subsets and T regulatory cells.
Blood 2004; 104: 2840-2848. http://dx.doi.org/10.1182/blood-2004-03-0859 PMid:15238416

- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005; 352: 804-815. http://dx.doi.org/10.1056/NEJMra041720 PMid:15728813

- Scrivener S, Goddard RV, Kaminski ER,
Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic
leukaemia. Leuk Lymphoma 2003; 44: 383-389. http://dx.doi.org/10.1080/1042819021000029993

- Christopoulos P, Pfeifer D, Bartholome K,
Follo M, Timmer J, Fisch P, Veelken H. Definition and characterization
of the systemic T-cell dysregulation in untreated indolent B-cell
lymphoma and very early CLL. Blood 2011; 117: 3836-3846. http://dx.doi.org/10.1182/blood-2010-07-299321 PMid:21270444

- Beyer M, Kochanek M, Darabi K, Popov A,
Jensen M, Endl E, Knolle PA, Thomas RK, von Bergwelt-Baildon M, Debey
S, Hallek M, Schultze JL. Reduced frequencies and suppressive function
of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic
leukemia after therapy with fludarabine. Blood 2005; 106: 2018-2025. http://dx.doi.org/10.1182/blood-2005-02-0642 PMid:15914560

- Giannopoulos K, Schmitt M, Kowal M,
Wlasiuk P, Bojarska-Junak A, Chen J, Rolinski J, Dmoszynska A.
Characterization of regulatory T cells in patients with B-cell chronic
lymphocytic leukemia. Oncol Rep 2008; 20: 677-682. PMid:18695923

- Deutsch V, Pierry C, Polliack A. Expansion
of regulatory T cells in B chronic lymphocytic leukemia: enhanced
‘brakes’ on host immunity. Leuk Lymphoma 2009; 50: 687-688. http://dx.doi.org/10.1080/10428190902954389

- Jak M, Mous R, Remmerswaal EBM, Spijker R,
Jaspers A, Yague A, Eldering E, van Lier RAW, Van Oers MHJ. Enhanced
formation and survival of CD4+CD25highFoxP3+ T-cells in chronic
lymphocytic leukemia. Leuk Lymphoma 2009; 50: 788-801. http://dx.doi.org/10.1080/10428190902803677

- D’Arena G, Laurenti L, Minervini MM,
Deaglio S, Bonello L, De Martino L, De Padua L, Savino L, Tarnani M, De
Feo V, Cascavilla N. Regulatory T-cell number is increate in chronic
lymphocytic leukemia patients and correlate with progressive disease.
Leuk Res 2011; 35: 363-368. http://dx.doi.org/10.1016/j.leukres.2010.08.010 PMid:20880586

- Giannopoulos K, Schmitt M, Wlasiuk P, Chen
J, Bojarska-Junak A, Kowal M, Rolinski J, Dmoszynska A. The high
frequency of T regulatory cells in patients with B-cell chronic
lymphocytic leukemia is diminished through treatment with thalidomide.
Leukemia 2008; 22: 222-224. http://dx.doi.org/10.1038/sj.leu.2404869 PMid:17657216

- Valencia X, Stephens G, Goldbach-Mansky R,
Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of
human CD4+CD25hi T-regulatory cells. Blood 2006; 108: 253-261. http://dx.doi.org/10.1182/blood-2005-11-4567 PMid:16537805 PMCid:1895836

- Ramsay AG, Gribben JG. Immune dysfunction
in chronic lymphocytic leukemia T cells and Lenalidomide as an
immunodulatory drug. Haematologica 2009; 94: 1198-1202. http://dx.doi.org/10.3324/haematol.2009.009274 PMid:19734414 PMCid:2738710

- Lee B-N, Gao H, Cohen EN, Badoux X, Wierda
WG, Estrov Z, Faderl SH, Keating MJ, Ferrajoli A, Reuben JM. Treatment
with Lenalidomide modulates T-cell immunophenotype and cytokine
production in patients with chronic lymphocytic leukemia. Cancer 2011;
117: 3999-4008. http://dx.doi.org/10.1002/cncr.25983 PMid:21858802

- Weiss L, Melchardt T, Egle A, Grabmer C,
Greil R, Tinhofer I. Regulatory T cells predict the time to initial
treatment in early stage chronic lymphocytic leukemia. Cancer 2011;
117: 2163-2169. http://dx.doi.org/10.1002/cncr.25752 PMid:21523729

- D’Arena G, D’Auria F, Simeon V, Laurenti
L, Deaglio S, Mansueto G, Del Principe MI, Statuto T, Pietrantuono G,
Guariglia R, Innocenti I, Martorelli MC, Villani O, De Feo V, Del Poeta
G, Musto P. A shorter time to the first treatment may be predicted by
the absolute number of regulatory T-cells in patients with Rai stage 0
chronic lymphocytic leukemia. Am J Hematol 2012; 87; 628-631. http://dx.doi.org/10.1002/ajh.23170 PMid:22460620

- Hallek M, Cheson BD, Catovsky D,
Caligaris-Cappio F, Dighiero G, D÷hner H, Hillmen P, Keating MJ,
Montserrat E, Rai KR, Kipps JK. Guidelines for the diagnosis and
treatment of chronic lymphocytic leukemia: a report from the
International Workshop on Chronic Lymphocytic Leukemia updating the
National Cancer Institute – Working Group 1996 Guidelines. Blood 2008;
111: 5446-5456. http://dx.doi.org/10.1182/blood-2007-06-093906 PMid:18216293 PMCid:2972576

- D’Arena G, Rossi G, Minervini MM, Savino
L, D’Auria F, Laurenti L, Del Principe MI, Deaglio S, Biagi A, De
Martino L, De Feo V, Cascavilla N, Musto P, Del Poeta G. Circulating
regulatory T cells in “clinical” monoclonal B-cell lymphocytosis. Int J
Immunopathol Pharmacol 2011; 24: 915-923. PMid:22230398

- Chatila TA. Role of regulatory T cells in human disease. J Allergy Clin Immunol 2005; 116: 949-959. http://dx.doi.org/10.1016/j.jaci.2005.08.047 PMid:16275360

- Colombo MP, Piconese S. Regulatory T-cell
inhibition versus depletion: the right choice in cancer immunotherapy.
Nature Rev 2007; 7: 880-887. http://dx.doi.org/10.1038/nrc2250 PMid:17957190

- Brusko TM, Putnam AL, Bluestone JA. Human
regulatory T cells: role in autoimmune disease and therapeutic
opportunities. Immunol Rev 2008; 223: 371388. http://dx.doi.org/10.1111/j.1600-065X.2008.00637.x PMid:18613848

- Nizar S, Copier J, Meyer B, Bodman-Smith
M, Galustian C, Kumar D, Dalgleish A. T-regulatory cell modulation: the
future of cancer immunotherapy? Br J Caner 2009; 100: 1697-1703. http://dx.doi.org/10.1038/sj.bjc.6605040 PMid:19384299 PMCid:2695683

- D’Arena G, Deaglio S, Laurenti L, De
Martino L, De Feo V, Fusco BM, Carella AM, Cascavilla N, Musto P.
Targeting regulatory t cells for anticancer therapy. Mini-Rev Med Chem
2011; 11: 480-485. http://dx.doi.org/10.2174/138955711795843365 PMid:21561409

- Wright GP, Ehrenstein MR, Stauss HJ.
Regulatory T-cell adoptive immunotherapy: potential for treatment of
autoimmunity. Expert Rev Clin Immunol 2011; 7: 213-225. http://dx.doi.org/10.1586/eci.10.96 PMid:21426259
