Pathogenesis of Malaria in Tissues and Blood
Beatrice Autino1§*, Yolanda Corbett2*, Francesco Castelli1,3 and Donatella Taramelli2
1University
Division of Infectious and Tropical Diseases, University of Brescia and
Spedali Civili General Hospital, Brescia (Italy), Piazza Spedali
Civili, 1 - 25123–Brescia, Italy
2Dipartimento di Scienze Farmacologiche e Biomolecolari, Università di Milano, Via Pascal 36, 20133 Milano Italy
3Chair of Infectious Diseases, University of Brescia, Italy
§ PhD trainee in Appropriate Methods and Technologies for International Development Co-operation, supported by the A. Archetti Fund
*equally contributed to the work
2Dipartimento di Scienze Farmacologiche e Biomolecolari, Università di Milano, Via Pascal 36, 20133 Milano Italy
3Chair of Infectious Diseases, University of Brescia, Italy
§ PhD trainee in Appropriate Methods and Technologies for International Development Co-operation, supported by the A. Archetti Fund
*equally contributed to the work
Correspondence
to:
Prof. Francesco Castelli. Director, University Division of Infectious
and Tropical Diseases, University of Brescia and Spedali Civili General
Hospital, Brescia (Italy). Piazza Spedali Civili, 1, 25123 – Brescia
(Italy). Tel. +39.030.3995664, Fax: +39.030.3702403. E-mail: castelli@med.unibs.it
Published: October 4, 2012
Received: September 6, 2012
Accepted: September 21, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012061, DOI 10.4084/MJHID.2012.061
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
The
clinical manifestations of severe malaria are several and occur in
different anatomical sites. Both parasite- and host-related factors
contribute to the pathogenicity of the severe forms of the disease.
Cytoadherence of infected red blood cells to the vascular endothelium
of different organs and rosetting are unique features of malaria
parasites which are likely to contribute to the vascular damage and the
consequent excessive inflammatory/immune response of the host. In
addition to cerebral malaria or severe anaemia, which are quite common
manifestation of severe malaria, clinical evidences of
thrombocytopenia, acute respiratory distress syndrome (ARDS), liver and
kidney disease, are reported. In primigravidae from endemic areas, life
threatening placental malaria may also be present.
In the following pages, some of the pathogenetic aspects will be briefly reviewed and then data on selected and less frequent manifestation of severe malaria, such as liver or renal failure or ARDS will be discussed.
In the following pages, some of the pathogenetic aspects will be briefly reviewed and then data on selected and less frequent manifestation of severe malaria, such as liver or renal failure or ARDS will be discussed.
Introduction
Malaria is a life-threatening disease which in 2010 killed more than 600,000 individuals, mainly children under 5 years of age, and pregnant women.[1] All the clinical symptoms of malaria are the consequence of infection of human erythrocyte by merozoites. Most of the fatal cases, which predominantly occur in P. falciparum infections, are due to severe anaemia or cerebral malaria, but different clinical manifestations also exist and vary in severity and outcome, depending on the parasite species, the organ involved and the access to care.
P. falciparum differs from other human malarial species in that infected red blood cells (IRBC) do not remain in the circulation for the entire life cycle. After 24–32 hours, when young parasites mature from the ring to the trophozoite stage, IRBC adhere to endothelial cells in the microcirculation of various organs. This phenomenon, termed “sequestration”, is believed to occur mainly to avoid splenic removal of IRBC. Sequestration causes microcirculatory obstruction, impaired tissue perfusion and inflammatory cells activation and it is linked to the severity of the disease.
At schizonts rupture, from 4 up to 36 daughter merozoites, depending on the Plasmodium species, are released into the circulation and invade fresh RBC to perpetuate the asexual life cycle. At the same time, a large amount of toxins and parasite products are also released and cause the activation of the innate immunity, the release of inflammatory mediators and the symptoms associated with the malaria attack, such as fever.
A combination of mechanical circulatory stress due to sequestration and excess inflammatory response contribute to the most severe manifestations of malaria including, but not limited to, cerebral malaria or anaemia. The present review will summarise some of the pathogenetic aspects of severe malaria and then it will report on selected and less frequent manifestation, such as renal failure or acute respiratory distress syndrome (ARDS).
Pathogenetic Characteristics of Severe Malaria
Cytoadherence. Cytoadherence, the ability of parasites to adhere to the vascular endothelium, was recognized as early as 1892 by Marchiafava and Bignami.[2] Mature forms of parasites (asexual stage and gametocytes) can adhere to the vascular endothelium of several organs (lung, heart, brain, lung, liver, and kidney), the subcutaneous adipose tissues and the placenta. This feature of the disease in vivo has been related exclusively to P. falciparum.[3,4] However, sequestration in vitro to some endothelial cell lines and placental cryosections has also been seen in reticulocytes infected with P. vivax.[5]
Parasite sequestration is thought to be the pathological base of the severe manifestation of malaria, including cerebral malaria.[6] It causes blood flow impairment leading to local hypoxia. It enhances parasite replication and the sticking of IRBC to non-infected red blood cells (rosetting, see below). Moreover, when parasite sequester, the effects of parasite toxins are more localized and also the stimulation of the host immune response, which may cause a focused production of inflammatory mediators and tissue damage. As a consequence, both RBC and IRBC become more rigid and less deformable.[7]
Sequestration is mostly mediated by mature parasite forms, approximately 20 hours after RBC invasion. The parasites produce new proteins that are exported to the IRBC surface and increase the adhesiveness of IRBC to the endothelium. During their 48-hour life cycle, the parasites can remain sequestered for 24 hours in the deep microvasculature. In this manner, they evade clearance by the spleen, and make the diagnosis more difficult since they are not seen in the peripheral blood.
Sequestration of P. falciparum has been attributed to different class of molecules of parasite origin and ligands present on the human endothelium. Among those, the P. falciparum histidine-rich protein (PfHRP) and the erythrocyte membrane protein 1 (PfEMP1), have received significant attention. PfHRP is related to the establishment of knobs, symmetric membrane arrangements which appear on the surface of infected RBC, while PfEMP1, a multimeric protein encoded by the var (variant) gene[3,4] protrudes from the knobs and plays a major role in sequestration and thus virulence (see BOX 1). To adhere to the endothelium, the parasites first adhere, roll and then become firmly attached to the endothelium adhesion molecules. Among these molecules, ICAM-1, a major sequestration receptor and involved in cerebral sequestration serves as a rolling receptor. On the other hand, CD36 gives stationary and stable adherence under flow.[8-10]
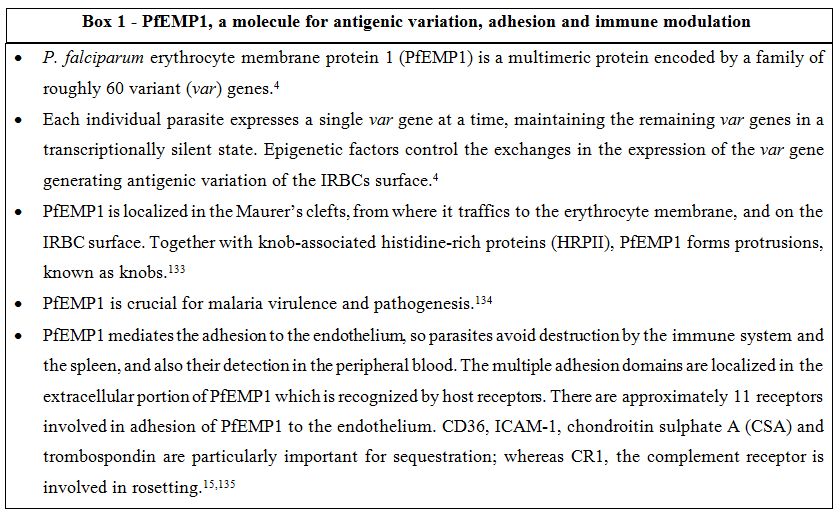
Box 1. PfEMP1, a molecule for antigenic variation, adhesion and immune modulation.
Sequestration is also seen during gestational malaria, when parasites adhere to the placenta. PfEMP1 is again the main adhesion receptor which adheres to the trophoblastic villous endothelium mainly through chondroitin-4-sulfate (CSA) and other sugars such as glycosaminoglycans and possibly hyaluronic acid (HA). As discussed later, malaria in pregnancy can be severe for mothers and induce fetal death especially during the first pregnancy, when women usually lack sufficient immunity against CSA-binding parasites.[11-14]
Rosetting. Rosetting is one of the forms of cytoadherence of late stages IRBC to non-parasitized red blood cells and /or platelets.[15] The IRBC ligand involved in rosette formation is PfEMP1, and three are the receptors associated with rosetting: complement receptor 1 (CR1), heparan sulfate (HS), and the ABO blood group.[15,16] PfEMP1 has been shown to bind to CR1, specifically at the C3b-binding site. The lectin-like DBL-domain of PfEMP1 can make strong adhesion with carbohydrate structures particularly A blood group antigen, favoring rosettes formation.[17] For this reason, non-O blood groups are considered significant risk factors for life-threatening malaria, through the mechanism of enhanced rosette formation.[18,19]
P. falciparum, P. vivax, and P. ovale are all able to form rosettes,[20,21] but only those caused by P. falciparum have been associated with severe malaria, and especially in African children they may enhance microcirculatory obstruction.[22] Rosetting has been related with parasite multiplication rate in a model of Saimiri sciureus.[23] More recently, it has been shown that 4-HNE, a biomembrane lipid peroxidation product driven by haem iron of the malarial pigment can be transferred from IRBC to normal RBC in rosettes favouring their removal by macrophages.[24] This could partly explain the rapid loss of normal non parasitized RBC in severe malaria anaemia.
Innate Immune Response. The immune response to the parasite is complex and not completely understood, and it is essentially both species and stage specific.[25] The activation of components of the innate immune system is crucial to control parasite replication, contributing to the subsequent elimination and resolution of the infection.[26] Neutrophils, monocytes/macrophages, dendritic cells, natural killer (NK) cells, NKT cells, and gamma T cells are all the cells of the innate immune system in charge of controlling the early progression of the disease through phagocytosis and/or production of inflammatory mediators. Much of the symptoms of malaria attacks such as fever, nausea, headaches, and others are the consequences of the inflammatory response orchestrated by the cells of the innate immune system, stimulated by parasites or their products at the rupture of the late stage infected erythrocytes.[25,27] An imbalance between the production of pro- or anti-inflammatory cytokines, such as TNF-α, IL6, IL1β, or IL-10 or mediators, like nitric oxide, may contribute to the pathogenesis of the severe form of the disease. Elevated levels of TNF-α are found in the serum of severe patients and have been correlated with cerebral malaria (CM).[28] In vitro, it has been found that TNF-α enhances the expression of ICAM-1,[29] which is also augmented in brain vessels of CM patients.[9] Evolutionarily conserved receptors, termed “pathogen recognition receptors” (PRRs), are present on the cells of native immunity and trigger the response upon the recognition of specific parasite molecules called “pathogen associated molecular patterns” (PAMPs).[30] Malaria PAMPs include the protein anchor, glycosyl-phosphatidyl inositol (GPI), or hemozoin (malaria pigment). PRRs related to malaria are the membrane bound Toll-like receptors (TLRs), the cytosolic receptors (such as NALP3, inflammasomes), and soluble receptors such as MBL.[31-34] For example, GPI released by IRBC at shizonts rupture stimulates the production of pro-infiammatory TNF-α by macrophages via the recognition of TLR2 and partly of TLR4 in a MyD88 dependent way.[31]
Specific Immune Response. Malaria is an important cause of morbidity, but not everyone infected with the malaria parasite becomes seriously ill or dies. In areas of stable endemicity, repeated exposure to the parasite leads to the acquisition of specific immunity, which restricts serious problems to young children; malaria in older subjects causes a relatively mild febrile illness. However, individuals with no previous experience of malaria become ill on their first exposure to the plasmodium parasite. They develop a febrile illness which may become severe and in a proportion of cases may lead to death.[35,36]
Immunity to malaria is provided by innate mechanisms, as we summarized above, and subsequently by the development of acquired immunity. Following repeated infections, people living in malaria endemic areas gradually acquire mechanisms, which helps limiting the inflammatory response to the parasite that causes the acute febrile symptoms. Sterilizing immunity is never achieved. Parasites have evolved to maintain a balanced relationship with their human hosts. In this sense they can partially escape from the host effector mechanisms, while hosts are able to develop partial immunity against the parasite. This type of immunity requires repeated infections, takes years to develop and usually lasts shortly. Natural acquired immunity is called premunition since low parasite burden often persists in the presence of circulating antibodies to the various stages, in the absence of clinical disease. In children without circulating antibodies to the parasite, premunition is lower. The poor and slowly developing immune response to malaria is partly due to parasite immune evasion strategies: antigenic polymorphism, shedding of parts of parasite proteins, cross-reactive antigen epitopes of developmental stages, prolonged exposure to endemic malaria and limited immunogenicity of antigens. In stable endemic areas, a heavy burden of morbidity and mortality falls on young children. Children born to immune mothers appear to be relatively immune to malaria for a period.[26,37] This is conferred by the prenatal or postnatal transfer of protective antibodies from mother to child. The acquired immunity is mediated by specific antibodies to several conserved and polymorphic proteins, and to the highly variable protein PfEMP1 expressed by the parasite at the trophozoites and schizonts stages and exported on the surface of infected erythrocytes.[38]
Specific Complications of Severe Malaria Infection
Anaemia. Anaemia is one of the most common causes of morbidity and mortality in malaria infection particularly in pregnant women and in children.[39] Pathogenesis of malarial anaemia has been intensively studied, even if it is not completely understood; hereby we summarise some of the pathogenetic aspects. Malarial anaemia could be acute or chronic; in holo-endemic areas chronic malarial anaemia is more common. Acute malarial anaemia could occur after massive erythrocytes lysis due to elevate parasitemia or to drug-induced or immune haemolysis.[40]
