Epidemiology of Malaria in Endemic Areas
Beatrice Autino1*, Alice Noris1, Rosario Russo2 and Francesco Castelli1,3
1University Division of Infectious and Tropical Diseases, University of Brescia and Spedali Civili General Hospital, Brescia (Italy), Piazza Spedali Civili, 1 - 25123–Brescia, Italy
2University Division of Infectious Diseases, University of Catania, Via Palermo 635 – 95100 Catania, Italy
3Chair of Infectious Diseases, University of Brescia, Italy
* PhD trainee in Appropriate Methods and Technologies for International Development Co-operation, supported by the A. Archetti Fund
2University Division of Infectious Diseases, University of Catania, Via Palermo 635 – 95100 Catania, Italy
3Chair of Infectious Diseases, University of Brescia, Italy
* PhD trainee in Appropriate Methods and Technologies for International Development Co-operation, supported by the A. Archetti Fund
Published: October 4, 2012
Received: September 6, 2012
Accepted: September 21, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012060, DOI 10.4084/MJHID.2012.060
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Malaria
infection is still to be considered a major public health problem in
those 106 countries where the risk of contracting the infection with
one or more of the Plasmodium species exists. According to estimates
from the World Health Organization, over 200 million cases and about
655.000 deaths have occurred in 2010. Estimating the real health and
social burden of the disease is a difficult task, because many of the
malaria endemic countries have limited diagnostic resources, especially
in rural settings where conditions with similar clinical picture may
coexist in the same geographical areas. Moreover, asymptomatic
parasitaemia may occur in high transmission areas after childhood, when
anti-malaria semi-immunity occurs. Malaria endemicity and control
activities are very complex issues, that are influenced by factors
related to the host, to the parasite, to the vector, to the environment
and to the health system capacity to fully implement available
anti-malaria weapons such as rapid diagnostic tests, artemisinin-based
combination treatment, impregnated bed-nets and insecticide residual
spraying while waiting for an effective vaccine to be made available.
Introduction
Malaria is one of the most important public health problem in term of morbidity and mortality, causing more than 200 million cases and 655.000 deaths every year.[1]
According to the World Health Organization (WHO) Malaria Report 2011, a total of 106 countries in the world are at risk of transmission of malaria infection (Figure 1).
A total of 216 million estimated malaria cases occurred in 2010, 81% of which were reported in the African Region, followed by South East Asia (13%) and Eastern Mediterranean Region (5%). The total number of malaria deaths was estimated to be 655.000 in 2010; 91% of whom occurred in the African Region, 6% in South-East Asia and 3% in Eastern Mediterranean Region (Table 1).
Although the proportion of people exposed to malaria parasites has decreased during the last century, the absolute number of people at risk for malaria infection increased from 0.8 billion in 1900 to 3.3 billion in 2010, as a consequence of the absolute increase of the population living in malaria-endemic regions.[1,2] However, between 2005 and 2010 malaria cases decreased from 244 million to 216 million; moreover, malaria mortality rates showed a global reduction of 26% between 2000 and 2010.
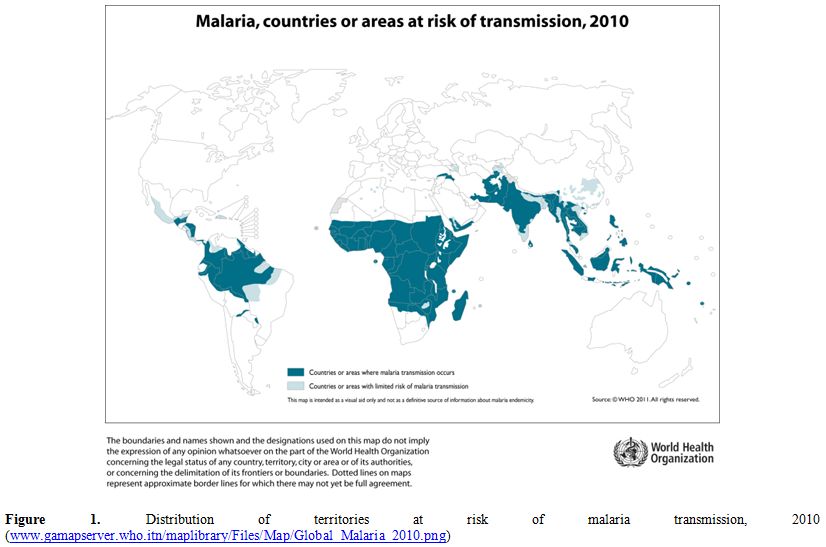
Figure 1. Distribution of territories at risk of malaria transmission, 2010
(www.gamapserver.who.itn/maplibrary/Files/Map/Global_Malaria_2010.png).
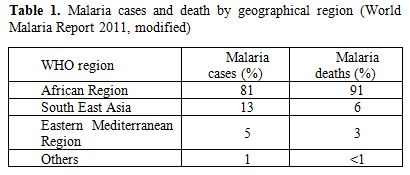
Table 1. Malaria cases and death by geographical region (World Malaria Report 2011, modified).
Malaria in humans is caused by 5 Plasmodium parasites: Plasmodium falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi. The current distribution of human-pathogenic Plasmodium species shows preponderance of P. falciparum in tropical Africa, while P. vivax prevails over P. falciparum in South America. Both P. falciparum and P. vivax are prevalent in south-eastern Asia and western Pacific. Although P. malariae may occur in all malarious areas, its prevalence is generally low. In tropical Africa, P. falciparum and P. malariae co-infection is sometimes encountered. P. ovale is widespread principally in tropical Africa whereas P. knowlesi infection occurs only in certain forested areas of South-East Asia.
Malaria burden is hard to estimate, particularly in low income countries where data collection and reporting quality is poor. Incomplete and discontinuous reports from single health facilities may alter final global malaria prevalence. Malaria cases are often under-diagnosed in hyper endemic countries, where mild symptoms of chronic malaria my possibly lead to misdiagnosis. On the contrary, over-diagnosis may also occur. In fact, not all reported malaria cases are confirmed by microscopy or others assay, such as rapid diagnostic tests (RDTs). Furthermore, in hyper endemic areas febrile illnesses from different causes might be misdiagnosed with malaria.[3] Anyway, WHO guidelines recommends that microscopy or RDTs should be used to confirm all malaria cases.
Another issue is the lack of population denominator that makes the real incidence of malaria difficult to assess. Data emerging from WHO reports just estimate malaria incidence and mortality, reporting malarial cases and malarial death from the different WHO regions, collected by Ministries of Health of different countries. These data do not reflect the real incidence in the general population. Nevertheless, they are good indicators to assess malarial control programmes and to estimate the impact of malaria infection in health systems.
Malariometry
The term endemicity is a proxy to indicate disease prevalence. Areas presenting the same level of endemicity often have similar characteristics of disease distribution, guiding malaria experts to design, implement and monitor control and prevention activities.[4]
Malaria endemicity is a very complex issue, that is influenced by factors related to the man-host interactions (agricultural activities, nocturnal activities, migration movements, wars, limited resources), to the parasite (different species, sporogonic cycle length, drug susceptibility), to the vector (density, larvae breeding sites, temperature, receptivity, feeding pattern, longevity, insecticide susceptibility) and to the environment (physical – biological – socio-economic). The detailed analysis of all such variables is beyond the scope of this brief article that will only report available data, referring the interested reader to more exhaustive and valuable treatises on the topic.
Moreover, malaria incidence may fluctuate according to seasonality. Figure 2 shows the months of start and end of malaria transmission over the year in the African continent.
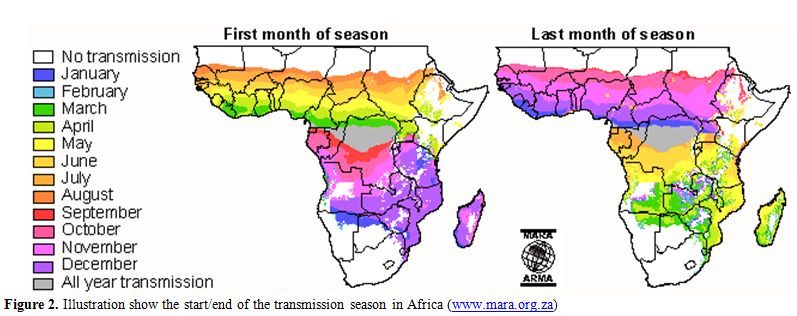
Figure 2. Illustration show the start/end of the transmission season in Africa (www.mara.org.za).
Different methods to classify malaria endemicity in a population exist. These methods includes (i) proportion of individuals in a population with a palpable enlargement of spleen (spleen rate [SR]), (ii) proportion of individuals in a population with a laboratory-confirmed parasite infection (parasite rate [PR]), (iii) number of infective bites per person (entomological inoculation rate [EIR]) and (iv) number of microscopically confirmed malaria cases detected during one year per unit population (annual parasite incidence [API]).[5]
Proportion of individuals with splenomegaly (SR) in a given population was the first method used to assess malaria endemicity during a malariometric survey in 1848 in India, where spleen dimension was assessed in selected population age groups. Thus, malariometry attention was focused on clinical manifestations of malaria. On the basis of splenomegaly prevalence rates in children from 2 to 9 years old, 4 different endemicity areas can be distinguished: holo-endemic areas, where proportion of people with splenomegaly is above 75%; hyper-endemic areas, where splenomegaly prevalence is between 51 and 75%; meso-endemic areas, with prevalence between 50 and 11%; hypo-endemic areas, where prevalence is below 11%.[5]
Parasite rate (PR) assesses the proportion of individuals with microscopically confirmed presence of asexual parasites in peripheral blood. It’s a technique that requires expert laboratory technicians and suffers of malaria seasonal variation.
Spleen and parasite rate are actually less used, whereas entomological inoculation rate (EIR) and annual parasite incidence (API) are utilized to prepare epidemiologic malaria maps that show malaria distribution in the world. Where data are unavailable, a model is required to predict malaria endemicity.[6-10] Many recent studies investigated a predictive framework known as model-based geostatistics (MBG) to asses malaria endemicity[11-15] and the prevalence of other vector-borne and intermediate host borne diseases.
Maps showing the global distribution of P. falciparum and P. vivax has recently been published by Malaria Atlas Project. These maps provide a geographical framework for monitoring malaria incidence and evaluation of impact on malaria control worldwide (Figures 3a and 3b).
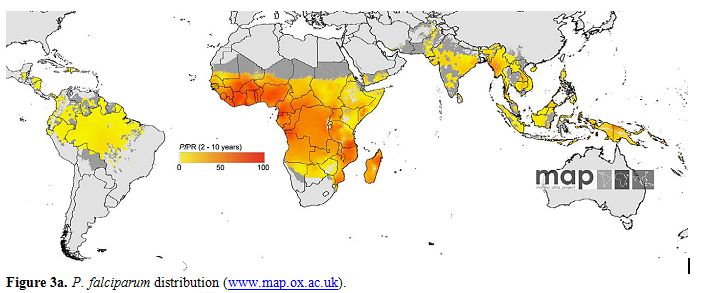
Figure 3a. P. falciparum distribution (www.map.ox.ac.uk).
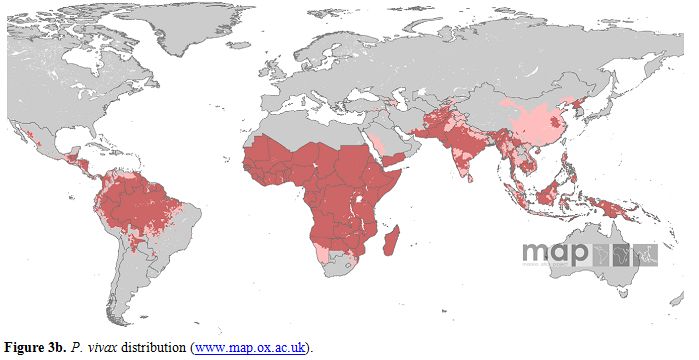
Figure 3a. P. vivax distribution (www.map.ox.ac.uk).
P. falciparum malaria endemicity has been mapped considering national malaria reports, medical intelligence and biological rules of transmission, such as temperature and aridity, important for Anopheles vectors spreading.[16,17] Thus, in 2007, the world was stratified into three spatial representation: (i) areas without P. falciparum malaria risk, (ii) unstable risk areas (P. falciparum annual parasite incidence [PfAPI]: < 0.1 per 1.000 people per annum [pa]) and (iii) stable risk areas (PfAPI > 0.1 per 1.000 people pa).[18] The global area at risk of stable P. falciparum malaria was quantified in 29.7 million km2, distributed into Africa (18.2 million km2, 61.1%), Americas (6.0 million km2, 20.3%) and Central and South East Asia regions (5.5 million km2, 18.6%). Of the 2.37 billion people exposed to P. falciparum transmission worldwide, 0.98 billion live in unstable risk areas,[16,17] whereas 1.383 billion live in stable risk areas, distributed into Africa (0.657 billion, 47.5%), Americas (0.041 billion, 2.9%) and Central and South East Asia (0.686 billion, 49.6%). Children are the most represented category, accounting for 32% of the population at risk in Americas and in Central and South East Asia. In Africa this percentage rise up to 43%.
P. vivax is transmitted in 95 tropical, subtropical and temperate countries.[19] People living at risk of P. vivax malaria infection are 2.85 billion, 91% living in Central and South East Asia region, 5.5% in America and 3,4% in Africa. As many as 57.1% of people exposed to P. vivax infection lives in unstable malaria areas.
Stable - unstable classification is another way to determine malaria endemicity. Macdonald defined malaria stability on the ground of the number of mosquitoes lifetime bites in the human host.[20] This vector-based index differentiated stable and unstable malaria. Vector-based classification is less used because of entomological-based metrics complexity, ethical concerns related to exposing human beings to malaria infection and measurement error issues.[5]