The potential mechanisms contributing to malarial anaemia can be divided into two categories: increased destruction of parasitized and un-parasitized erythrocytes (immune-mediated lysis, phagocytosis, splenic sequestration) and decrease of RBC production (dyserythropoiesis and bone marrow suppression, inadequate reticulocyte production, effects of inflammatory cytokines, effects of parasite factors). Co-infection with bacteremia, HIV-1 and hookworm, malnutrition and repeated malarial infections in holo-endemic countries may also contribute to decrease haemoglobin levels.[39,41-43]
Parasitized red cells rupture caused by plasmodium cycles and clearance of deformed parasitized and un-parasitized erythrocytes are the principal causes of malarial anaemia. Phospholipid asymmetry, membrane rigidity and reduced deformability are the mechanisms involved in the premature removal of un-parasitized red cell.[7,41,44] Phagocytosis and complement activation are the principal mechanisms of non-specific immune mediated clearance of erythrocytes in malaria infection.[45] Moreover, uninfected red cells membrane proteins may be altered by reactive oxygen species (ROS) and other factors, becoming target for auto-antibodies.[4] It has been suggested that the spleen may play a double role in malaria infection: on the one hand it could contribute to severe anaemia, by excess removal of IRBC and uninfected RBC; on the other hand it may protect from severe cerebral malaria.[46]
Dyserythropoiesis plays an important role in the pathogenesis of anaemia; examination of bone marrow from children with severe anaemia showed hypercellularity, mild to normal erythroid hyperplasia and abnormal features of late erythroid progenitors. Hemozoin and its phagocytosis by bone marrow macrophages has been proposed to cause dys-erythropoiesis either through direct accumulation in the bone marrow and generation of reactive toxic species or activation of the innate immune response.[42]
The immune response is central in the pathogenesis of malarial anaemia; parasitized red cells, hemozoin and malarial antigens activate monocyte and lymphocyte response. Pro-inflammatory and anti-inflammatory mediators, including TNF-α, IFN-γ, IL-23 and IL-1, chemokines and growth factor are produced and contribute to anaemia. On the contrary, IL-12 and IL-10 seems to be protective cytokines since low levels are found in severe malarial anaemia.[40,43] Macrophage migration inhibiting factor (MIF) is associated with severe anaemia and bone marrow suppression.[47] Nitric oxide is an inhibitor of erythropoiesis.[40,48]
Erythropoietin (EPO) levels are increased during malaria anaemia, but erythroid progenitors response is not adequate, particularly during chronic malaria infection, resulting in low reticulocytosis.[43] It has been shown in a rodent model that exogenous EPO could stimulate splenic erythroblasts. However, their maturation is impaired due to altered iron metabolism and haemoglobin production.[49] Ineffective erythropoiesis, erythrophagocytosis and iron delocalisation are the most important causes of reticulopenia.
Pro-inflammatory cytokines play an important role also in iron delocalisation pathway of malarial anaemia. TNFα induces re-localisation of ferroportin, an important protein abundant in the reticulo-endothelial system that mediates macrophage iron release and intestinal iron absorption. Relocalisation of ferroportin induces decrease of iron absorption and release from macrophage cells;[45] hepcidin, a protein released during chronic disease, also induces reduction of ferroportin and its levels are increased during severe malaria anaemia.[50]
STAT6, a member of signal transducer and activator of transcription family proteins, seems also to be involved in malarial anaemia through the activation of the regulatory cytokines, in particular IL-4 and IFN-γ, resulting in erythropoietic suppression during blood stage malaria.[51,52] A recent review found an interesting correlation between malarial anaemia and micronutrient malnutrition: vitamin A and E, iron, zinc, riboflavin and folate deficiency may play a role in worsening the anaemia mediated by alteration of immunity, dyserythropoiesis and iron metabolism.[39] It has also been suggested that high catecholamine concentration could alter the functions of the erythrocyte membrane, providing an additional erythrocyte clearance mechanism.[53]
Thrombocytopenia. Thrombocytopenia is very common in malaria, usually during the early stage of P. falciparum and P. vivax infections. Incidence is high both in children and in adults.[54-56] Thrombocytopenia in pregnant women is not well documented, but a recent study performed in Thailand showed that platelet counts were lower in pregnant than in non-pregnant women.[57] The pathogenesis of malaria thrombocytopenia is complex and may be related to coagulation disturbances, splenomegaly and platelet destruction by macrophages, bone marrow alterations, antibody-mediated platelet destruction, oxidative stress and platelets aggregation. These processes are well described in a recent review.[58] A study performed in Indonesia showed that patients with P. falciparum and P. vivax malaria had lower platelet count, higher von Willebrand factor (VWF) concentration, lower ADAMTS13 activity and ADAMTS13 antigen concentrations.[59] Higher VWF seems to correlate with platelet binding, leading to thrombocytopenia. In contrast, another study demonstrated that sGP1b, the external domain of platelet receptor for VWF, increased early in the blood of malaria patients thus preventing excessive platelet adhesion.[60] Despite thrombocytopenia is very common, hemorrhagic events are rare and usually are associated with severe thrombocytopenia or disseminated intravascular coagulation (DIC).[61-63] Further study are needed to understand the relationship between malaria, platelet counts and hemorrhagic events.
ARDS. Deep breathing, respiratory distress and pulmonary oedema are some of the clinical feature defining severe malaria according with WHO classification.[64] In adults and pregnant women, rather than children, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are the most common clinical presentation burdened by an elevated mortality rate as shown in a recent study performed in India where severe P. falciparum malaria mortality rate was 35,4% and mortality was principally due to shock, acute renal failure, seizure and ARDS.[65] Despite ARDS is mostly observed as a complication of P. falciparum infection, case reports due to P. vivax,[66-69] P. ovale[70] and P. knowlesi[71,72] were also published. Malaria associated ARDS due to P. vivax caused three maternal deaths in a cohort of 221 patient in India during one year of observation.[66] ARDS can occur before or after specific treatment.[67] P. knowlesi, the fifth plasmodium species infecting humans, mostly in Southeast Asia, may cause life threatening diseases, as well.[73] A prospective study conducted in Malaysia demonstrated that the most frequent complication of P. knowlesi infection was respiratory distress.[72] Malaria is presently considered one of the most common risk factor of ARDS and acute lung injury (ALI) in the tropics[74] and the second cause of ARDS after sepsis.[75]
Little is known about the pathogenesis of malaria associated ALI/ARDS. Inflammatory mediators and increased endothelial permeability may play an important role, while parasite sequestration may take a minor role. Pulmonary manifestations of uncomplicated malaria were analysed by Anstey et al. showing that patients frequently have subclinical impairment of lung function, such as small airways obstruction, impaired alveolar ventilation, reduced gas exchange and increased pulmonary phagocytic activity.[76] All these features underline the activation of inflammatory pathways and could explain part of the pathogenetic mechanisms of respiratory distress occurring during severe malaria. Studies with rodent malaria models were performed to understand the pathogenetic mechanisms of ALI/ARDS. Epiphanio et al. in DBA/2 mice infected by P. berghei ANKA[77] observed that 60% of mice had dyspnea, airways obstruction and hypoxemia; pleural effusion, pulmonary hemorrhagies and oedema were also found as pathological findings, demonstrating the role of inflammation in ALI development. Moreover, high levels of vascular endothelial growth factor (VEGF) were found in mice with ALI, supporting the importance of increased vascular permeability in malaria respiratory failure. Another study[78] which utilized DBA/2 mice infected with P. berghei K173, showed that proteins and inflammatory cells mainly CD4+ and CD8+ lymphocytes, neutrophils and monocytes accumulate in the lungs of infected mice. These results were confirmed by P.E. Van den Steen et al.,[79] who also measured the cytokines and chemokines associated with ARDS, showing an increased expression in the lungs of TNF-α, IL-10, IFN γ, CXCL10 and CXCL11, as well as monocyte and neutrophil chemo-attractant chemokines (CCL2, KC). IRBC were are also observed in the lung vessels, but at a lower extent compared with the massive sequestration in the brain.[80] Infected mice lungs had an increased water content, demonstrating the development of oedema;[78,79] this could be related to the decreased expression of epithelial sodium channel, ENaC, due to hypoxia, resulting from malaria associated anaemia, or to TNFα mediated down-regulation.[78] In a rodent model malaria associated ARDS, dexamethasone inhibited the infiltration of macrophages and CD8 T cells into the lungs, suggesting that adjunct therapy with anti-inflammatory drugs could also be useful in the clinical setting.[79]
Most of the studies with P. falciparum are based on in vitro models or post mortem observations. It was shown in vitro that P. falciparum merozoite proteins could increase endothelial permeability, while P. falciparum IRBC did not show the same properties suggesting that the effects of the parasite on the pulmonary endothelium are probably mediated by the activity of Src-family kinases.[81] Morphologic changes were noted in the proteins of the tight junctions and adherent junction in association with increased endothelial permeability and development of pulmonary oedema.[81] A post mortem study performed in children with cerebral malaria demonstrated that sequestration of P. falciparum -IRBC occurs in the lungs, even if to a lesser extent than in the brain, skin or intestine.[80]
Different pathological presentations of ARDS were observed among the different species of human malaria: the greatest severity and frequency of cases are due to P. falciparum and could be partially attributed to the sequestration and rosetting of infected RBC in the pulmonary microcirculation;[67] heavy parasitaemia and WBC agglutinates were associated to ARDS in P. vivax malaria.[66] L. Price et al. suggested that the pathogenesis of lung injury by P. falciparum include cytokine-induced damage or direct effects of sequestration of parasitized erythrocytes, while ARDS caused by P. vivax may be due principally to dysregulation of cytokines production.[69] Elevated parasitemia in ARDS in P. knowlesi infection suggests parasite-specific effects that increase pulmonary capillary permeability,[71] but hypoxemia and metabolic acidosis may also contribute.[72]
Liver involvement. According to the World Health Organization liver dysfunction is an uncommon occurrence in malaria, while jaundice is not unusual. The incidence of jaundice and liver dysfunction in P. falciparum malaria varied from 5.3% to 62% and from 2.5% to 21%, respectively, in different reports,[82-84] while malarial hepatitis is rare in P. vivax infection.[85] Case-fatality rate in malaria-related hepatic failure is elevated, up to 40%[84-86] when high parasite density is associated with jaundice and liver dysfunction.[87] Liver is involved in malaria at two stages: during the pre-erythrocytic cycle and the erythrocytic phase. The first step is linked to the binding of the merozoite circumsporozoite protein CSP-A and TRAP protein to the hepatocytes via the heparan sulphate glycosylaminoglycans GAG[88] and promotes minimal liver damage. In the erythrocytic phase, jaundice is a common remark and it is directly caused by the infection (malarial hepatitis, intravascular hemolysis of parasitized RBC, septicemic hepatitis), or by indirect causes (microangiopathic hemolysis associated with DIC, G6PD-related hemolysis, antimalarial drug induced-hemolysis) or completely unrelated (coexisting acute viral hepatitis, underlying chronic hepatitis).[88] Intravascular hemolysis of parasitized and non parasitized RBC causes an increase of unconjugated bilirubinemia with mild to moderate jaundice;[89] conjugated hyperbilirubinemia indicates hepatocyte dysfunction. The pathogenesis of hepatic dysfunction is not completely known; reduction in portal venous flow as a consequence of microocclusion of portal venous branches by parasitized erythrocytes, intrahepatic cholestasis due to reticuloendothelial blockage and hepatic microvilli dysfunction, suppression of bilirubin excretion due to effect of parasitemia or endotoxemia or metabolic acidosis, apoptosis and oxidative stress are all mechanisms involved in hepatic damage.[88,90] Histopathological findings reported in literature are: congestion of hepatocytes, swollen hepatocytes, centrizonal necrosis, Kupffer cell hyperplasia, deposition of brown malarial pigment, portal infiltration with lymphocytes, steatosis, parasitized RBC, cholestasis, spotty and submassive necrosis.[88-91] These evidences demonstrate inflammatory as well as direct plasmodial effects in the damage to hepatocytes.
Kidney involvement. Kidneys in malaria are involved in two different manners: acute and chronic diseases. Acute renal failure (ARF) is one of the most challenging diseases in tropical countries and malaria plays an important epidemiological role.[92-95] The mortality due to malaria ARF is high.[96] A study performed in Yemen showed that malaria was the first cause of death in patient with ARF.[97] Malaria acute renal failure (MARF) is more common in non-immune adults and in older children.[98] MARF is mostly associated to P. falciparum infection and is more frequent in low transmission areas;[98-101] nevertheless MARF could be caused also by P. vivax.[100,102] P. malariae causes chronic and progressive glomerulopathy, known as quartan malaria nephropathy (QMN); only few cases of MARF caused by P. malariae has been published.[103]