Endemic Areas Distribution
Different malaria endemic areas have different epidemiological situations and also the feasible targets may differ. According to WHO, the following terminology should be adopted when referring to malaria endemic status:
Malaria control: reducing the malaria disease burden to a level at which it is no longer a public health problem.
Malaria elimination: the interruption of local mosquito-borne malaria transmission; reduction to zero of the incidence of infection caused by human malaria parasites in a defined geographical area as a result of deliberate efforts; continued measures to prevent re-establishment of transmission are required.
Malaria eradication: permanent reduction to zero of the worldwide incidence of infection caused by a particular malaria parasite species. Intervention measures are no longer needed once eradication has been achieved.
Hereunder we summarize data from WHO Reports, showing malaria trends in different WHO Regions. Trends in malarial cases and deaths reflect control programmes, such as distribution of insecticide-treated nets (ITN), long lasting insecticidal nets (LLIN), use of indoor residual spraying (IRS) and artemisinin combination treatment (ACT).
On the ground of slide positivity rate (SPR) and of the population at risk of malaria, WHO distinguishes areas with advance malaria control activities in (I) pre-elimination phase, (II) elimination phase, (III) prevention of reintroduction and (IV) malaria-free stages as shown in table 2.[1]
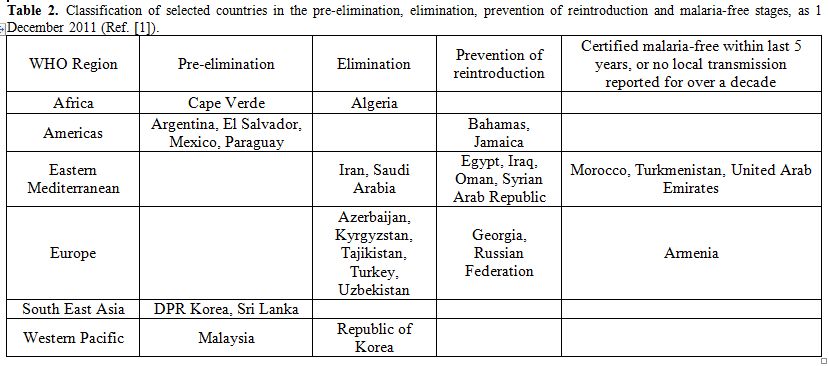
Table 2. Classification of selected countries in the pre-elimination, elimination, prevention of reintroduction and malaria-free stages, as 1 December 2011 (Ref. [1]).
Most malaria cases and deaths occur in the African Region. As a consequence of implementation programs, high burden countries of African Region, such as Madagascar, Sao Tome and Principe, Eritrea, Rwanda and Zambia, showed a decrease in malaria cases up to 50% between 2000 and 2009.[21] Rwanda showed a decrease by 74% of confirmed malarial cases between 2005 and 2010 and slide positivity rate decreased from 35% to 9%. Moreover, number of malaria hospital admissions and malaria deaths showed a decrease of 65% and 55% respectively. Zanzibar, belonging to United Republic of Tanzania, showed a dramatic decrease of malaria admissions and deaths due not only to the efficacy of control strategies, but also to favourable geographic position. In low-transmission countries of African Region control strategies have also been performed. Thanks to these strategies, Algeria is in the malaria elimination phase, Capo Verde in pre-elimination phase.[1]
In 15 countries of the WHO Region of the Americas, where P. vivax is the most represented species, reductions of more than 50% in the number of the reported cases were observed. During 2010, malaria transmission occurred in 21 countries, of which 17 are in the control stage and 4 are in the pre-elimination stage. Bahamas and Jamaica are in the prevention of reintroduction phase. In Ecuador, malaria cases dropped from 105.000 in 2000 to 4.120 in 2009, a reduction of 96% due to IRS, LLINs distribution, strengthening of malaria diagnosis and treatment and also due to Global Found, UNICEF, USAID and government funds invested in malaria control.[1]
In 2010, 2.4 million confirmed malaria cases were reported in WHO South-East Asia Region. India accounts for 66% of confirmed cases, even though a reduction of 28% of the cases between 2000 and 2010 was observed. In 2010, malaria deaths were 2.426 as reported from eight countries of the region, most of all reported in India. Democratic People’s Republic of Korea and Sri Lanka are actually in pre-elimination phase. Bangladesh, Bhutan, the Democratic Republic of Timor-Leste, India, Indonesia, Myanmar, Nepal and Thailand are in the control phase.
In the WHO European Region, the number of autochthonous cases decreased from 32.394 in 2000 to 176 in 2010. All malaria cases are now attributable to P. vivax infection; no P. falciparum cases occurred since 2008. Malaria cases were identified in Azerbaijan, Kyrgyzstan, Tajikistan, Turkey and Uzbekistan. Georgia reported no cases in 2010 and Turkmenistan was declared malaria-free in October 2010. A particular case is represented by Greece, a country that was declared malaria-free from 1974.
Since June 2011 a total of 63 autochthonous malaria cases have been reported,[21] all due to P. vivax infection. Cases occurred mostly in the southern region of the country, specifically of the Evrotas delta area of Laconia district in agricultural area with large migrant populations.[21,22] Other cases occurred in the Evia/Euboea (island east of the Central Greece region), Eastern Attiki, Voitia and Larissa districts.[21]
In the WHO Eastern Mediterranean Region, Islamic Republic of Iran and Saudi Arabia are in the elimination phase, while Egypt, Iraq, Oman and Syrian Arab Republic are in prevention of reintroduction phase. Morocco was confirmed malaria-free in May 2010. Afghanistan, Djibouti, Pakistan, Somalia, Sudan, South Sudan and Yemen are in the control stage, and they still represent high malaria transmission areas.
As many as 262.000 confirmed cases were reported from the WHO Western Pacific Region in 2010. Papua New Guinea, Cambodia and Solomon Island account for 70% of these malarial cases. China, Philippines, Republic of Korea and Vietnam showed a decrease in malaria cases up to 50% between 2000 and 2010, while other countries showed a more slowly decrease (e.g. Cambodia, Lao People’s Democratic Republic, Malaysia, Solomon Island, Vanuatu).
Plasmodium Species Distribution
Plasmodium species are differently distributed in the world. Prevalence of malaria cases and deaths differs during different seasons, as mentioned before. Prevalence data must be related to season and to endemicity of each countries.
The following paragraphs report available epidemiological data obtained in different geographical areas for the different Plasmodia species. While recognizing the value of the robust epidemiological research reviewed, we would like to underline that comparison among studies is virtually impossible because of lack of homogeneity in diagnostic methods, seasonality and methodological approach. The interested reader is then invited to refer to the selected articles for further information and details.
a) Plasmodium falciparum
Anopheles Vectors
Malaria is transmitted exclusively through the bites of Anopheles mosquitoes. There are 512 Anopheles species recognized worldwide and 50 only provisionally designated and awaiting description.[68] Seventy Anopheles species are able to transmit Plasmodium parasite to human hosts.[69] Anopheles mosquitoes breed in water and each species has its own breeding preference. Transmission is more intense in places where mosquito lifespan is longer (parasite has time to complete its development inside the mosquito) and where anthropophilic mosquitoes prevail. Forty-one of the 512 Anopheles species are defined by experts “Dominant Vector Species” (DVS). DVS are the most important malarial vector worldwide, providing the majority of human malaria cases. Characteristics of dominant vector species are their propensity for humans feeding, longevity, abundance and elevate vectorial capacity.[70] Many studies were recently conducted to define Anopheles distribution in Africa,[71,72] Americas,[73,74] Europe,[75] Central and South East Asia[76] and worldwide.[77]
Africa has the most effective and efficient DVS of human malaria, the Anopheles gambiae complex; thus some areas account the highest entomological inoculation rates and the highest malaria prevalence worldwide.[17,78,79] There are 4 principal species belonging to An. gambiae complex: An. gambiae, An. arabiensis, An. merus and An. melas. Other three highly anthropophilic DVS are spread in African region: An. funestus, An. moucheti and An. nili.[80]
Despite European and the Middle Eastern regions are low malaria transmission areas, existence of DVS may play a potential role in re-introduction of malaria.[78,81] An. atroparvus is mostly diffused in these regions.[80] Nine DVS were found in the Americas, where An. darlingi is considered one of the most efficient malaria vectors.[82]
In Asian-Pacific region 19 DVS were found; some of them are predominant in the Arabian Peninsula (e.g. An. stephensi and the An. culicifacies complex), others in the Indian subcontinent, China and Korea (e.g. An. lesteri), in the Solomon Islands and Vanuatu (e.g. the An. farauti complex) and finally in Queensland and the Northern Territory of Australia (e.g. the An. farauti complex). Myanmar appeared to contain the greatest number of DVS, of wich An. aconitus, An. annularis, An. barbirostris complex, An. culicifacies complex, An. dirus complex, An. maculatus group, An. minimus complex, An. sinensis complex, An. stephensi, An. subpictus complex and, in some coastal site, An. sundaicus complex.[83]
Environmental factors play an important role in vector distribution and malaria biodiversity. Climate seasonality, rainfall patterns, temperature, humidity, presence of vegetation and surface water all are directly related to the malaria transmission cycle. In addition, human activities such as agriculture, irrigation, deforestation, urbanization, population movements, dam/road constructions and wars are also connected to transmission levels and malaria epidemiology.[84]
Conclusion
Malaria is still considered a global health problem and a major killer. Morbidity and mortality burden of malaria could be reduced strengthening prevention, improving malaria diagnosis, using correct therapies based on artemisinin combination and adopting strategies aimed at preventing drug resistances.
Real malaria incidence is difficult to obtain. However, it is possible to make reliable estimates thanks to the data supplied by Ministries of Health of different countries and to accurate prevalence studies. Determination of real incidence of malaria and determination of the real Plasmodium species distribution are two different issues that could help expert in eradication of malaria, the real and unique goal in the fight against malaria.
Malaria is one of the most important public health problem in term of morbidity and mortality, causing more than 200 million cases and 655.000 deaths every year.[1]
According to the World Health Organization (WHO) Malaria Report 2011, a total of 106 countries in the world are at risk of transmission of malaria infection (Figure 1).
A total of 216 million estimated malaria cases occurred in 2010, 81% of which were reported in the African Region, followed by South East Asia (13%) and Eastern Mediterranean Region (5%). The total number of malaria deaths was estimated to be 655.000 in 2010; 91% of whom occurred in the African Region, 6% in South-East Asia and 3% in Eastern Mediterranean Region (Table 1).
Although the proportion of people exposed to malaria parasites has decreased during the last century, the absolute number of people at risk for malaria infection increased from 0.8 billion in 1900 to 3.3 billion in 2010, as a consequence of the absolute increase of the population living in malaria-endemic regions.[1,2] However, between 2005 and 2010 malaria cases decreased from 244 million to 216 million; moreover, malaria mortality rates showed a global reduction of 26% between 2000 and 2010.