Quartan malaria nephropathy (QMN) is frequently seen in African children and it is clinically associated with oedema and hypertension; urine analysis shows often proteinuria and microhematuria.[101,104] Pathogenesis of QMN is linked to subendothelial deposits of immune complexes containing IgG, IgM, C3 and in the 25-33% of cases also malaria antigens.[101,104] It is thought to be the consequence of the activation of TH2 type T lymphocytes. The pathogenesis may also include genetic and acquired factors, such as autoimmunity, co-infection with Epstein-Barr virus and malnutrition.[104] Immune complexes deposition provokes glomerular damage, resulting in proliferative glomerulonephritis; the pathology, initially focal, becomes diffuse and progressive, reaching sclerosis. Different histopatological patterns revealed by renal biopsy induced some authors to conclude that the association between P. malariae infection and renal involvement was only coincidental.[101] P. falciparum acute renal failure is more common in adults; its pathogenesis is complex and it includes mechanical and immunologic factors, volume depletion, hypoxia, hyperparasitemia and other factors.[98,101,105] High parasite density was associated to acute renal failure.[101,87] Parasitized erythrocyte sequestration was found in kidneys of adults who died from severe P. falciparum malaria;[106] sequestration of parasitized erythrocytes is lower in the kidney than in the brain.[98,106] A study performed in Mali, showed that rosetting is present in all severe malaria clinical forms, including acute renal failure.[22] Hemolysis, causing endothelial activation and hemodynamic alteration, can lead to acute tubular necrosis and acute interstitial nephritis.[104]
Hypoperfusion, due to the loss of liquids and to the absence of volume restoration, leads to renal ischemia.[98] TNFα, reactive oxygen species and inducible nitric oxide also play an important role in determining the haemodynamic alteration.[104] Mononuclear cell infiltration was reported in glomerular and in peritubular capillaries of adults with ARF, while immune complexes were not reported.[106] It was hypothesized that the release of malaria antigens activates monocyte cells, to release proinflammatory cytokines and activate TH1 cell mediated response, causing acute interstitial nephritis.[104] The role of cytokines such us INFγ, IL-1α, IL-6, GM-CSF was studied in murine malaria infection and an association of nephritis with up-regulation of pro-inflammatory and dysregulation of anti-inflammatory cytokines was found.[107]
Rarely, acute renal failure has been associated to rhabdomyolysis in P. falciparum and P.vivax infections, probably due to the sequestration of parasitized red cells in the skeletal capillaries and consequent vessels occlusion.[108] In the pathogenesis of ARF related to rhabdomyolysis, the myoglobin nephrotoxic effect play the principal role; hypovolemia, hypotension, fever, acidosis and the use of non-steroidal anti-inflammatory drugs may worsened the renal function.[109,110]
Black water fever (BWF), a rare but severe complication of severe malaria, is characterized by fever, intravascular haemolysis with haemoglobinuria, dark urines and often acute renal failure.[111-113] Haemoglobin release during massive haemolysis causes renal impairment. Drugs as quinine, halophantrine and mefloquine, and G6PD deficiency, have been suggested to be the trigger of BWF.
Placenta involvement. Malaria during pregnancy is associated to high morbidity and mortality both for the mother and the child.[114-117] Mother could develop severe malaria and severe anemia, and is much exposed to obstetric adverse events.[115,117-119] Placental malaria has been associated with elevated risk of miscarriage, preterm delivery, intrauterine growth retardation, low birth weight, fetal anemia, congenital malaria and perinatal mortality.[114,116,120,121] Placental malaria is quite common during P. falciparum infection, less common in P. vivax malaria; P. falciparum and P. vivax placental mixed-infection can occur.[122] P. vivax placental malaria may lead to the same adverse events as P. falciparum infection, even if with milder consequences.[121-124]
In high endemic areas, while adults are less susceptible, pregnant women are commonly susceptible to malaria infection because pregnancy causes a transient depression of cell mediated immunity. Age under 25 years and primiparity are both risk factors for developing placental malaria;[115,120] moreover the risk increases in the first and second trimester of pregnancy. A meta-analysis carried out in four sub-Saharan African countries demonstrated a reduction of placental malaria in multi-gravidae women with blood group 0; however, statistically significance were demonstrated in only two of these studies.[125]
Alterations of materno-fetal blood exchange are the basis of placental malaria. During infection, parasitized red cells both from P. falciparum and P. vivax are sequestered within the placenta and they accumulate in intervillous spaces; trophozoite and schizont forms also accumulate in the placenta.[13] The presence of IRBC activates mononuclear cells which release chemokines to recruit additional phagocytic cells in the intervillous spaces. Elevated TNF α and IL-10 levels were also described and were associated with poor pregnancy outcomes.[13] IRBC, leukocyte infiltration, fibrin and hemozoin depositions contribute to increase the thickness of the trophoblast basement membrane and to alter the intervillous and perivillous spaces, causing reduction of oxygen and nutrient transport to the fetus.[13,126]
P. falciparum parasitized erythrocytes adhere to chondroitin sulphate A expressed in placenta; thus, placenta selects for strains of P. falciparum with a CSA-binding phenotype. Primigravidae who have not been exposed to P. falciparum CSA-binding phenotype are still susceptible to placental infection, while multigravidae have developed antibodies during the first successful pregnancies.[115] P. vivax parasitized erythrocytes can also cytoadhere to the placenta but their mechanism is not clarified, yet.[5,124]
Molecular studies demonstrate that IRBC binding to chondroitin sulphate A (CSA), expressed on the apical membrane of the placental syncytiotrophoblast epithelium, is mediated by VAR2CSA antigen, a variant of the PfEMP1 family proteins. There are multiple genes encoding for different VAR2CSA antigens; a study performed in Cameroon shows that the parasites infecting pregnant women living in high transmission areas have an increased copy number of var2csa genes compared to non-pregnant women. The multiplicity of var2csa-type genes confers to P. falciparum parasites a greater capacity for antigenic variation and evasion of immune response.[127] A recent study describes a new flow cytometry-based adhesion assay that use apical epithelial plasma membrane vesicles and IRBC isolated from patients.[128] Data from this study showed that the vesicles prepared from various placental regions could adhere to IRBC in different percentage but with the same adhesion intensity; moreover, parasite molecules, other than VAR2CSA, can also mediate placental adhesion, suggesting that a different molecular pathway can occur. These findings were confirmed by another study that showed that transcripts from var genes, different from var2csa, were found in 67% of placental isolates, revealing the importance of other adhesion molecules during pregnancy.[129]
Complement activation may play an important pathogenetic role during placental malaria. C5a, a factor derived from the complement cascade, is increased in peripheral blood and placental blood of pregnant women with malaria compared with pregnant women without malaria infection.[130] Factors derived from the activation of the complement cascade are likely to have a role in inflammation during placental malaria, in particular they could stimulate the release of pro-inflammatory cytokines and chemokines by monocytes and neutrophils.[130,131] Moreover, C5a could play an important role in dysregulation of angiogenesis since it seems to stimulate monocyte production of the anti-angiogenic factor sFlt-1, a soluble variant of VEGFR-1. The sFlt-1 binds to and sequesters placental growth factor and VEGF, leading to vascular and placental alteration.[130,131] Muehlenbachs et al. performed a study in Tanzanian women showing that the FLT1 genotype was associated to pregnancy loss, low birth weight, placental inflammatory gene expression and high Flt1 levels.[132]
Conclusions
The pathogenicity of severe malaria infection is complex and it is regulated by both parasite and host factors. Cytoadherence of IRBC to the vascular endothelium and rosetting are unique features of malaria parasites which are likely to contribute substantially to the vascular damage and the consequent excessive inflammatory/immune response of the host. This can occur in many different organs, a feature that can partly explain the complexity of the clinical manifestations occurring in severe malaria. In this context, adequate clinical management of malaria patients requires first an accurate diagnosis, then appropriate antimalarial treatment, associated with adjunct supportive therapies which need to be adapted to the different clinical presentation of the disease.
Malaria is a life-threatening disease which in 2010 killed more than 600,000 individuals, mainly children under 5 years of age, and pregnant women.[1] All the clinical symptoms of malaria are the consequence of infection of human erythrocyte by merozoites. Most of the fatal cases, which predominantly occur in P. falciparum infections, are due to severe anaemia or cerebral malaria, but different clinical manifestations also exist and vary in severity and outcome, depending on the parasite species, the organ involved and the access to care.
P. falciparum differs from other human malarial species in that infected red blood cells (IRBC) do not remain in the circulation for the entire life cycle. After 24–32 hours, when young parasites mature from the ring to the trophozoite stage, IRBC adhere to endothelial cells in the microcirculation of various organs. This phenomenon, termed “sequestration”, is believed to occur mainly to avoid splenic removal of IRBC. Sequestration causes microcirculatory obstruction, impaired tissue perfusion and inflammatory cells activation and it is linked to the severity of the disease.
At schizonts rupture, from 4 up to 36 daughter merozoites, depending on the Plasmodium species, are released into the circulation and invade fresh RBC to perpetuate the asexual life cycle. At the same time, a large amount of toxins and parasite products are also released and cause the activation of the innate immunity, the release of inflammatory mediators and the symptoms associated with the malaria attack, such as fever.
A combination of mechanical circulatory stress due to sequestration and excess inflammatory response contribute to the most severe manifestations of malaria including, but not limited to, cerebral malaria or anaemia. The present review will summarise some of the pathogenetic aspects of severe malaria and then it will report on selected and less frequent manifestation, such as renal failure or acute respiratory distress syndrome (ARDS).
Pathogenetic Characteristics of Severe Malaria
Cytoadherence. Cytoadherence, the ability of parasites to adhere to the vascular endothelium, was recognized as early as 1892 by Marchiafava and Bignami.[2] Mature forms of parasites (asexual stage and gametocytes) can adhere to the vascular endothelium of several organs (lung, heart, brain, lung, liver, and kidney), the subcutaneous adipose tissues and the placenta. This feature of the disease in vivo has been related exclusively to P. falciparum.[3,4] However, sequestration in vitro to some endothelial cell lines and placental cryosections has also been seen in reticulocytes infected with P. vivax.[5]
Parasite sequestration is thought to be the pathological base of the severe manifestation of malaria, including cerebral malaria.[6] It causes blood flow impairment leading to local hypoxia. It enhances parasite replication and the sticking of IRBC to non-infected red blood cells (rosetting, see below). Moreover, when parasite sequester, the effects of parasite toxins are more localized and also the stimulation of the host immune response, which may cause a focused production of inflammatory mediators and tissue damage. As a consequence, both RBC and IRBC become more rigid and less deformable.[7]
Sequestration is mostly mediated by mature parasite forms, approximately 20 hours after RBC invasion. The parasites produce new proteins that are exported to the IRBC surface and increase the adhesiveness of IRBC to the endothelium. During their 48-hour life cycle, the parasites can remain sequestered for 24 hours in the deep microvasculature. In this manner, they evade clearance by the spleen, and make the diagnosis more difficult since they are not seen in the peripheral blood.
Sequestration of P. falciparum has been attributed to different class of molecules of parasite origin and ligands present on the human endothelium. Among those, the P. falciparum histidine-rich protein (PfHRP) and the erythrocyte membrane protein 1 (PfEMP1), have received significant attention. PfHRP is related to the establishment of knobs, symmetric membrane arrangements which appear on the surface of infected RBC, while PfEMP1, a multimeric protein encoded by the var (variant) gene[3,4] protrudes from the knobs and plays a major role in sequestration and thus virulence (see BOX 1). To adhere to the endothelium, the parasites first adhere, roll and then become firmly attached to the endothelium adhesion molecules. Among these molecules, ICAM-1, a major sequestration receptor and involved in cerebral sequestration serves as a rolling receptor. On the other hand, CD36 gives stationary and stable adherence under flow.[8-10]