Figure 1. Distribution of territories at risk of malaria transmission, 2010
(www.gamapserver.who.itn/maplibrary/Files/Map/Global_Malaria_2010.png).

Table 1. Malaria cases and death by geographical region (World Malaria Report 2011, modified).
Malaria in humans is caused by 5 Plasmodium parasites: Plasmodium falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi. The current distribution of human-pathogenic Plasmodium species shows preponderance of P. falciparum in tropical Africa, while P. vivax prevails over P. falciparum in South America. Both P. falciparum and P. vivax are prevalent in south-eastern Asia and western Pacific. Although P. malariae may occur in all malarious areas, its prevalence is generally low. In tropical Africa, P. falciparum and P. malariae co-infection is sometimes encountered. P. ovale is widespread principally in tropical Africa whereas P. knowlesi infection occurs only in certain forested areas of South-East Asia.
Malaria burden is hard to estimate, particularly in low income countries where data collection and reporting quality is poor. Incomplete and discontinuous reports from single health facilities may alter final global malaria prevalence. Malaria cases are often under-diagnosed in hyper endemic countries, where mild symptoms of chronic malaria my possibly lead to misdiagnosis. On the contrary, over-diagnosis may also occur. In fact, not all reported malaria cases are confirmed by microscopy or others assay, such as rapid diagnostic tests (RDTs). Furthermore, in hyper endemic areas febrile illnesses from different causes might be misdiagnosed with malaria.[3] Anyway, WHO guidelines recommends that microscopy or RDTs should be used to confirm all malaria cases.
Another issue is the lack of population denominator that makes the real incidence of malaria difficult to assess. Data emerging from WHO reports just estimate malaria incidence and mortality, reporting malarial cases and malarial death from the different WHO regions, collected by Ministries of Health of different countries. These data do not reflect the real incidence in the general population. Nevertheless, they are good indicators to assess malarial control programmes and to estimate the impact of malaria infection in health systems.
Malariometry
The term endemicity is a proxy to indicate disease prevalence. Areas presenting the same level of endemicity often have similar characteristics of disease distribution, guiding malaria experts to design, implement and monitor control and prevention activities.[4]
Malaria endemicity is a very complex issue, that is influenced by factors related to the man-host interactions (agricultural activities, nocturnal activities, migration movements, wars, limited resources), to the parasite (different species, sporogonic cycle length, drug susceptibility), to the vector (density, larvae breeding sites, temperature, receptivity, feeding pattern, longevity, insecticide susceptibility) and to the environment (physical – biological – socio-economic). The detailed analysis of all such variables is beyond the scope of this brief article that will only report available data, referring the interested reader to more exhaustive and valuable treatises on the topic.
Moreover, malaria incidence may fluctuate according to seasonality. Figure 2 shows the months of start and end of malaria transmission over the year in the African continent.

Figure 2. Illustration show the start/end of the transmission season in Africa (www.mara.org.za).
Different methods to classify malaria endemicity in a population exist. These methods includes (i) proportion of individuals in a population with a palpable enlargement of spleen (spleen rate [SR]), (ii) proportion of individuals in a population with a laboratory-confirmed parasite infection (parasite rate [PR]), (iii) number of infective bites per person (entomological inoculation rate [EIR]) and (iv) number of microscopically confirmed malaria cases detected during one year per unit population (annual parasite incidence [API]).[5]
Proportion of individuals with splenomegaly (SR) in a given population was the first method used to assess malaria endemicity during a malariometric survey in 1848 in India, where spleen dimension was assessed in selected population age groups. Thus, malariometry attention was focused on clinical manifestations of malaria. On the basis of splenomegaly prevalence rates in children from 2 to 9 years old, 4 different endemicity areas can be distinguished: holo-endemic areas, where proportion of people with splenomegaly is above 75%; hyper-endemic areas, where splenomegaly prevalence is between 51 and 75%; meso-endemic areas, with prevalence between 50 and 11%; hypo-endemic areas, where prevalence is below 11%.[5]
Parasite rate (PR) assesses the proportion of individuals with microscopically confirmed presence of asexual parasites in peripheral blood. It’s a technique that requires expert laboratory technicians and suffers of malaria seasonal variation.
Spleen and parasite rate are actually less used, whereas entomological inoculation rate (EIR) and annual parasite incidence (API) are utilized to prepare epidemiologic malaria maps that show malaria distribution in the world. Where data are unavailable, a model is required to predict malaria endemicity.[6-10] Many recent studies investigated a predictive framework known as model-based geostatistics (MBG) to asses malaria endemicity[11-15] and the prevalence of other vector-borne and intermediate host borne diseases.
Maps showing the global distribution of P. falciparum and P. vivax has recently been published by Malaria Atlas Project. These maps provide a geographical framework for monitoring malaria incidence and evaluation of impact on malaria control worldwide (Figures 3a and 3b).