Box 1. PfEMP1, a molecule for antigenic variation, adhesion and immune modulation.
Sequestration is also seen during gestational malaria, when parasites adhere to the placenta. PfEMP1 is again the main adhesion receptor which adheres to the trophoblastic villous endothelium mainly through chondroitin-4-sulfate (CSA) and other sugars such as glycosaminoglycans and possibly hyaluronic acid (HA). As discussed later, malaria in pregnancy can be severe for mothers and induce fetal death especially during the first pregnancy, when women usually lack sufficient immunity against CSA-binding parasites.[11-14]
Rosetting. Rosetting is one of the forms of cytoadherence of late stages IRBC to non-parasitized red blood cells and /or platelets.[15] The IRBC ligand involved in rosette formation is PfEMP1, and three are the receptors associated with rosetting: complement receptor 1 (CR1), heparan sulfate (HS), and the ABO blood group.[15,16] PfEMP1 has been shown to bind to CR1, specifically at the C3b-binding site. The lectin-like DBL-domain of PfEMP1 can make strong adhesion with carbohydrate structures particularly A blood group antigen, favoring rosettes formation.[17] For this reason, non-O blood groups are considered significant risk factors for life-threatening malaria, through the mechanism of enhanced rosette formation.[18,19]
P. falciparum, P. vivax, and P. ovale are all able to form rosettes,[20,21] but only those caused by P. falciparum have been associated with severe malaria, and especially in African children they may enhance microcirculatory obstruction.[22] Rosetting has been related with parasite multiplication rate in a model of Saimiri sciureus.[23] More recently, it has been shown that 4-HNE, a biomembrane lipid peroxidation product driven by haem iron of the malarial pigment can be transferred from IRBC to normal RBC in rosettes favouring their removal by macrophages.[24] This could partly explain the rapid loss of normal non parasitized RBC in severe malaria anaemia.
Innate Immune Response. The immune response to the parasite is complex and not completely understood, and it is essentially both species and stage specific.[25] The activation of components of the innate immune system is crucial to control parasite replication, contributing to the subsequent elimination and resolution of the infection.[26] Neutrophils, monocytes/macrophages, dendritic cells, natural killer (NK) cells, NKT cells, and gamma T cells are all the cells of the innate immune system in charge of controlling the early progression of the disease through phagocytosis and/or production of inflammatory mediators. Much of the symptoms of malaria attacks such as fever, nausea, headaches, and others are the consequences of the inflammatory response orchestrated by the cells of the innate immune system, stimulated by parasites or their products at the rupture of the late stage infected erythrocytes.[25,27] An imbalance between the production of pro- or anti-inflammatory cytokines, such as TNF-α, IL6, IL1β, or IL-10 or mediators, like nitric oxide, may contribute to the pathogenesis of the severe form of the disease. Elevated levels of TNF-α are found in the serum of severe patients and have been correlated with cerebral malaria (CM).[28] In vitro, it has been found that TNF-α enhances the expression of ICAM-1,[29] which is also augmented in brain vessels of CM patients.[9] Evolutionarily conserved receptors, termed “pathogen recognition receptors” (PRRs), are present on the cells of native immunity and trigger the response upon the recognition of specific parasite molecules called “pathogen associated molecular patterns” (PAMPs).[30] Malaria PAMPs include the protein anchor, glycosyl-phosphatidyl inositol (GPI), or hemozoin (malaria pigment). PRRs related to malaria are the membrane bound Toll-like receptors (TLRs), the cytosolic receptors (such as NALP3, inflammasomes), and soluble receptors such as MBL.[31-34] For example, GPI released by IRBC at shizonts rupture stimulates the production of pro-infiammatory TNF-α by macrophages via the recognition of TLR2 and partly of TLR4 in a MyD88 dependent way.[31]
Specific Immune Response. Malaria is an important cause of morbidity, but not everyone infected with the malaria parasite becomes seriously ill or dies. In areas of stable endemicity, repeated exposure to the parasite leads to the acquisition of specific immunity, which restricts serious problems to young children; malaria in older subjects causes a relatively mild febrile illness. However, individuals with no previous experience of malaria become ill on their first exposure to the plasmodium parasite. They develop a febrile illness which may become severe and in a proportion of cases may lead to death.[35,36]
Immunity to malaria is provided by innate mechanisms, as we summarized above, and subsequently by the development of acquired immunity. Following repeated infections, people living in malaria endemic areas gradually acquire mechanisms, which helps limiting the inflammatory response to the parasite that causes the acute febrile symptoms. Sterilizing immunity is never achieved. Parasites have evolved to maintain a balanced relationship with their human hosts. In this sense they can partially escape from the host effector mechanisms, while hosts are able to develop partial immunity against the parasite. This type of immunity requires repeated infections, takes years to develop and usually lasts shortly. Natural acquired immunity is called premunition since low parasite burden often persists in the presence of circulating antibodies to the various stages, in the absence of clinical disease. In children without circulating antibodies to the parasite, premunition is lower. The poor and slowly developing immune response to malaria is partly due to parasite immune evasion strategies: antigenic polymorphism, shedding of parts of parasite proteins, cross-reactive antigen epitopes of developmental stages, prolonged exposure to endemic malaria and limited immunogenicity of antigens. In stable endemic areas, a heavy burden of morbidity and mortality falls on young children. Children born to immune mothers appear to be relatively immune to malaria for a period.[26,37] This is conferred by the prenatal or postnatal transfer of protective antibodies from mother to child. The acquired immunity is mediated by specific antibodies to several conserved and polymorphic proteins, and to the highly variable protein PfEMP1 expressed by the parasite at the trophozoites and schizonts stages and exported on the surface of infected erythrocytes.[38]
Specific Complications of Severe Malaria Infection
Anaemia. Anaemia is one of the most common causes of morbidity and mortality in malaria infection particularly in pregnant women and in children.[39] Pathogenesis of malarial anaemia has been intensively studied, even if it is not completely understood; hereby we summarise some of the pathogenetic aspects. Malarial anaemia could be acute or chronic; in holo-endemic areas chronic malarial anaemia is more common. Acute malarial anaemia could occur after massive erythrocytes lysis due to elevate parasitemia or to drug-induced or immune haemolysis.[40]
The potential mechanisms contributing to malarial anaemia can be divided into two categories: increased destruction of parasitized and un-parasitized erythrocytes (immune-mediated lysis, phagocytosis, splenic sequestration) and decrease of RBC production (dyserythropoiesis and bone marrow suppression, inadequate reticulocyte production, effects of inflammatory cytokines, effects of parasite factors). Co-infection with bacteremia, HIV-1 and hookworm, malnutrition and repeated malarial infections in holo-endemic countries may also contribute to decrease haemoglobin levels.[39,41-43]
Parasitized red cells rupture caused by plasmodium cycles and clearance of deformed parasitized and un-parasitized erythrocytes are the principal causes of malarial anaemia. Phospholipid asymmetry, membrane rigidity and reduced deformability are the mechanisms involved in the premature removal of un-parasitized red cell.[7,41,44] Phagocytosis and complement activation are the principal mechanisms of non-specific immune mediated clearance of erythrocytes in malaria infection.[45] Moreover, uninfected red cells membrane proteins may be altered by reactive oxygen species (ROS) and other factors, becoming target for auto-antibodies.[4] It has been suggested that the spleen may play a double role in malaria infection: on the one hand it could contribute to severe anaemia, by excess removal of IRBC and uninfected RBC; on the other hand it may protect from severe cerebral malaria.[46]
Dyserythropoiesis plays an important role in the pathogenesis of anaemia; examination of bone marrow from children with severe anaemia showed hypercellularity, mild to normal erythroid hyperplasia and abnormal features of late erythroid progenitors. Hemozoin and its phagocytosis by bone marrow macrophages has been proposed to cause dys-erythropoiesis either through direct accumulation in the bone marrow and generation of reactive toxic species or activation of the innate immune response.[42]
The immune response is central in the pathogenesis of malarial anaemia; parasitized red cells, hemozoin and malarial antigens activate monocyte and lymphocyte response. Pro-inflammatory and anti-inflammatory mediators, including TNF-α, IFN-γ, IL-23 and IL-1, chemokines and growth factor are produced and contribute to anaemia. On the contrary, IL-12 and IL-10 seems to be protective cytokines since low levels are found in severe malarial anaemia.[40,43] Macrophage migration inhibiting factor (MIF) is associated with severe anaemia and bone marrow suppression.[47] Nitric oxide is an inhibitor of erythropoiesis.[40,48]
Erythropoietin (EPO) levels are increased during malaria anaemia, but erythroid progenitors response is not adequate, particularly during chronic malaria infection, resulting in low reticulocytosis.[43] It has been shown in a rodent model that exogenous EPO could stimulate splenic erythroblasts. However, their maturation is impaired due to altered iron metabolism and haemoglobin production.[49] Ineffective erythropoiesis, erythrophagocytosis and iron delocalisation are the most important causes of reticulopenia.
Pro-inflammatory cytokines play an important role also in iron delocalisation pathway of malarial anaemia. TNFα induces re-localisation of ferroportin, an important protein abundant in the reticulo-endothelial system that mediates macrophage iron release and intestinal iron absorption. Relocalisation of ferroportin induces decrease of iron absorption and release from macrophage cells;[45] hepcidin, a protein released during chronic disease, also induces reduction of ferroportin and its levels are increased during severe malaria anaemia.[50]
STAT6, a member of signal transducer and activator of transcription family proteins, seems also to be involved in malarial anaemia through the activation of the regulatory cytokines, in particular IL-4 and IFN-γ, resulting in erythropoietic suppression during blood stage malaria.[51,52] A recent review found an interesting correlation between malarial anaemia and micronutrient malnutrition: vitamin A and E, iron, zinc, riboflavin and folate deficiency may play a role in worsening the anaemia mediated by alteration of immunity, dyserythropoiesis and iron metabolism.[39] It has also been suggested that high catecholamine concentration could alter the functions of the erythrocyte membrane, providing an additional erythrocyte clearance mechanism.[53]
Thrombocytopenia. Thrombocytopenia is very common in malaria, usually during the early stage of P. falciparum and P. vivax infections. Incidence is high both in children and in adults.[54-56] Thrombocytopenia in pregnant women is not well documented, but a recent study performed in Thailand showed that platelet counts were lower in pregnant than in non-pregnant women.[57] The pathogenesis of malaria thrombocytopenia is complex and may be related to coagulation disturbances, splenomegaly and platelet destruction by macrophages, bone marrow alterations, antibody-mediated platelet destruction, oxidative stress and platelets aggregation. These processes are well described in a recent review.[58] A study performed in Indonesia showed that patients with P. falciparum and P. vivax malaria had lower platelet count, higher von Willebrand factor (VWF) concentration, lower ADAMTS13 activity and ADAMTS13 antigen concentrations.[59] Higher VWF seems to correlate with platelet binding, leading to thrombocytopenia. In contrast, another study demonstrated that sGP1b, the external domain of platelet receptor for VWF, increased early in the blood of malaria patients thus preventing excessive platelet adhesion.[60] Despite thrombocytopenia is very common, hemorrhagic events are rare and usually are associated with severe thrombocytopenia or disseminated intravascular coagulation (DIC).[61-63] Further study are needed to understand the relationship between malaria, platelet counts and hemorrhagic events.
ARDS. Deep breathing, respiratory distress and pulmonary oedema are some of the clinical feature defining severe malaria according with WHO classification.[64] In adults and pregnant women, rather than children, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are the most common clinical presentation burdened by an elevated mortality rate as shown in a recent study performed in India where severe P. falciparum malaria mortality rate was 35,4% and mortality was principally due to shock, acute renal failure, seizure and ARDS.[65] Despite ARDS is mostly observed as a complication of P. falciparum infection, case reports due to P. vivax,[66-69] P. ovale[70] and P. knowlesi[71,72] were also published. Malaria associated ARDS due to P. vivax caused three maternal deaths in a cohort of 221 patient in India during one year of observation.[66] ARDS can occur before or after specific treatment.[67] P. knowlesi, the fifth plasmodium species infecting humans, mostly in Southeast Asia, may cause life threatening diseases, as well.[73] A prospective study conducted in Malaysia demonstrated that the most frequent complication of P. knowlesi infection was respiratory distress.[72] Malaria is presently considered one of the most common risk factor of ARDS and acute lung injury (ALI) in the tropics[74] and the second cause of ARDS after sepsis.[75]
Little is known about the pathogenesis of malaria associated ALI/ARDS. Inflammatory mediators and increased endothelial permeability may play an important role, while parasite sequestration may take a minor role. Pulmonary manifestations of uncomplicated malaria were analysed by Anstey et al. showing that patients frequently have subclinical impairment of lung function, such as small airways obstruction, impaired alveolar ventilation, reduced gas exchange and increased pulmonary phagocytic activity.[76] All these features underline the activation of inflammatory pathways and could explain part of the pathogenetic mechanisms of respiratory distress occurring during severe malaria. Studies with rodent malaria models were performed to understand the pathogenetic mechanisms of ALI/ARDS. Epiphanio et al. in DBA/2 mice infected by P. berghei ANKA[77] observed that 60% of mice had dyspnea, airways obstruction and hypoxemia; pleural effusion, pulmonary hemorrhagies and oedema were also found as pathological findings, demonstrating the role of inflammation in ALI development. Moreover, high levels of vascular endothelial growth factor (VEGF) were found in mice with ALI, supporting the importance of increased vascular permeability in malaria respiratory failure. Another study[78] which utilized DBA/2 mice infected with P. berghei K173, showed that proteins and inflammatory cells mainly CD4+ and CD8+ lymphocytes, neutrophils and monocytes accumulate in the lungs of infected mice. These results were confirmed by P.E. Van den Steen et al.,[79] who also measured the cytokines and chemokines associated with ARDS, showing an increased expression in the lungs of TNF-α, IL-10, IFN γ, CXCL10 and CXCL11, as well as monocyte and neutrophil chemo-attractant chemokines (CCL2, KC). IRBC were are also observed in the lung vessels, but at a lower extent compared with the massive sequestration in the brain.[80] Infected mice lungs had an increased water content, demonstrating the development of oedema;[78,79] this could be related to the decreased expression of epithelial sodium channel, ENaC, due to hypoxia, resulting from malaria associated anaemia, or to TNFα mediated down-regulation.[78] In a rodent model malaria associated ARDS, dexamethasone inhibited the infiltration of macrophages and CD8 T cells into the lungs, suggesting that adjunct therapy with anti-inflammatory drugs could also be useful in the clinical setting.[79]