Figure 3a. P. falciparum distribution (www.map.ox.ac.uk).

Figure 3a. P. vivax distribution (www.map.ox.ac.uk).
P. falciparum malaria endemicity has been mapped considering national malaria reports, medical intelligence and biological rules of transmission, such as temperature and aridity, important for Anopheles vectors spreading.[16,17] Thus, in 2007, the world was stratified into three spatial representation: (i) areas without P. falciparum malaria risk, (ii) unstable risk areas (P. falciparum annual parasite incidence [PfAPI]: < 0.1 per 1.000 people per annum [pa]) and (iii) stable risk areas (PfAPI > 0.1 per 1.000 people pa).[18] The global area at risk of stable P. falciparum malaria was quantified in 29.7 million km2, distributed into Africa (18.2 million km2, 61.1%), Americas (6.0 million km2, 20.3%) and Central and South East Asia regions (5.5 million km2, 18.6%). Of the 2.37 billion people exposed to P. falciparum transmission worldwide, 0.98 billion live in unstable risk areas,[16,17] whereas 1.383 billion live in stable risk areas, distributed into Africa (0.657 billion, 47.5%), Americas (0.041 billion, 2.9%) and Central and South East Asia (0.686 billion, 49.6%). Children are the most represented category, accounting for 32% of the population at risk in Americas and in Central and South East Asia. In Africa this percentage rise up to 43%.
P. vivax is transmitted in 95 tropical, subtropical and temperate countries.[19] People living at risk of P. vivax malaria infection are 2.85 billion, 91% living in Central and South East Asia region, 5.5% in America and 3,4% in Africa. As many as 57.1% of people exposed to P. vivax infection lives in unstable malaria areas.
Stable - unstable classification is another way to determine malaria endemicity. Macdonald defined malaria stability on the ground of the number of mosquitoes lifetime bites in the human host.[20] This vector-based index differentiated stable and unstable malaria. Vector-based classification is less used because of entomological-based metrics complexity, ethical concerns related to exposing human beings to malaria infection and measurement error issues.[5]
Endemic Areas Distribution
Different malaria endemic areas have different epidemiological situations and also the feasible targets may differ. According to WHO, the following terminology should be adopted when referring to malaria endemic status:
Malaria control: reducing the malaria disease burden to a level at which it is no longer a public health problem.
Malaria elimination: the interruption of local mosquito-borne malaria transmission; reduction to zero of the incidence of infection caused by human malaria parasites in a defined geographical area as a result of deliberate efforts; continued measures to prevent re-establishment of transmission are required.
Malaria eradication: permanent reduction to zero of the worldwide incidence of infection caused by a particular malaria parasite species. Intervention measures are no longer needed once eradication has been achieved.
Hereunder we summarize data from WHO Reports, showing malaria trends in different WHO Regions. Trends in malarial cases and deaths reflect control programmes, such as distribution of insecticide-treated nets (ITN), long lasting insecticidal nets (LLIN), use of indoor residual spraying (IRS) and artemisinin combination treatment (ACT).
On the ground of slide positivity rate (SPR) and of the population at risk of malaria, WHO distinguishes areas with advance malaria control activities in (I) pre-elimination phase, (II) elimination phase, (III) prevention of reintroduction and (IV) malaria-free stages as shown in table 2.[1]

Table 2. Classification of selected countries in the pre-elimination, elimination, prevention of reintroduction and malaria-free stages, as 1 December 2011 (Ref. [1]).
Most malaria cases and deaths occur in the African Region. As a consequence of implementation programs, high burden countries of African Region, such as Madagascar, Sao Tome and Principe, Eritrea, Rwanda and Zambia, showed a decrease in malaria cases up to 50% between 2000 and 2009.[21] Rwanda showed a decrease by 74% of confirmed malarial cases between 2005 and 2010 and slide positivity rate decreased from 35% to 9%. Moreover, number of malaria hospital admissions and malaria deaths showed a decrease of 65% and 55% respectively. Zanzibar, belonging to United Republic of Tanzania, showed a dramatic decrease of malaria admissions and deaths due not only to the efficacy of control strategies, but also to favourable geographic position. In low-transmission countries of African Region control strategies have also been performed. Thanks to these strategies, Algeria is in the malaria elimination phase, Capo Verde in pre-elimination phase.[1]
In 15 countries of the WHO Region of the Americas, where P. vivax is the most represented species, reductions of more than 50% in the number of the reported cases were observed. During 2010, malaria transmission occurred in 21 countries, of which 17 are in the control stage and 4 are in the pre-elimination stage. Bahamas and Jamaica are in the prevention of reintroduction phase. In Ecuador, malaria cases dropped from 105.000 in 2000 to 4.120 in 2009, a reduction of 96% due to IRS, LLINs distribution, strengthening of malaria diagnosis and treatment and also due to Global Found, UNICEF, USAID and government funds invested in malaria control.[1]
In 2010, 2.4 million confirmed malaria cases were reported in WHO South-East Asia Region. India accounts for 66% of confirmed cases, even though a reduction of 28% of the cases between 2000 and 2010 was observed. In 2010, malaria deaths were 2.426 as reported from eight countries of the region, most of all reported in India. Democratic People’s Republic of Korea and Sri Lanka are actually in pre-elimination phase. Bangladesh, Bhutan, the Democratic Republic of Timor-Leste, India, Indonesia, Myanmar, Nepal and Thailand are in the control phase.
In the WHO European Region, the number of autochthonous cases decreased from 32.394 in 2000 to 176 in 2010. All malaria cases are now attributable to P. vivax infection; no P. falciparum cases occurred since 2008. Malaria cases were identified in Azerbaijan, Kyrgyzstan, Tajikistan, Turkey and Uzbekistan. Georgia reported no cases in 2010 and Turkmenistan was declared malaria-free in October 2010. A particular case is represented by Greece, a country that was declared malaria-free from 1974.
Since June 2011 a total of 63 autochthonous malaria cases have been reported,[21] all due to P. vivax infection. Cases occurred mostly in the southern region of the country, specifically of the Evrotas delta area of Laconia district in agricultural area with large migrant populations.[21,22] Other cases occurred in the Evia/Euboea (island east of the Central Greece region), Eastern Attiki, Voitia and Larissa districts.[21]
In the WHO Eastern Mediterranean Region, Islamic Republic of Iran and Saudi Arabia are in the elimination phase, while Egypt, Iraq, Oman and Syrian Arab Republic are in prevention of reintroduction phase. Morocco was confirmed malaria-free in May 2010. Afghanistan, Djibouti, Pakistan, Somalia, Sudan, South Sudan and Yemen are in the control stage, and they still represent high malaria transmission areas.
As many as 262.000 confirmed cases were reported from the WHO Western Pacific Region in 2010. Papua New Guinea, Cambodia and Solomon Island account for 70% of these malarial cases. China, Philippines, Republic of Korea and Vietnam showed a decrease in malaria cases up to 50% between 2000 and 2010, while other countries showed a more slowly decrease (e.g. Cambodia, Lao People’s Democratic Republic, Malaysia, Solomon Island, Vanuatu).
Plasmodium Species Distribution
Plasmodium species are differently distributed in the world. Prevalence of malaria cases and deaths differs during different seasons, as mentioned before. Prevalence data must be related to season and to endemicity of each countries.
The following paragraphs report available epidemiological data obtained in different geographical areas for the different Plasmodia species. While recognizing the value of the robust epidemiological research reviewed, we would like to underline that comparison among studies is virtually impossible because of lack of homogeneity in diagnostic methods, seasonality and methodological approach. The interested reader is then invited to refer to the selected articles for further information and details.
a) Plasmodium falciparum
P. falciparum is widespread in nearly all malaria endemic countries. A study identified 2.37 billion people at risk of P. falciparum transmission worldwide, 26% located in the African Region and 62% in South Eeast Asian and Western Pacific regions.[17] In Africa, many epidemiological studies suggest that P. falciparum
is the most prevalent malarial species. Blood samples were collected
between 1998 and 2006 from nine different African countries and
analyzed by PCR for the presence of each of the four human malaria
parasites.[23] Out of 2.588 samples, 1.737 were positive for Plasmodium species and 1.711 (98,5%) were positive for P. falciparum
considering both mono and mixed infection. Another study performed in 4
villages in Mulanda sub-county, in eastern Uganda, showed a prevalence
of P. falciparum infection of 94% during rainy season, from July to December, using thin film diagnosis.[24] A study performed in metropolitan Lagos, Nigeria, showed a microscopic prevalence of P. falciparum species of 88,5% in pregnant women attending antenatal care clinic, during one observation year.[25] In Asia, P. falciparum and P. vivax are the two prevalent species. In India, P. falciparum is mostly widespread in Orissa state[27,28] while in the west of the country mixed infections are predominant.[27] In Bangladesh, samples collected during 3 years from febrile patients and analyzed by species-specific PCR showed a P. falciparum prevalence of 81,5%.[29] In Cambodia, falciparum prevalence among residents of 8 villages was about 59% using a new PCR technique.[30] In Thailand (Tak, Chantaburi, Prachuab Khirikhan, Yala and Narathiwat Provinces), PCR research of P. falciparum among febrile patients from October 2006 to September 2007 showed a prevalence of 43,5% both in mono and in mixed infection.[31]
Samples collected from 146 selected patients with uncomplicated malaria
in 2008 in southern Myanmar underwent PCR analysis to investigate
malaria parasites: the prevalence of P. falciparum was 52,1% considering mono and mixed infection.[32] In South America P. vivax is the predominant species, followed by P. falciparum (25.7%).[33] Most of the malaria cases occur in Brazil; the others are distributed in 20 other countries of Central and South America.[33]
b) Plasmodium vivaxIn Central and Western Africa P. vivax
infection is rare because of the high prevalence of the red blood cells
Duffy negative phenotype in the population, interfering with P. vivax merozoite entry into the red blood cells. A large study carried out in nine African countries failed to detect P. vivax species.[23] Despite these studies, however, there are some evidence of P. vivax transmission in West and Central Africa. In Congo, specific P. vivax
antibodies were researched in 409 samples from patients coming from an
health center located on the west coast, where Duffy antigen is
expected to be > 95%. Out of the 409 samples, 55 (13%) tested
positive for specific P. vivax antibodies.[34] Another study from Kenya demonstrated the presence of P. vivax among mosquitoes; in addition, P. vivax DNA was amplified and sequenced in blood of two Duffy negative children.[35] In eastern and southern Africa only 5% of malaria infections are attributable to P. vivax.[36] In Asia, P. vivax and P. falciparum are the predominant species.[37] In India, isolate P. vivax infection is widespread in the southern state of Tamil Nadu, while mixed-species infections are prevalent in the west.[27] In Bangladesh, samples collected from febrile patients underwent PCR research of Plasmodium species, showing a prevalence of P. vivax infection of 15,3% in mono-infected patients and of 27,5% in mixed infections.[29] In Cambodia, prevalence of P. vivax infection detected by PCR method in samples collected in September 2001 was 15%.[30] Studies performed in Thailand and in Myanmar showed that P. vivax is the most prevalent malarial species,[31,32] such as in the WHO Eastern Mediterranean Region, in particular in Afghanistan, Islamic Republic of Iran and Turkey.[38-41] In Central and South America P. vivax is the predominant plasmodium species, accounting for 71-81% of all malaria cases.[33,35] Studies demonstrate that P. vivax accounts for 83,7% of malarial infections in Brazil,[43] for 70% of infections in Colombia[44] and for 90% of infections in Ecuador.[45]
c) Plasmodium ovaleThe real burden of P. ovale malaria is difficult to asses because its diagnosis is difficult. Plasmodium ovale may be encountered in sub-Saharan Africa and in Asia.[46]
A recent study from Mozambique tested malaria prevalence among febrile
patients: only 2 of 111 malaria positive patients presented P. ovale mono-infection, while 4 P. ovale and P. falciparum mixed infections were also detected.[47] In Congo, a cross-sectional, population-based cluster household survey of adults aged 15–59 years demonstrated that P. ovale parasitemia was rare and its prevalence in mono-infection was only 0,1%.[26]
In a recent multicenter study, blood samples were collected from the
indigenous population of nine African countries and malaria parasites
were searched by PCR method. Of 1.737 samples, 67 were positive for P. ovale: 12 single infections, 51 mixed with P. falciparum and 4 triple infections with P. falciparum and P. malariae. When samples from Rwanda, Mozambique, Angola and Sao Tome were excluded, P. ovale infection represented 3,9% of all malaria cases.[23] Another study performed in Congo-Brazzaville, Uganda and Equatorial Guinea, concluded that two P. ovale species, P. o. curtisi and P. o. wallikeri, are both widespread in Africa; in Uganda and Equatorial Guinea the prevalence of P. ovale spp. in population-based samples was found to be between 1% and 6%.[48]
There are many evidence of P. ovale infection in Asia. Samples collected during 2007/2008 in Bangladesh from 379 febrile patients who underwent microscopic, DNA extraction and nested PCR analysis: 3 of the 189 positive samples (1,6%) were positive for P. ovale.[29] Nested PCR detected P. ovale parasites in 1,3% of blood samples collected from 1.356 inhabitants of eight villages of Rattanakiri Province (Cambodia).[30] In a study performed in Myanmar, P. ovale was detected by PCR technique in 4,9% of malaria positive samples; most of cases were co-infections with P. falciparum, P. vivax and/or P. malariae.[49] Case reports of P. ovale infection were recently published from Gujarat, India,[50] Malaysia[51] and Sri Lanka.[52] P. ovale infection is present in Papua, Indonesia[53] and in Thailand[31], while it is very rare in Philippines, where has been reported only in the island of Palawan.[54]
d) Plasmodium malariaeThere are many evidence of P. ovale infection in Asia. Samples collected during 2007/2008 in Bangladesh from 379 febrile patients who underwent microscopic, DNA extraction and nested PCR analysis: 3 of the 189 positive samples (1,6%) were positive for P. ovale.[29] Nested PCR detected P. ovale parasites in 1,3% of blood samples collected from 1.356 inhabitants of eight villages of Rattanakiri Province (Cambodia).[30] In a study performed in Myanmar, P. ovale was detected by PCR technique in 4,9% of malaria positive samples; most of cases were co-infections with P. falciparum, P. vivax and/or P. malariae.[49] Case reports of P. ovale infection were recently published from Gujarat, India,[50] Malaysia[51] and Sri Lanka.[52] P. ovale infection is present in Papua, Indonesia[53] and in Thailand[31], while it is very rare in Philippines, where has been reported only in the island of Palawan.[54]
P. malariae
is spread in sub-Saharan Africa, in southeast Asia, in Indonesia, in
many islands in western Pacific and in areas of the Amazon Basin of
South America. Its distribution overlaps with that of P. falciparum.[55]
In a recent study, blood samples were collected from the indigenous
population of nine African countries and malaria parasites were
searched by PCR method. Plasmodium malariae was found in 147 of the 1.737 positive blood samples, 14 as mono-infections, 129 as mixed infections with P. falciparum and 4 as triple infections with P. ovale and P. falciparum.[23] Excluding samples from Rwanda, Mozambique, Angola and Sao Tome, P. malariae infections represented 8.5% of all malaria infections.[23]
In Nigeria, between November 2001 and October 2002, a total of 350
pregnant women attending the ante-natal clinics were randomly recruited
and blood samples were collected. Of 350 blood samples, 96 (27,4%) were
positive for malaria parasite and 11 (11,5%) were P. malariae positive as tested by microscopy.[25]
During the rainy season, blood samples were collected from resident
people of four village in Mulanda, Uganda. Of 709 malaria positive
samples, 6% were positive for P. malariae.[24] In the state of Orissa, India, the prevalence of P. malariae detected by PCR method was 44,6% during the peak season of malaria incidence.[28] A recent case report was published demonstrating the presence of P. malariae in Bangladesh.[56] In Papua New Guinea, prevalence of P. malariae
was detected by nested PCR, by quantitative PCR and by PCR-ligase
detection reaction-fluorescent microsphere assay (PCR-LDR-FMA); the
results were 3,3%, 4,7% and 7,7% respectively.[57] Plasmodium malariae infection was confirmed in Thailand[58] and in Yemen.[42] P. malariae is present in Brazil, in French Guiana and in Venezuela.[43,59,60] A report recently published confirmed P. malariae presence in Haitian refugees in Jamaica.[61]
e) P. knowlesiPlasmodium knowlesi
infection is localized only in the South East Asia Region and interest
both monkeys, where it was first reported, and humans. Forest areas are
the reservoirs of P. knowlesi,
that was first reported in humans in 1965 in a man who had worked in
the jungle of Pahang, Peninsular Malaysia. An analysis of stored blood
films identified cases of Plasmodium knowlesi infection occurring since 1996 in Sarawak region, Malaysian Borneo.[62]
In Sabah region, Malaysian Borneo, samples were collected from February
to November 2010 from patients with suspected malaria. Nested PCR was
performed in all 243 samples collected; of 107 samples positive for
malaria parasite, 63 were positive for P. knowlesi, demonstrating an high incidence of P. knowlesi infection in the interior division of Sabah.[63]
Another study performed in Thailand screened 1.874 samples collected
from febrile patients during a period of one year. Of the 1.751 sample
positive for malaria parasites, 10 were positive for P. knowlesi, that mostly occurred in males with uncomplicated malaria coming from southern and southwestern regions of Thailand.[31] P. knowlesi mono and mixed infection were also demonstrated in Myanmar,[32] in the Philippines[64] and in Singapore.[65] According to a three years prospective study performed in Cambodia, two P. knowlesi malaria cases were diagnosed by nested PCR.[66] A recent study performed in Vietnam showed the presence of P. knowlesi in three of ninety-five samples of patients with P. malaria mono or mixed infection, demonstrating for the first time the presence of P. knowlesi malaria in Vietnam.[67]
Anopheles Vectors
Malaria is transmitted exclusively through the bites of Anopheles mosquitoes. There are 512 Anopheles species recognized worldwide and 50 only provisionally designated and awaiting description.[68] Seventy Anopheles species are able to transmit Plasmodium parasite to human hosts.[69] Anopheles mosquitoes breed in water and each species has its own breeding preference. Transmission is more intense in places where mosquito lifespan is longer (parasite has time to complete its development inside the mosquito) and where anthropophilic mosquitoes prevail. Forty-one of the 512 Anopheles species are defined by experts “Dominant Vector Species” (DVS). DVS are the most important malarial vector worldwide, providing the majority of human malaria cases. Characteristics of dominant vector species are their propensity for humans feeding, longevity, abundance and elevate vectorial capacity.[70] Many studies were recently conducted to define Anopheles distribution in Africa,[71,72] Americas,[73,74] Europe,[75] Central and South East Asia[76] and worldwide.[77]
Africa has the most effective and efficient DVS of human malaria, the Anopheles gambiae complex; thus some areas account the highest entomological inoculation rates and the highest malaria prevalence worldwide.[17,78,79] There are 4 principal species belonging to An. gambiae complex: An. gambiae, An. arabiensis, An. merus and An. melas. Other three highly anthropophilic DVS are spread in African region: An. funestus, An. moucheti and An. nili.[80]
Despite European and the Middle Eastern regions are low malaria transmission areas, existence of DVS may play a potential role in re-introduction of malaria.[78,81] An. atroparvus is mostly diffused in these regions.[80] Nine DVS were found in the Americas, where An. darlingi is considered one of the most efficient malaria vectors.[82]
In Asian-Pacific region 19 DVS were found; some of them are predominant in the Arabian Peninsula (e.g. An. stephensi and the An. culicifacies complex), others in the Indian subcontinent, China and Korea (e.g. An. lesteri), in the Solomon Islands and Vanuatu (e.g. the An. farauti complex) and finally in Queensland and the Northern Territory of Australia (e.g. the An. farauti complex). Myanmar appeared to contain the greatest number of DVS, of wich An. aconitus, An. annularis, An. barbirostris complex, An. culicifacies complex, An. dirus complex, An. maculatus group, An. minimus complex, An. sinensis complex, An. stephensi, An. subpictus complex and, in some coastal site, An. sundaicus complex.[83]
Environmental factors play an important role in vector distribution and malaria biodiversity. Climate seasonality, rainfall patterns, temperature, humidity, presence of vegetation and surface water all are directly related to the malaria transmission cycle. In addition, human activities such as agriculture, irrigation, deforestation, urbanization, population movements, dam/road constructions and wars are also connected to transmission levels and malaria epidemiology.[84]
Conclusion
Malaria is still considered a global health problem and a major killer. Morbidity and mortality burden of malaria could be reduced strengthening prevention, improving malaria diagnosis, using correct therapies based on artemisinin combination and adopting strategies aimed at preventing drug resistances.
Real malaria incidence is difficult to obtain. However, it is possible to make reliable estimates thanks to the data supplied by Ministries of Health of different countries and to accurate prevalence studies. Determination of real incidence of malaria and determination of the real Plasmodium species distribution are two different issues that could help expert in eradication of malaria, the real and unique goal in the fight against malaria.
References
- World Malaria Report 2011. http://www.who.int/malaria/world_malaria_report_2011/97892415 64403_eng.pdf
- Hay SI, Guerra CA, Tatem AJ, Noor AM, Neve
Rw. The global distribution and population at risk of malaria: past,
present, and future. Lancet Infect Dis. 2004; 4 (6): 327–336. http://dx.doi.org/10.1016/S1473-3099(04)01043-6