Most of the studies with P. falciparum are based on in vitro models or post mortem observations. It was shown in vitro that P. falciparum merozoite proteins could increase endothelial permeability, while P. falciparum IRBC did not show the same properties suggesting that the effects of the parasite on the pulmonary endothelium are probably mediated by the activity of Src-family kinases.[81] Morphologic changes were noted in the proteins of the tight junctions and adherent junction in association with increased endothelial permeability and development of pulmonary oedema.[81] A post mortem study performed in children with cerebral malaria demonstrated that sequestration of P. falciparum -IRBC occurs in the lungs, even if to a lesser extent than in the brain, skin or intestine.[80]
Different pathological presentations of ARDS were observed among the different species of human malaria: the greatest severity and frequency of cases are due to P. falciparum and could be partially attributed to the sequestration and rosetting of infected RBC in the pulmonary microcirculation;[67] heavy parasitaemia and WBC agglutinates were associated to ARDS in P. vivax malaria.[66] L. Price et al. suggested that the pathogenesis of lung injury by P. falciparum include cytokine-induced damage or direct effects of sequestration of parasitized erythrocytes, while ARDS caused by P. vivax may be due principally to dysregulation of cytokines production.[69] Elevated parasitemia in ARDS in P. knowlesi infection suggests parasite-specific effects that increase pulmonary capillary permeability,[71] but hypoxemia and metabolic acidosis may also contribute.[72]
Liver involvement. According to the World Health Organization liver dysfunction is an uncommon occurrence in malaria, while jaundice is not unusual. The incidence of jaundice and liver dysfunction in P. falciparum malaria varied from 5.3% to 62% and from 2.5% to 21%, respectively, in different reports,[82-84] while malarial hepatitis is rare in P. vivax infection.[85] Case-fatality rate in malaria-related hepatic failure is elevated, up to 40%[84-86] when high parasite density is associated with jaundice and liver dysfunction.[87] Liver is involved in malaria at two stages: during the pre-erythrocytic cycle and the erythrocytic phase. The first step is linked to the binding of the merozoite circumsporozoite protein CSP-A and TRAP protein to the hepatocytes via the heparan sulphate glycosylaminoglycans GAG[88] and promotes minimal liver damage. In the erythrocytic phase, jaundice is a common remark and it is directly caused by the infection (malarial hepatitis, intravascular hemolysis of parasitized RBC, septicemic hepatitis), or by indirect causes (microangiopathic hemolysis associated with DIC, G6PD-related hemolysis, antimalarial drug induced-hemolysis) or completely unrelated (coexisting acute viral hepatitis, underlying chronic hepatitis).[88] Intravascular hemolysis of parasitized and non parasitized RBC causes an increase of unconjugated bilirubinemia with mild to moderate jaundice;[89] conjugated hyperbilirubinemia indicates hepatocyte dysfunction. The pathogenesis of hepatic dysfunction is not completely known; reduction in portal venous flow as a consequence of microocclusion of portal venous branches by parasitized erythrocytes, intrahepatic cholestasis due to reticuloendothelial blockage and hepatic microvilli dysfunction, suppression of bilirubin excretion due to effect of parasitemia or endotoxemia or metabolic acidosis, apoptosis and oxidative stress are all mechanisms involved in hepatic damage.[88,90] Histopathological findings reported in literature are: congestion of hepatocytes, swollen hepatocytes, centrizonal necrosis, Kupffer cell hyperplasia, deposition of brown malarial pigment, portal infiltration with lymphocytes, steatosis, parasitized RBC, cholestasis, spotty and submassive necrosis.[88-91] These evidences demonstrate inflammatory as well as direct plasmodial effects in the damage to hepatocytes.
Kidney involvement. Kidneys in malaria are involved in two different manners: acute and chronic diseases. Acute renal failure (ARF) is one of the most challenging diseases in tropical countries and malaria plays an important epidemiological role.[92-95] The mortality due to malaria ARF is high.[96] A study performed in Yemen showed that malaria was the first cause of death in patient with ARF.[97] Malaria acute renal failure (MARF) is more common in non-immune adults and in older children.[98] MARF is mostly associated to P. falciparum infection and is more frequent in low transmission areas;[98-101] nevertheless MARF could be caused also by P. vivax.[100,102] P. malariae causes chronic and progressive glomerulopathy, known as quartan malaria nephropathy (QMN); only few cases of MARF caused by P. malariae has been published.[103]
Quartan malaria nephropathy (QMN) is frequently seen in African children and it is clinically associated with oedema and hypertension; urine analysis shows often proteinuria and microhematuria.[101,104] Pathogenesis of QMN is linked to subendothelial deposits of immune complexes containing IgG, IgM, C3 and in the 25-33% of cases also malaria antigens.[101,104] It is thought to be the consequence of the activation of TH2 type T lymphocytes. The pathogenesis may also include genetic and acquired factors, such as autoimmunity, co-infection with Epstein-Barr virus and malnutrition.[104] Immune complexes deposition provokes glomerular damage, resulting in proliferative glomerulonephritis; the pathology, initially focal, becomes diffuse and progressive, reaching sclerosis. Different histopatological patterns revealed by renal biopsy induced some authors to conclude that the association between P. malariae infection and renal involvement was only coincidental.[101] P. falciparum acute renal failure is more common in adults; its pathogenesis is complex and it includes mechanical and immunologic factors, volume depletion, hypoxia, hyperparasitemia and other factors.[98,101,105] High parasite density was associated to acute renal failure.[101,87] Parasitized erythrocyte sequestration was found in kidneys of adults who died from severe P. falciparum malaria;[106] sequestration of parasitized erythrocytes is lower in the kidney than in the brain.[98,106] A study performed in Mali, showed that rosetting is present in all severe malaria clinical forms, including acute renal failure.[22] Hemolysis, causing endothelial activation and hemodynamic alteration, can lead to acute tubular necrosis and acute interstitial nephritis.[104]
Hypoperfusion, due to the loss of liquids and to the absence of volume restoration, leads to renal ischemia.[98] TNFα, reactive oxygen species and inducible nitric oxide also play an important role in determining the haemodynamic alteration.[104] Mononuclear cell infiltration was reported in glomerular and in peritubular capillaries of adults with ARF, while immune complexes were not reported.[106] It was hypothesized that the release of malaria antigens activates monocyte cells, to release proinflammatory cytokines and activate TH1 cell mediated response, causing acute interstitial nephritis.[104] The role of cytokines such us INFγ, IL-1α, IL-6, GM-CSF was studied in murine malaria infection and an association of nephritis with up-regulation of pro-inflammatory and dysregulation of anti-inflammatory cytokines was found.[107]
Rarely, acute renal failure has been associated to rhabdomyolysis in P. falciparum and P.vivax infections, probably due to the sequestration of parasitized red cells in the skeletal capillaries and consequent vessels occlusion.[108] In the pathogenesis of ARF related to rhabdomyolysis, the myoglobin nephrotoxic effect play the principal role; hypovolemia, hypotension, fever, acidosis and the use of non-steroidal anti-inflammatory drugs may worsened the renal function.[109,110]
Black water fever (BWF), a rare but severe complication of severe malaria, is characterized by fever, intravascular haemolysis with haemoglobinuria, dark urines and often acute renal failure.[111-113] Haemoglobin release during massive haemolysis causes renal impairment. Drugs as quinine, halophantrine and mefloquine, and G6PD deficiency, have been suggested to be the trigger of BWF.
Placenta involvement. Malaria during pregnancy is associated to high morbidity and mortality both for the mother and the child.[114-117] Mother could develop severe malaria and severe anemia, and is much exposed to obstetric adverse events.[115,117-119] Placental malaria has been associated with elevated risk of miscarriage, preterm delivery, intrauterine growth retardation, low birth weight, fetal anemia, congenital malaria and perinatal mortality.[114,116,120,121] Placental malaria is quite common during P. falciparum infection, less common in P. vivax malaria; P. falciparum and P. vivax placental mixed-infection can occur.[122] P. vivax placental malaria may lead to the same adverse events as P. falciparum infection, even if with milder consequences.[121-124]
In high endemic areas, while adults are less susceptible, pregnant women are commonly susceptible to malaria infection because pregnancy causes a transient depression of cell mediated immunity. Age under 25 years and primiparity are both risk factors for developing placental malaria;[115,120] moreover the risk increases in the first and second trimester of pregnancy. A meta-analysis carried out in four sub-Saharan African countries demonstrated a reduction of placental malaria in multi-gravidae women with blood group 0; however, statistically significance were demonstrated in only two of these studies.[125]
Alterations of materno-fetal blood exchange are the basis of placental malaria. During infection, parasitized red cells both from P. falciparum and P. vivax are sequestered within the placenta and they accumulate in intervillous spaces; trophozoite and schizont forms also accumulate in the placenta.[13] The presence of IRBC activates mononuclear cells which release chemokines to recruit additional phagocytic cells in the intervillous spaces. Elevated TNF α and IL-10 levels were also described and were associated with poor pregnancy outcomes.[13] IRBC, leukocyte infiltration, fibrin and hemozoin depositions contribute to increase the thickness of the trophoblast basement membrane and to alter the intervillous and perivillous spaces, causing reduction of oxygen and nutrient transport to the fetus.[13,126]
P. falciparum parasitized erythrocytes adhere to chondroitin sulphate A expressed in placenta; thus, placenta selects for strains of P. falciparum with a CSA-binding phenotype. Primigravidae who have not been exposed to P. falciparum CSA-binding phenotype are still susceptible to placental infection, while multigravidae have developed antibodies during the first successful pregnancies.[115] P. vivax parasitized erythrocytes can also cytoadhere to the placenta but their mechanism is not clarified, yet.[5,124]
Molecular studies demonstrate that IRBC binding to chondroitin sulphate A (CSA), expressed on the apical membrane of the placental syncytiotrophoblast epithelium, is mediated by VAR2CSA antigen, a variant of the PfEMP1 family proteins. There are multiple genes encoding for different VAR2CSA antigens; a study performed in Cameroon shows that the parasites infecting pregnant women living in high transmission areas have an increased copy number of var2csa genes compared to non-pregnant women. The multiplicity of var2csa-type genes confers to P. falciparum parasites a greater capacity for antigenic variation and evasion of immune response.[127] A recent study describes a new flow cytometry-based adhesion assay that use apical epithelial plasma membrane vesicles and IRBC isolated from patients.[128] Data from this study showed that the vesicles prepared from various placental regions could adhere to IRBC in different percentage but with the same adhesion intensity; moreover, parasite molecules, other than VAR2CSA, can also mediate placental adhesion, suggesting that a different molecular pathway can occur. These findings were confirmed by another study that showed that transcripts from var genes, different from var2csa, were found in 67% of placental isolates, revealing the importance of other adhesion molecules during pregnancy.[129]
Complement activation may play an important pathogenetic role during placental malaria. C5a, a factor derived from the complement cascade, is increased in peripheral blood and placental blood of pregnant women with malaria compared with pregnant women without malaria infection.[130] Factors derived from the activation of the complement cascade are likely to have a role in inflammation during placental malaria, in particular they could stimulate the release of pro-inflammatory cytokines and chemokines by monocytes and neutrophils.[130,131] Moreover, C5a could play an important role in dysregulation of angiogenesis since it seems to stimulate monocyte production of the anti-angiogenic factor sFlt-1, a soluble variant of VEGFR-1. The sFlt-1 binds to and sequesters placental growth factor and VEGF, leading to vascular and placental alteration.[130,131] Muehlenbachs et al. performed a study in Tanzanian women showing that the FLT1 genotype was associated to pregnancy loss, low birth weight, placental inflammatory gene expression and high Flt1 levels.[132]
Conclusions
The pathogenicity of severe malaria infection is complex and it is regulated by both parasite and host factors. Cytoadherence of IRBC to the vascular endothelium and rosetting are unique features of malaria parasites which are likely to contribute substantially to the vascular damage and the consequent excessive inflammatory/immune response of the host. This can occur in many different organs, a feature that can partly explain the complexity of the clinical manifestations occurring in severe malaria. In this context, adequate clinical management of malaria patients requires first an accurate diagnosis, then appropriate antimalarial treatment, associated with adjunct supportive therapies which need to be adapted to the different clinical presentation of the disease.
References
- World Health Organization (WHO) World Malaria Report 2011.
- Marchiafava F, Bignami A. Sulle febbri malariche estivo-autunnali. 1892. Rome: Loescher
- Kyes S, Horrocks P, and Newbold C.
Antigenic variation at the infected red cell surface in malaria. Annu
Rev Microbiol 2001, 55: 673-707. http://dx.doi.org/10.1146/annurev.micro.55.1.673 PMid:11544371