- Sullivan D. Uncertainty in Mapping Malaria Epidemiology: Implications for Control. Epidemiol Rev. 2010; 1: 175-187. http://dx.doi.org/10.1093/epirev/mxq013 PMid:20581219 PMCid:2912605

- Malaria endemicity. http://www.map.ox.ac.uk/explore/about-malaria/malaria-endemicity/
- Hay SI, Smitha DL and Snow RW. Measuring
malaria endemicity from intense to interrupted transmission. Lancet
Infect Dis. 2008; 8 (6): 369–378. http://dx.doi.org/10.1016/S1473-3099(08)70069-0

- Kleinschmidt I, Bagayoko M, Clarke GPY,
Craig M, Le Sueur D. A spatial statistical approach to malaria mapping.
Int J Epidemiol. 2000; 29: 355–361. http://dx.doi.org/10.1093/ije/29.2.355 PMid:10817136

- Kleinschmidt I, Omumbo J, Briet O, van de
Giesen N, Sogoba N, Mensah NK, Windmeijer P, Moussa M, Teuscher T. An
empirical malaria distribution map for West Africa. Trop Med Int
Health. 2001; 6: 779–786. http://dx.doi.org/10.1046/j.1365-3156.2001.00790.x PMid:11679126

- Rogers DJ, Randolph SE, Snow RW, Hay SI. Satellite imagery in the study and forecast of malaria. Nature. 2002; 415: 710–715. http://dx.doi.org/10.1038/415710a PMid:11832960 PMCid:3160466

- Omumbo JA, Hay SI, Snow RW, Tatem AJ,
Rogers DJ. Modelling malaria risk in East Africa at high-spatial
resolution. Trop Med Int Health. 2005; 10: 557–566. http://dx.doi.org/10.1111/j.1365-3156.2005.01424.x PMid:15941419 PMCid:3191364

- Rogers DJ. Models for vectors and vector-borne diseases. Adv Parasitol. 2006; 62: 1-35. http://dx.doi.org/10.1016/S0065-308X(05)62001-5

- Gemperli A, Vounatsou P, Kleinschmidt I,
Bagayoko M, Lengeler C, Smith C. Spatial patterns of infant mortality
in Mali: the effect of malaria endemicity. Am J Epidemiol. 2004; 159:
64–72. http://dx.doi.org/10.1093/aje/kwh001 PMid:14693661

- Gemperli A, Vounatsou P, Sogoba N, Smith
T. Malaria mapping using transmission models: application to survey
data from Mali. Am J Epidemiol. 2006; 163: 289–297. http://dx.doi.org/10.1093/aje/kwj026 PMid:16357113

- Rattanasiri S, Bohning D, Rojanavipart P,
Athipanyakom S. A mixture model application in disease mapping of
malaria. Southeast Asian. J Trop Med Public Health. 2004; 35: 38–47.
PMid:15272743

- Gosoniu L, Vounatsou P, Sogoba N, Smith T.
Bayesian modelling of geostatistical malaria risk data. Geospat Health.
2006; 1: 127–139. PMid:18686238

- Noor AM, Clements ACA, Gething PW, Moloney
G, Borle M, Shewchuk T, Fieno SI, Neve RW. Spatial prediction of
Plasmodium falciparum prevalence in Somalia. Malar J. 2008; 7:159. http://dx.doi.org/10.1186/1475-2875-7-159 PMid:18717998 PMCid:2531188

- Guerra CA. Mapping the contemporary global
distribution limits of malaria using empirical data and expert opinion.
Oxford, University of Oxford. 2007; 258.
- Guerra CA, Gikandi PW, Tatem AJ, Noor AM,
Smith DL, Hay SI, Snow RW. The limits and intensity of Plasmodium
falciparum transmission: implications for malaria control and
elimination worldwide. PLoS Medicine. 2008; 5 (2): e38. http://dx.doi.org/10.1371/journal.pmed.0050038 PMid:18303939 PMCid:2253602

- Hay SI, Guerra CA, Gething PW, Patil AP,
Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, Brooker S, Smith
DL, Moyeed RA, Snow RW. A world malaria map: Plasmodium falciparum
endemicity in 2007. PLoS Med. 2009; 6 (3): e1000048. http://dx.doi.org/10.1371/journal.pmed.1000048 PMid:19323591 PMCid:2659708

- Guerra CA, Howes RE, Patil AP, Gething PW,
Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar
IR, Baird JK, Snow RW, Hay SI. The International Limits and Population
at Risk of Plasmodium vivax Transmission in 2009. PLoS Negl Trop Dis.
2010; 4(8): e774. http://dx.doi.org/10.1371/journal.pntd.0000774 PMid:20689816 PMCid:2914753

- Macdonald, G. The epidemiology and control
of malaria. Oxford University Press; London: 1957. Local features of
malaria; p. 63-99.
- Malaria recommendation update: Greece. http://www.cdc.gov/malaria/malariagreece.htm
- European Centre for Disease Prevention and
Control. Autochthonous Plasmodium vivax malaria in Greece. RISK
ASSESSMENT - UPDATE. European Centre for Disease Prevention and
Control, Stockholm, 2011.
- Culleton RL, Mita T, Ndounga M, Unger H,
Cravo PVL, Paganotti GM, Takahashi N, Kaneko A, Eto H, Tinto H, Karema
C, D'Alessandro U, Do Rosário V, Kobayakawa T, Ntoumi F, Carter R,
Tanabe K. Failure to detect Plasmodium vivax in West and Central Africa
by PCR species typing. Malar J. 2008; 7: 174. http://dx.doi.org/10.1186/1475-2875-7-174 PMid:18783630 PMCid:2546428

- Pullan RL, Bukirwa H, Staedke SG, Snow RW,
Brooker S. Plasmodium infection and its risk factors in eastern Uganda.
Malar J. 2010; 9: 2. http://dx.doi.org/10.1186/1475-2875-9-2 PMid:20044942 PMCid:2822788

- Iriemenam NC, Dosunmu AO, Oyibo WA,
Fagbenro-Beyioku AF. Knowledge, attitude, perception of malaria and
evaluation of malaria parasitaemia among pregnant women attending
antenatal care clinic in metropolitan Lagos, Nigeria. J Vector Borne
Dis. 2011; 48: 12–17. PMid:21406732

- Taylor SM, Messina JP, Hand CC, Juliano
JJ, Uwonga J, Tshefu AK, Atua B, Emch M, Meshnick SR. Molecular malaria
epidemiology: mapping and burden estimates for the Democratic Republic
of the Congo, 2007. PLoS ONE. 2011; 6(1): e16420.

- Das A, Anvikar AR, Cator LJ, Dhiman RC,
Eapen A, Mishra N, B. Nagpal N, Nanda N, Raghavendra K, Read AF, Sharma
SK, Singh OP, Singh V, Sinni P, Srivastava HC, Sullivan SA, Sutton PL,
Thomas MB, Carlton JM, Valecha N. Malaria in India: The Center for the
Study of Complex Malaria in India. Acta Trop. 2011; 28.

- Dhangadamajhi G, Kar SK, Ranjit MR. High
prevalence and gender bias in distribution of Plasmodium malariae
infection in central east-coast India. Trop Biomed. 2009; 26(3):
326–333. PMid:20237447

- Fuehrer H, Starzengruber P, Swoboda P,
Khan WA, Matt J, Ley B, Thriemer K, Haque R, Yunus EB, Hossain SM,
Walochnik J, Noedl H. Indigenous Plasmodium ovale Malaria in
Bangladesh. Am J Trop Med. Hyg. 2010; 83(1): 75–78. http://dx.doi.org/10.4269/ajtmh.2010.09-0796 PMid:20595481 PMCid:2912579

- Steenkeste N, Rogers WO, Okell L, Jeanne
I, Incardona S, Duval L, Chy S, Hewitt S, Chou M, Socheat D, Babin F,
Ariey F, Rogier C. Sub-microscopic malaria cases and mixed malaria
infection in a remote area of high malaria endemicity in Rattanakiri
province, Cambodia: implication for malaria elimination. Mal J. 2010;
9: 108. http://dx.doi.org/10.1186/1475-2875-9-108 PMid:20409349 PMCid:2868861

- Putaporntip C, Hongsrimuang T, Seethamchai
S, Kobasa T, Limkittikul K, Cui L, Jongwutiwes S. Differential
prevalence of Plasmodium infections and cryptic Plasmodium knowlesi
malaria in humans in Thailand. J Infect Dis. 2009; 199: 1143-50. http://dx.doi.org/10.1086/597414 PMid:19284284

- Jiang N, Chang Q, X. Sun, H. Lu, J. Zhang,
M. Wahlgren, Q. Chen. Co-infections with Plasmodium knowlesi and other
malaria parasites, Myanmar. Emerg Infect Dis. 2010; 16(9): 1476-1478. http://dx.doi.org/10.3201/eid1609.100339 PMid:20735938 PMCid:3294981

- Arevalo-Herrera M, L. Quiñones M, Guerra
C, Céspedes N, Giron S, Ahumada M, Piñeros JG, Padilla N, Terrientes Z,
Rosas A, Padilla JC, Escalante A, Beier JC, Herrera S. Malaria in
selected non-Amazonian countries of Latin America. Acta Trop. 2011;
121(3):303-14. http://dx.doi.org/10.1016/j.actatropica.2011.06.008 PMid:21741349

- Culleton R, Ndounga M, Yildiz Zeyrek F,
Coban C, Casimiro PN, Takeo S, Tsuboi T, Yadava A, Carter R, Tanabe K.
Evidence for the transmission of Plasmodium vivax in the Republic of
the Congo, West Central Africa. J Infect Dis. 2009; 200: 1465–9. http://dx.doi.org/10.1086/644510 PMid:19803728

- Ryan J, Stoute JA, Amon J, Dunton RF,
Mtalib R, Koros J, Owour B, Luckhart S, Wirtz RA, Barnwell JW,
Rosenberg R. Evidence for transmission of Plasmodium vivax among a
Duffy antigen negative population in Western Kenya. Am J Trop Med. Hyg.
2006; 75 (4): 575–581. PMid:17038676

- Price RN, Tjitra E, Guerra CA, Yeung S,
White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop
Med Hyg. 2007; 77 (6): 79–87. PMid:18165478 PMCid:2653940

- Dash AP, Valecha N, Anvikar AR, Kumar A. Malaria in India: challenges and opportunities. J Biosci. 2008; 33 (4): 583-592. http://dx.doi.org/10.1007/s12038-008-0076-x PMid:19208983

- Beljaev AE. The malaria situation in the WHO eastern Mediterranean region. Med Parazitol (Mosk). 2000; 2: 12-15.

- Brooker S, Leslie T, Kolaczinski K, Mohsen
E, Mehboob N, Saleheen S, Khudonazarov J, Freeman T, Clements A,
Rowland M, Kolaczinski J. Spatial Epidemiology of Plasmodium vivax,
Afghanistan. Emerg Infect Dis. 2006; 12 (10): 1600-1602. http://dx.doi.org/10.3201/eid1210.060051 PMid:17176583 PMCid:1773016

- Zakeri S, Najafabadi ST, Zare A, Djadid
ND. Detection of malaria parasites by nested PCR in south-eastern,
Iran: evidence of highly mixed infections in Chahbahar district. Malar
J. 2002; 1: 2. http://dx.doi.org/10.1186/1475-2875-1-2 PMid:12057020 PMCid:111500

- Celikbas AK, Ergönül O, Baykam N, Eren S,
Güven T, Dokuzoguz B. Malaria in Turkey and 14 years of clinical
experience. Mikrobiyol Bul. 2006; 40 (3): 237-43. PMid:17001853

- Al-Mekhlafi MQ, Mahdy MAK, Azazy AA, Fong
MY. Molecular epidemiology of Plasmodium species prevalent in Yemen
based on 18 s rRNA. Parasit Vectors. 2010; 3: 110. http://dx.doi.org/10.1186/1756-3305-3-110

- Oliveira-Ferreira J, Lacerda MVG, Brasil
P, Ladislau JLB, Tauil PL, Tadeu Daniel-Ribeiro C. Malaria in Brazil:
an overview. Malar J. 2010; 9: 115. http://dx.doi.org/10.1186/1475-2875-9-115 PMid:20433744 PMCid:2891813

- Padilla Rodríguez JC, Álvarez Uribe G,
Montoya Araújo R, Chaparro Narváez P, Herrera Valencia S. Epidemiology
and control of malaria in Colombia. Mem Inst Oswaldo Cruz. 2011; 106:
114-122. PMid:21881765

- Manock SR, Jacobsen KH, de Bravo NB,
Russell KL, Negrete M, Olson JG, Sanchez JL, P. Blair J, Smalligan RD,
Quist BK, Espín JF, Espinoza WR, MacCormick F, Fleming LC, Kochel T.
Etiology of Acute Undifferentiated Febrile Illness in the Amazon Basin
of Ecuador. Am J Trop Med Hyg. 2009; 81(1): 146-151. PMid:19556580

- Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clin Microbiol Rev. 2005; 18 (3): 570-581. http://dx.doi.org/10.1128/CMR.18.3.570-581.2005 PMid:16020691 PMCid:1195966

- Macedo de Oliveira A, Mutemba R, Morgan J,
Streat E, Roberts J, Menon M, Mabunda S. Prevalence of malaria among
patients attending Public Health facilities in Maputo city, Mozambique.
Am J Trop Med Hyg. 2011; 85(6): 1002-7. http://dx.doi.org/10.4269/ajtmh.2011.11-0365 PMid:22144434

- Oguike MC, Betson M, Burke M, Nolder D,
Russell Stothard J, Kleinschmidt I, Proietti C, Bousema T, Ndounga M,
Tanabe K, Ntege E, Cullettan R, Sutherland CJ. Plasmodium ovale curtisi
and Plasmodium ovale wallikeri circulate simultaneously in African
communities. Int J Parasitol. 2011; 41(6-10): 677-683.

- Win TT, Lin K, Mizuno S, Zhou M, Liu Q,
Ferreira MU, Tantular IS, Kojima S, Ishii A, Kawamoto F. Wide
distribution of Plasmodium ovale in Myanmar. Trop Med Int Health. 2002;
7(3): 231–239. http://dx.doi.org/10.1046/j.1365-3156.2002.00857.x PMid:11903985

- Marathe, Date V, Shah HN, Tripathi JR.
Plasmodium ovale – a case report from Gujarat. J Vect Borne Dis. 2006;
43: 206–208. PMid:17175709

- Lim YA, Mahmud R, Hoong Chew C, Heng Chua
TTK. Plasmodium ovale infection in Malaysia: first imported case. Malar
J. 2010; 9: 272. http://dx.doi.org/10.1186/1475-2875-9-272 PMid:20929588 PMCid:2959071

- Wickremasinghe R, Galapaththy GNL,
Fernando WAP, de Monbrison F, Wijesinghe RS, Mendis KN, Picot S,
Ringwald P, Wickremasinghe AR. Short report: an indigenous case of
Plasmodium ovale infection in Sri Lanka. Am J Trop Med. Hyg. 2008;
78(2): 206–207. PMid:18256413

- Siswantoro H, Russell B, Ratcliff A,
Prasetyorini B, Chalfein F, Marfurt J, Kenangalem E, Wuwung M, Piera
KA, Ebsworth EP, Anstey NM, Tjitra E, Price RN. In vivo and in vitro
efficacy of cloroquine against Plasmodium malariae and P. ovale in
Papua, Indonesia. Antimicrob Agents Chemother. 2011; 55(1): 197–202. http://dx.doi.org/10.1128/AAC.01122-10 PMid:20937779 PMCid:3019630

- Cabrera BD, Arambulo PV. Malaria in the Republic of the Philippines. A review. Acta Trop. 1977; 34(3): 265-79. PMid:21558