- Scherf A, Lopez-Rubio JJ, and Riviere L. Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol 2008, 62: 445-470. http://dx.doi.org/10.1146/annurev.micro.61.080706.093134 PMid:18785843

- Carvalho B O, Lopes SC, Nogueira PA,
Orlandi PP, Bargieri DY, Blanco YC, Mamoni R, Leite JA, Rodrigues MM,
Soares IS, Oliveira TR, Wunderlich G, Lacerda MV, del Portillo HA,
Araújo MO, Russell B, Suwanarusk R, Snounou G, Rénia L, and Costa FT.
On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect
Dis 2010, 202: 638-647. http://dx.doi.org/10.1086/654815 PMid:20617923

- Grau GE, Craig AG. Cerebral malaria
pathogenesis: revisiting parasite and host contributions. Future
Microbiol. 2012 Feb;7(2):291-302. http://dx.doi.org/10.2217/fmb.11.155 PMid:22324996

- Dondorp AM, Pongponratn E, and White NJ.
Reduced microcirculatory flow in severe falciparum malaria:
pathophysiology and electron-microscopic pathology. Acta Trop 2004, 89:
309-317. http://dx.doi.org/10.1016/j.actatropica.2003.10.004 PMid:14744557

- Chakravorty SJ, and Craig A. The role of ICAM-1 in Plasmodium falciparum cytoadherence. Eur J Cell Biol 2005, 84: 15-27. http://dx.doi.org/10.1016/j.ejcb.2004.09.002 PMid:15724813

- Turner GD, Morrison H, Jones M, Davis TM,
Looareesuwan S, Buley ID, Gatter KC, Newbold CI, Pukritayakamee S, and
Nagachinta B. An immunohistochemical study of the pathology of fatal
malaria. Evidence for widespread endothelial activation and a potential
role for intercellular adhesion molecule-1 in cerebral sequestration.
Am J Pathol 1994,145: 1057-1069 PMid:7526692 PMCid:1887431

- Milner DA Jr. Rethinking cerebral malaria pathology. Curr Opin Infect Dis. 2010, 23:456-63. http://dx.doi.org/10.1097/QCO.0b013e32833c3dbe PMid:20613511

- Fried M, Domingo GJ, Gowda CD, Mutabingwa
TK, and Duffy PE. Plasmodium falciparum: chondroitin sulfate A is the
major receptor for adhesion of parasitized erythrocytes in the
placenta. Exp Parasitol 2006, 113: 36-42 http://dx.doi.org/10.1016/j.exppara.2005.12.003 PMid:16430888

- Reeder JC, Cowman AF, Davern KM, Beeson
JG, Thompson JK, Rogerson SJ, and Brown GV. The adhesion of Plasmodium
falciparum-infected erythrocytes to chondroitin sulfate A is mediated
by P. falciparum erythrocyte membrane protein 1. Proc Natl Acad Sci
USA. 1999, 96: 5198-5202. http://dx.doi.org/10.1073/pnas.96.9.5198

- Rogerson SJ, Hviid L, Duffy PE, Leke RF,
Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet
Infect Dis, 2007, 7: 105-117. http://dx.doi.org/10.1016/S1473-3099(07)70022-1

- von Itzstein, M, Plebanski M, Cooke BM,
and Coppel RL. Hot, sweet and sticky: the glycobiology of Plasmodium
falciparum. Trends Parasitol 2008, 24: 210-218. http://dx.doi.org/10.1016/j.pt.2008.02.007 PMid:18420458

- Rowe JA, Claessens A, Corrigan RA, and
Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to
human cells: molecular mechanisms and therapeutic implications. Expert
Rev Mol Med 2009, 11: e16. http://dx.doi.org/10.1017/S1462399409001082 PMid:19467172 PMCid:2878476

- Chen Q, Barragan A, Fernandez V, Sundström
A, Schlichtherle M, Sahlén A, Carlson J, Datta S, and Wahlgren M.
Identification of Plasmodium falciparum erythrocyte membrane protein 1
(PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum.
J Exp Med 1998,187: 15-23. http://dx.doi.org/10.1084/jem.187.1.15 PMid:9419207 PMCid:2199182

- Vogt, AM, Barragan A, Chen Q, Kironde F,
Spillmann D, and Wahlgren M. Heparan sulfate on endothelial cells
mediates the binding of Plasmodium falciparum-infected erythrocytes via
the DBL1alpha domain of PfEMP1. Blood 2003, 101: 2405-2411. http://dx.doi.org/10.1182/blood-2002-07-2016 PMid:12433689

- Barragan, A, Kremsner PG, Wahlgren M, and
Carlson J. Blood group A antigen is a coreceptor in Plasmodium
falciparum rosetting. Infect Immun 2000,68: 2971-2975 http://dx.doi.org/10.1128/IAI.68.5.2971-2975.2000 PMid:10768996 PMCid:97511

- Rowe, J A, Handel IG, Thera MA, Deans AM,
Lyke KE, Koné A, Diallo DA, Raza A, Kai O, Marsh K, Plowe CV, Doumbo
OK, and Moulds JM. Blood group O protects against severe Plasmodium
falciparum malaria through the mechanism of reduced rosetting. Proc
Natl Acad Sci USA 2007, 104: 17471-17476. http://dx.doi.org/10.1073/pnas.0705390104 PMid:17959777 PMCid:2077280

- Udomsanpetch, R, Thanikkul K,
Pukrittayakamee S, and White NJ. Rosette formation by Plasmodium vivax.
Trans R Soc Trop Med Hyg 1995, 89: 635-637. http://dx.doi.org/10.1016/0035-9203(95)90422-0

- Angus, BJ, Thanikkul K, Silamut K, White
NJ, and Udomsangpetch R. Short report: Rosette formation in Plasmodium
ovale infection. Am J Trop Med Hyg 1996, 55: 560-561. PMid:8940990

- Doumbo, OK, Thera MA, A. K. Koné AK, Raza
A, Tempest LJ, Lyke KE, Plowe CV, and Rowe JA. High levels of
Plasmodium falciparum rosetting in all clinical forms of severe malaria
in African children. Am J Trop Med Hyg 2009, 81: 987-993. http://dx.doi.org/10.4269/ajtmh.2009.09-0406 PMid:19996426 PMCid:2877664

- Le Scanf, C, Vigan-Womas I, Contamin H,
Guillotte M, Bischoff E, and Mercereau-Puijalon O. Rosetting is
associated with increased Plasmodium falciparum in vivo multiplication
rate in the Saimiri sciureus monkey. Microbes Infect 2008, 10: 447-451.
http://dx.doi.org/10.1016/j.micinf.2007.12.012 PMid:18400545

- Uyoga, S, Skorokhod OA, Opiyo M, Orori EN,
Williams TN, Arese P, and Schwarzer E. Transfer of 4-hydroxynonenal
from parasitized to non-parasitized erythrocytes in rosettes. Proposed
role in severe malaria anemia. Br J Haematol. 2012, [Epub ahead of
print] http://dx.doi.org/10.1111/j.1365-2141.2011.09015.x PMid:22352722 PMCid:3412292

- Stevenson, MM, and Riley EM. Innate immunity to malaria. Nat Rev Immunol 2004, 4: 169-180. http://dx.doi.org/10.1038/nri1311 PMid:15039754

- Urban, BC, Ing R, and Stevenson MM. Early
interactions between blood-stage plasmodium parasites and the immune
system. Curr Top Microbiol Immunol 2005, 297: 25-70. http://dx.doi.org/10.1007/3-540-29967-X_2

- Taramelli, D, Recalcati S, Basilico N,
Olliaro P, and Cairo G. Macrophage preconditioning with synthetic
malaria pigment reduces cytokine production via heme iron-dependent
oxidative stress. Lab. Invest 2000, 80, 1-8. http://dx.doi.org/10.1038/labinvest.3780189 PMid:11140691

- Kwiatkowski, D, Hill AV, Sambou I, Twumasi
P, Castracane J, Manogue KR, Cerami A, Brewster DR, and Greenwood BM.
TNF concentration in fatal cerebral, non-fatal cerebral, and
uncomplicated Plasmodium falciparum malaria. Lancet 1990, 336:
1201–1204. http://dx.doi.org/10.1016/0140-6736(90)92827-5

- Treeratanapiboon, L, Psathaki, K, Wegener,
J, Looareesuwan, S, Galla, HJ, Udomsangpetch, R, In vitro study of
malaria parasite induced disruption of blood–brain barrier. Biochem.
Biophys. Res. Commun. 2005, 335, 810–818. http://dx.doi.org/10.1016/j.bbrc.2005.07.151 PMid:16105659

- Janeway, CA, and Medzhitov, R. Innate immunity recognition. Ann Rev Immunol. 2002, 20:197-216 http://dx.doi.org/10.1146/annurev.immunol.20.083001.084359 PMid:11861602

- Krishnegowda, G, Hajjar AM, Zhu J,
Douglass EJ, Uematsu S, Akira S, Woods AS, and Gowda DC. Induction of
proinflammatory responses in macrophages by the
glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling
receptors, glycosylphosphatidylinositol (GPI) structural requirement,
and regulation of GPI activity. J Biol Chem 2005,280: 8606-8616. http://dx.doi.org/10.1074/jbc.M413541200 PMid:15623512

- Coban, C, Ishii KJ, Kawai T, Hemmi H, Sato
S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, Horii T, and
Akira S. Toll-like receptor 9 mediates innate immune activation by the
malaria pigment hemozoin. J Exp Med 2005, 201: 19-25. http://dx.doi.org/10.1084/jem.20041836 PMid:15630134 PMCid:2212757

- Shio, MT, Tiemi Shio M, Eisenbarth SC,
Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS,
Descoteaux A, Flavell RA, and Olivier M. Malarial hemozoin activates
the NLP3 inflammasome through Lyn and Syk kinases. PLoS Pathog 2009, 5:
e1000559. http://dx.doi.org/10.1371/journal.ppat.1000559 PMid:19696895 PMCid:2722371

- Luty AJ, Kun JF, and Kremsner PG.
Mannose-Binding Lectin Plasma Levels and Gene Polymorphisms in
Plasmodium falciparum Malaria. J Infect. Dis 1998, 178: 1221-1224. http://dx.doi.org/10.1086/515690 PMid:9806066

- Riley EM, Wahl S, Perkins DJ, Schofield L. Regulating immunity to malaria. Parasite Immunol. 2006,28:35-49. http://dx.doi.org/10.1111/j.1365-3024.2006.00775.x PMid:16438675

- Langhorne, JF, Ndungu M, Sponaas AM, and
Marsh K. Immunity to malaria: more questions than answers. Nat Immunol
2008,9: 725-732. http://dx.doi.org/10.1038/ni.f.205 PMid:18563083

- Fried M, Nosten F, Brockman A, Brabin BJ, and Duffy PE. Maternal antibodies block malaria. Nature 1998,395: 851-852. http://dx.doi.org/10.1038/27570 PMid:9804416

- Miller, LH, Baruch DI, K. Marsh K, and Doumbo OK. The pathogenic basis of malaria. Nature 2002, 415: 673-679. http://dx.doi.org/10.1038/415673a PMid:11832955

- Nussenblatt V, Semba RD. Micronutrient malnutrition and the pathogenesis of malarial anemia. Acta Trop. 2002,82: 321-37 http://dx.doi.org/10.1016/S0001-706X(02)00049-9

- Ghosh K, Ghosh K. Pathogenesis of anemia in malaria: a concise review. Parasitol Res. 2007; 101: 1463-9 http://dx.doi.org/10.1007/s00436-007-0742-1 PMid:17874326

- Haldar K, Mohandas N. Malaria, erythrocytic infection and anemia. Hematology Am Soc Hematol Educ Program. 2009; 1: 87-93 http://dx.doi.org/10.1182/asheducation-2009.1.87 PMid:20008186 PMCid:2933134

- Skorokhod OA, Caione L, Marrocco T,
Migliardi G, Barrera V, Arese P, Piacibello W, Schwarzer E. Inhibition
of erythropoiesis in malaria anemia: role of hemozoin and
hemozoin-generated 4-hydroxynonenal. Blood. 2010,116:4328-37. http://dx.doi.org/10.1182/blood-2010-03-272781 PMid:20686121

- Perkins DJ, Were T, Davenport GC, Kempaiah
P, Hittner JB, Ong’echa JM. Severe malarial anemia: innate immunity and
pathogenesis. Int J Biol Sci. 2011, 7: 1427-42 http://dx.doi.org/10.7150/ijbs.7.1427 PMid:22110393 PMCid:3221949

- Pasvol G, Clough B, and Carlsson J. Malaria and red cell membrane. Blood 1992, 6: 183-92 http://dx.doi.org/10.1016/0268-960X(92)90014-H

- Nweneka CV, Doherty CP, Cox S, Prentice A.
Iron delocalization in the pathogenesis of malarial anaemia. Trans R
Soc Trop Med Hyg. 2010, 104: 175-84 http://dx.doi.org/10.1016/j.trstmh.2009.08.007 PMid:19783267

- Buffet PA, Safeukui I, Milon G,
Marcereau-Puijalon O, David PH. Retention of erythrocytes in the
spleen: a double-edged process in human malaria. Curr Opin Hematol.
2009; 16: 157-64 http://dx.doi.org/10.1097/MOH.0b013e32832a1d4b PMid:19384231

- McDevitt MM, Xie J, Shanmugasundaram G,
Griffith J, Liu A, McDonald C, Thuma P, Gordeuk VR, Metz CN, Mitchell
R, Keefer J, David J, Leng L, Bucala R. A critical role for the host
mediator macrophage migration inhibitory factor in the pathogenesis of
malarial anaemia. J Exp Med. 2006; 203: 1185-96 http://dx.doi.org/10.1084/jem.20052398 PMid:16636133 PMCid:2121202

- Pradhan P. Malarial anaemia and nitric
oxide induced megaloblastic anaemia: a review on the causes of malarial
anaemia. J Vector Borne Dis. 2009; 46: 100-8 PMid:19502689

- Chang KH, Tam M, Stevenson MM.
Inappropriately low reticulocytosis in severe malarial anemia
correlates with suppression in the development of late erythroid
precursor. Blood. 2004; 103: 3727-35 http://dx.doi.org/10.1182/blood-2003-08-2887 PMid:14739226

- de Mast Q, Syafruddin D, Keijmel S,
Riekerink TO, Deky O, Asih PB, Swinkels DW, van ser Ven AJ. Increased
serum hepcidin and alterations in blood iron parameters associated with
asymptomatic P. falciparum and P. vivax malaria. Haematologica. 2010;
95: 1068-74 http://dx.doi.org/10.3324/haematol.2009.019331 PMid:20133896 PMCid:2895029

- Robson KJH, Weatherall DJ. Malarial anemia and STAT6. Haematologica. 2009; 94: 157-9 http://dx.doi.org/10.3324/haematol.2008.002311 PMid:19181787 PMCid:2635405

- Thawani N, Tam M, Stevenson MM.
STAT6-mediated suppression of erythropoiesis in an experimental model
of malarial anemia. Haematologica. 2008; 94: 195-204. http://dx.doi.org/10.3324/haematol.13422 PMid:19109218 PMCid:2635397

- Haldar K, Murphy SC, Milner DA, Taylor TE.
Malaria: mechanisms of erythrocytic infection and pathological
correlates of severe disease. Ann Rev Pathol Mech Dis. 2007, 2: 217-49 http://dx.doi.org/10.1146/annurev.pathol.2.010506.091913 PMid:18039099

- Maina RN, Walsh D, Gaddy C, Hongo G,
Waitumbi J, Otieno L, Jones D, Ogutu BR. Impact of Plasmodium
falciparum infection on haematological parameters in children living in
Western Kenya. Malar J. 2010, 9: S4 http://dx.doi.org/10.1186/1475-2875-9-S3-S4 PMid:21144084 PMCid:3002140

- Kochar DK, Das A, Kochar A, Middha S,
Acharya J, Tanwar GS, Gupta A, Pakalapati D, Garg S, Saxena V, Subudhi
AK, Boopathi PA, Sirohi P, Kochar SK. Thrombocytopenia in Plasmodium
falciparum, Plasmodium vivax and mixed infection malaria: a study from
Bikaner (Northwestern India). Platelets. 2010, 21: 623-7 http://dx.doi.org/10.3109/09537104.2010.505308 PMid:21050055

- Leowattana W, Tangpukdee N, Thar SK,
Nakasiri S, Srivilarit S, Kano S, Wilairatana P, Krudsood S. Changes in
platelet count in uncomplicated and severe falciparum malaria.
Southeast Asian J Trop Med Public Health. 2010, 41: 1035-41
PMid:21073022

- Tan SO, McGready R, Zwang J, Pimanpanarak
M, Sriprawat K, Thwai KL, Moo Y, Ashley EA, Edwards B, Singhasivanon P,
White NJ, Nosten F. Thrombocytopaenia in pregnant women with malaria on
the Thai-Burmese border. Malar J. 2008, 7:209 http://dx.doi.org/10.1186/1475-2875-7-209 PMid:18922167 PMCid:2579302