- Collins WE, Jeffery GM. Plasmodium malariae: parasite and disease. Clin Microbiol Rev. 2007; 20 (4):. 579–592. http://dx.doi.org/10.1128/CMR.00027-07 PMid:17934075 PMCid:2176047

- Rahman W, Chotivanich K, Silamut K,
Tanomsing N, Hossain A, Faiz MA, Dondorp AM, Maude RJ. Plasmodium
malariae in Bangladesh. Trans R Soc Trop Med Hyg. 2010; 104(1): 78–80. http://dx.doi.org/10.1016/j.trstmh.2009.06.014 PMid:19818463 PMCid:2793369

- Rosanas-Urgell A, Mueller D, Betuela I,
Barnadas C, Iga J, Zimmerman PA, del Portillo HA, Siba P, Mueller I,
Felger I. Comparison of diagnostic methods for the detection and
quantification of the four sympatric Plasmodium species in field
samples from Papua New Guinea. Malar J. 2010; 9: 361. http://dx.doi.org/10.1186/1475-2875-9-361 PMid:21156052 PMCid:3016373

- Forney JR, Magill AJ, Wongsrichanalai C,
Sirichaisinthop J, Bautista CT, Heppner DG, R. S. Miller, Ockenhouse
CF, Gubanov A, Shafer R, Dewitt CC, Quino-Ascurra HA, Kester KE, Kain
KC, Walsh DS, Ballou WR, Gasser RA. Malaria Rapid Diagnostic devices:
performance characteristics of the ParaSight F device determined in a
multisite field study. J Clin Microbiol. 2001; 39(8): 2884-2890. http://dx.doi.org/10.1128/JCM.39.8.2884-2890.2001 PMid:11474008 PMCid:88255

- Carme B, Ardillon V, Girod R, Grenier C,
Joubert M, Djossou F, Ravachol F. Update on the epidemiology of malaria
in French Guiana. Med Trop Mars. 2009;69 (1): 19-25.

- Metzger WG, Vivas-Martìnez S, Rodriguez I,
Gonçalves J, Bongard E, Fanello CI, Vivas L, Magris M. Malaria
diagnosis under field conditions in the Venezuelan Amazon. Trans R Soc
Trop Med Hyg. 2008; 102(1): 20-4. http://dx.doi.org/10.1016/j.trstmh.2007.08.007 PMid:17919672

- Lindo JF, Horner Bryce J, Bullock Ducasse
M, Howitt C, Barrett DM, Morales JL, Ord R, Burke M, Chiodini PL,
Sutherland CJ. Plasmodium malariae in Haitian refugees, Jamaica. Emerg
Infect Dis 2007; 13(6): 931-3. http://dx.doi.org/10.3201/eid1306.061227 PMid:17553241 PMCid:2792841

- Lee KS, Cox-Singh J, Brooke G, Matusop A,
Singh B. Plasmodium knowlesi from archival blood films: further
evidence that human infections are widely distributed and not newly
emergent in Malaysian Borneo. Int J Parasitol 2009; 39: 1125-28. http://dx.doi.org/10.1016/j.ijpara.2009.03.003 PMid:19358848 PMCid:2722692

- Jooven-Neoh WF, Lung Chong K, Vui Ling
Wong CM, Ying Lau T. Incidence of malaria in the interior division of
Sabah, Malaysian Borneo, based on Nested PCR. J Parasitol Res. 2011;
2011: 104284.

- Luchavez J, Espino F, Curameng P, Espina
R, Bell D, Chiodini P, Nolder D, Sutherland C, Lee K, Singh B. Human
infection with Plasmodium knowlesi, the Philippines. Emerg Infect Dis.
2008; 14(5): 811-813. http://dx.doi.org/10.3201/eid1405.071407 PMid:18439369 PMCid:2600254

- Jeslyn WPS, Huat TC, Vernoon L, Zhi Irene
LM, Kim Sung L, Piao Jarrod L, Singh B, Ching NL. Molecular
epidemiological investigation of Plasmodium knowlesi in humans and
macaques in Singapore. Vector Borne and Zoonotic Dis. 2011; 11(2):
131-135. http://dx.doi.org/10.1089/vbz.2010.0024 PMid:20586605 PMCid:3033207

- Khim N, Siv S, Kim S, Mueller T,
Fleischmann E, Singh B, Divis PCS, Steenkeste N, Duval L, Bouchier C,
Duong S, Ariey F, Ménard D. Plasmodium knowlesi infection in humans,
Cambodia 2007-2010. Emerg Infect Dis. 2011; 17(10): 1900-1902. http://dx.doi.org/10.3201/eid1710.110355 PMid:22000366 PMCid:3310675

- Van den Eede P, Van HN, Van Overmeir C,
Vythilingam I, Ngo Duc T, Hung LX, Manh HN, Anné J, D’Alessandro U,
Erhart A. Human Plasmodium Knowlesi infections in young children in
central Vietnam. Malar J. 2009; 8: 249. http://dx.doi.org/10.1186/1475-2875-8-249 PMid:19878553 PMCid:2773789

- Harbach RE. Mosquito taxonomic inventory. http://mosquito-taxonomic-inventory.info
- Gilles HM, Warrell DA, Essential Malariology. Fourth edition, London, Arnold. 2002; 59-84.
- Takken W, Scott TW. Ecological Aspects for Application of Genetically Modified Mosquitoes. Dordrecht, Kluwer Academic Publishers. 2003; 75–90.
- Moffett A, Shackelford N, Sarkar S.
Malaria in Africa: vector species’ niche models and relative risk maps.
PLoS One 2007; 2: e824. http://dx.doi.org/10.1371/journal.pone.0000824 PMid:17786196 PMCid:1950570

- Moffett A, Strutz S, Guda N, González C,
Ferro MC, Sánchez-Cordero V, Sarkar S. A global public database of
disease vector and reservoir distributions. PLoS Negl Trop Dis. 2009;
3: e378. http://dx.doi.org/10.1371/journal.pntd.0000378 PMid:19333367 PMCid:2656641

- Levine RS, Peterson AT, Benedict MQ.
Distribution of members of Anopheles quadrimaculatus Say s.l. (Diptera:
Culicidae) and implications for their roles in malaria transmission in
the United States. J Med Entomol. 2004; 41: 607–613. http://dx.doi.org/10.1603/0022-2585-41.4.607 PMid:15311451

- Foley DH, Weitzman AL, Miller SE, Faran
ME, Rueda LM, et al. The value of georeferenced collection records for
predicting patterns of mosquito species richness and endemism in the
Neotropics. Ecol Entomol. 2008; 33: 12–23.

- Kuhn KG, Campbell-Lendrum DH, Davies CR. A
continental risk map for malaria mosquito (Diptera: Culicidae) vectors
in Europe. J Med Entomol. 2002; 39: 621–630. http://dx.doi.org/10.1603/0022-2585-39.4.621

- Manguin S, Garros C, Dusfour I, Harbach
RE, Coosemans M. Bionomics, taxonomy, and distribution of the major
malaria vector taxa of Anopheles subgenus Cellia in Southeast Asia: an
updated review. Infect Genet Evol. 2008; 8: 489–503. http://dx.doi.org/10.1016/j.meegid.2007.11.004 PMid:18178531

- Manguin S, Carnevale P, Mouchet J, Coosemans M, Julvez J, et al. Biodiversity of malaria in the world. Montrouge, France, John Libbey Eurotext. 2008; 464.
- Hay SI, Guerra CA, Gething PW, Patil
AP,Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, BrookerS, Smith
DL, Moyeed RA, Snow RW. A world malaria map: Plasmodium falciparum
endemicity in 2007. PLoS Med 2009, 6: e1000048. PMid:19323591
PMCid:2659708

- Coluzzi M. The clay feet of the malaria
giant and its African roots: hypotheses and inferences about origin,
spread and control of Plasmodium falciparum. Parassitologia. 1999,
41:277-283. PMid:10697869

- Sinka ME, Bangs MJ, Manguin S, Coetzee M,
Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW,
Okara RM, Van Boeckel T, Godfray HC, Harbach RE, Hay SI. The dominant
Anopheles vectors of human malaria in Africa, Europe and the Middle
East: occurrence data, distribution maps and bionomic précis. Parasit
Vectors. 2010; 3:117. http://dx.doi.org/10.1186/1756-3305-3-117

- Sainz-Elipe S, Latorre JM, Escosa R, Masia
M, Fuentes MV, Mas-Coma S, Bargues MD. Malaria resurgence risk in
southern Europe: climate assessment in an historically endemic area of
rice fields at the Mediterranean shore of Spain. Malar J. 2010, 9:221. http://dx.doi.org/10.1186/1475-2875-9-221 PMid:20673367 PMCid:2924348

- Sinka ME, Rubio-Palis Y, Manguin S, Patil
AP, Temperley WH, Gething PW, Van Boeckel T, Kabaria CW, Harbach RE,
Hay SI. The dominant Anopheles vectors of human malaria in the
Americas: occurrence data, distribution maps and bionomic précis.
Parasit Vectors. 2010; 3:72. http://dx.doi.org/10.1186/1756-3305-3-72

- Sinka ME, Bangs MJ, Manguin S,
Chareonviriyaphap T, Patil AP, Temperley WH, Gething PW, Elyazar IR,
Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human
malaria in the Asia-Pacific region: occurrence data, distribution maps
and bionomic précis. Parasit Vectors. 2011, 4:89 http://dx.doi.org/10.1186/1756-3305-4-89

- Machault V, Vignolles C, Borchi F,
Vounatsou P, Pages F, Briolant S, Lacaux JP, Rogier C. The use of
remotely sensed environmental data in the study of malaria. Geospatial
Health. 2011; 5 (2): 151-168. PMid:21590665