- Lacerda MV, Mourão MP, Coelho HC, Santos JB. Thrombocytopenia in malaria: who cares? Mem Inst Oswaldo Cruz. 2011, 106: 52-63 http://dx.doi.org/10.1590/S0074-02762011000900007

- de Mast Q, Groot E, Asih PB, Syafruddin D,
Oosting M, Sebastian S, Ferwerda B, Netea MG, de Groot PG, van der Ven
AJ, Fijnheer R. ADAMTS13 deficiency with elevated levels of ultra-large
and active von Willebrand factor in P. falciparum and P. vivax malaria.
Am J Trop Med Hyg. 2009; 80: 492-8 PMid:19270304

- de Mast Q, de Groot PG, van Heerde WL,
Roestenberg M, van Velzen JF, Verbruggen B, Roest M, McCall M, Nieman
AE, Westendorp J, Syafruddin D, Fijnheer R, van Dongen-Lases EC,
Sauerwein RW, van der Ven AJ. Thrombocytopenia in early malaria is
associated with GP1b shedding in absence of systemic platelet
activation and consumptive coagulopathy. Br J Haematol. 2010; 151:
495-503 http://dx.doi.org/10.1111/j.1365-2141.2010.08399.x PMid:20955404

- Chaudhary SC, Sonkar SK, Kumar V, Gupta A.
Falciparum malaria presenting as subdural haematoma. J Assoc Physicians
India. 2011; 59: 325-6 PMid:21751613

- Seshadri P, Dev AV, Viggeswarpu S,
Sathyendra S, Peter JV. Acute pancreatitis and subdural haematoma in a
patient with severe falciparum malaria: case report and review of
literature. Malar J. 2008, 7:97 http://dx.doi.org/10.1186/1475-2875-7-97 PMid:18510778 PMCid:2426706

- Misra DP, Das S, Pattnaik M, Singh SC,
Jena RK. Relationship of hepatic and renal dysfunction with
haemorrheological parameters in Plasmodium falciparum malaria. J Assoc
Physicians India. 2011; 59: 552-6 PMid:22334967

- WHO. Guidelines for the treatment of malaria. 2nd Ed., Geneva, World Health Organization. 2010
- Sahu S, Mohanty NK, Rath J, Patnaik SB.
Spectrum of malaria complications in an intensive care unit. Singapore
Med J. 2010; 51: 226-9 PMid:20428736

- Sharma A, Khanduri U. How benign is benign tertian malaria? J Vector Born Dis. 2009, 46: 141-4 PMid:19502694

- Saleri N, Gulletta M, Matteelli A,
Caligaris S, Tomasoni LR, Antonini B, Perandin F, Castelli F. Acute
Respiratory Distress Syndrome in Plasmodium vivax malaria from
Venezuela. Journal of Travel Medicine, 2006; 13: 112-113 http://dx.doi.org/10.1111/j.1708-8305.2006.00024.x PMid:16553597

- Agarwal R, Nath A, Gupta D. Noninvasive ventilation in Plasmodium vivax related ALI/ARDS. Intern Med. 2007; 46: 2007-11 http://dx.doi.org/10.2169/internalmedicine.46.0401 PMid:18084125

- Price L, Planche T, Rayner C, Krishna S.
Acute respiratory distress syndrome in Plasmodium vivax malaria: case
report and review of the literature. Trans R Soc Trop Med Hyg. 2007;
101: 655-9 http://dx.doi.org/10.1016/j.trstmh.2007.02.014 PMid:17433389

- Haydoura S, Mazboudi O, Charafeddine K,
Bouakl I, Baban TA, Taher AT, Kanj SS. Transfusion-related Plasmodium
ovale malaria complicated by acute respiratory distress syndrome (ARDS)
in a non endemic country. Parasitol Int. 2011; 60: 114-6 http://dx.doi.org/10.1016/j.parint.2010.10.005 PMid:20971212

- Daneshvar C, Davis TM, Cox-Sing J, Rafa'ee
MZ, Zakaria SK, Divis PC, Singh B. Clinical and laboratory features of
human Plasmodium knowlesi infection. Clin Infect Dis. 2009; 49: 852-60 http://dx.doi.org/10.1086/605439 PMid:19635025 PMCid:2843824

- William T, Menon J, Rajahram G, Chan L, Ma
G, Donaldson S, Khoo S, Fredrick C, Jelip J, Anstey NM, Yeo TW. Severe
Plasmodium knowlesi malaria in a terziary care hospital, Sabah,
Malaysia. Emerg Infect Dis. 2011; 17: 1248-55 http://dx.doi.org/10.3201/eid1707.101017 PMid:21762579 PMCid:3381373

- Sabbatani S, Fiorino S, Manfredi R. The
emerging of the fifth malaria parasite (Plasmodium knowlesi). A public
health concern? Braz Infect Dis. 2010; 14: 299-309 http://dx.doi.org/10.1590/S1413-86702010000300019 PMid:20835518

- Mohan A, Sharma SK, Bollineni S. Acute
lung injury and acute respiratory distress syndrome in malaria. J
Vector Borne Dis. 2008; 45: 179-93 PMid:18807374

- Gupta D, Ramanathan RP, Aggarwal AN,
Jindal SK. Assessment of factors predicting outcome of acute
respiratory distress syndrome in North India. Respirology. 2001, 6:
125-30 http://dx.doi.org/10.1046/j.1440-1843.2001.00324.x PMid:11422891

- Anstey NM, Jacups SP, Cain T, Pearson T,
Ziesing PJ, Fisher DA, Marks BJ, Maguire GP. Pulmonary manifestations
of uncomplicated falciparum and vivax malaria: cough, small airways
obstruction, impaired gas transfer, and increased pulmonary phagocytic
activity. J Infect Dis. 2002, 185: 1326-34 http://dx.doi.org/10.1086/339885 PMid:12001051

- Ephiphanio S, Campos MG, Pamplona A,
Carapau D, Pena AC, Ataìde R, Monteiro CA, Félix N, Costa-Silva A,
Marinho CR, Dias S, Mota MM. VEGF promotes malaria-associated acute
lung injury in mice. PLoS Pathog. 2010, 6: e1000916 http://dx.doi.org/10.1371/journal.ppat.1000916 PMid:20502682 PMCid:2873913

- Hee L, Dinudom A, Mitchell AJ, Grau GE,
Cook DI, Hunt NH, Ball HJ. Reduced activity of the epithelial sodium
channel in malaria-induced pulmonary oedema in mice. Int J Parasitol.
2011, 41: 81-8 http://dx.doi.org/10.1016/j.ijpara.2010.07.013 PMid:20816846

- van den Steen PA, Geurts N, Deroost K, van
Aelst I, Verhenne S, Heremans H, van Damme J, Opdenakker G.
Immunopathology and dexamethasone therapy in a new model for
malaria-associated acute respiratory distress syndrome. Am J Respir
Crit Care Med. 2010, 181: 957-68 http://dx.doi.org/10.1164/rccm.200905-0786OC PMid:20093644

- Seydel KB, Milner DA, Kamiza SB, Molyneux
ME, Taylor TE. The distribution and intensity of parasite sequestration
in comatose Malawian children. J Infect Dis. 2006; 194: 208-15 http://dx.doi.org/10.1086/505078 PMid:16779727 PMCid:1515074

- Gillrie MR, Krishnegowda G, Lee K, Buret
AG, Robbins SM, Looareesuwan S, Gowda DC, Ho M. Src-family
kinase-dependent disruption of endothelial barrier function by
Plasmodium falciparum merozoite proteins. Blood. 2007; 110: 3426-35 http://dx.doi.org/10.1182/blood-2007-04-084582 PMid:17693580 PMCid:2200906

- Anand AC, Ramji C, Narula AS, Singh W.
Malarial hepatitis: a heterogeneous syndrome? Natl Med J India. 1992,
5: 59-62 PMid:1304265

- Mazumder R, Mishra RK, Mazumder H,
Mukherjee P. Jaundice in falciparum malaria - some prospective
observations. J Indian Med Assoc. 2002, 100: 312-4 PMid:12418632

- Murthy GL, Sahay RK, Sreenivas DV,
Sundaram C, Shantaram V. Hepatitis in falciparum malaria. Trop
Gastroenterol. 1998, 19: 152-4 PMid:10228440

- Sung YH, Park JM. A case of malarial hepatitis by Plasmodium vivax. Korean J Gastroenterol. 2010; 56: 329-33 http://dx.doi.org/10.4166/kjg.2010.56.5.329 PMid:21099242

- Das SN, Mohapatra B, Mohanty R, Dash PC,
Kar K, Dash PK. Malarial hepatitis as a component of multiorgan failure
– a bad prognostic sign. J Indian Med Assoc. 2007; 105: 247-50
PMid:17915792

- Ali H, Ahsan T, Mahmood T, Bakht SF,
Faroog MU, Ahmed N. Parasite density and the spectrum of clinical
illness in falciparum malaria. J Coll Physicians Surg Pak. 2008; 18:
362-8 PMid:18760048

- Anand AC, Puri P. Jaundice in malaria. J Gastroenterol Hepatol. 2005, 20: 1322-32 http://dx.doi.org/10.1111/j.1440-1746.2005.03884.x PMid:16105116

- Kochar DK, Singh P, Agarwal P, Kochar SK,
Pokharna R, Sareen PK. Malarial hepatitis. J Assoc Physicians India
2003, 51: 1069-72 PMid:15260391

- Bhalla A, Suri V, Singh V. Malarial hepatopathy. J Postgrad Med. 2006, 52: 315-20 PMid:17102560

- Kochar DK, Agarwal P, Kochar SK, Jain R,
Rawat N, Pokharna RK, Kachhawa S, Srivastava T. Hepatocyte dysfunction
and hepatic encephalopathy in Plasmodium falciparum malaria. Q J Med.
2003, 96: 505-12 http://dx.doi.org/10.1093/qjmed/hcg091 PMid:12881593

- Naicker S, Aboud O, Gharbi MB. Epidemiology of acute kidney injury in Africa. Semin Nephrol. 2008, 28: 348-53 http://dx.doi.org/10.1016/j.semnephrol.2008.04.003 PMid:18620957

- Lombardi R, Yu L, Younes-Ibrahim M, Schor
N, Burdmann EA. Epidemiology of acute kidney injury in Latin America.
Semin Nephrol. 2008; 28: 320-9 http://dx.doi.org/10.1016/j.semnephrol.2008.04.001 PMid:18620955

- Jha V, Chungh KS. Community-acquired acute kidney injury in Asia. Semin Nephrol. 2008; 28: 330-47 http://dx.doi.org/10.1016/j.semnephrol.2008.04.002 PMid:18620956

- Assounga AG, Assambo-Kieku C, Mafoua A,
Moyen G, Nzingoula S. Etiology and outcome of acute renal failure in
children in Congo-Brazzaville. Saudi J Kidney Dis Transplant. 2000; 11:
40-3 PMid:18209297

- Mishra SK, Mahanta KC, Mohanty S. Malaria
associated acute renal failure – experience from Rourkela, eastern
India. J Indian Med Assoc. 2008; 106: 640-2, 654 PMid:19552096

- Rohani MA, Aliawshaei H, Aduolimi E. Acute
renal failure in Yemeni patients. Saudi J Kidney Dis Transpl. 2011; 22:
829-33 PMid:21743244

- Das BS. Renal failure in malaria. J Vector Borne Dis. 2008; 45: 83-97 PMid:18592837

- Basu G, Chrispal A, Boorugu H, Gopinath
KG, Chandy S, Prakash JAJ, Thomas K, Abraham AM, John GT. Acute kidney
injury in tropical acute febrile illness in a tertiary care centre –
RIFLE criteria validation. Nephrol Dial Transplant. 2011, 26: 524-31 http://dx.doi.org/10.1093/ndt/gfq477 PMid:20702532

- Kanodia KV, Shah PR, Vanikar AV, Kasat P,
Gumber M, Trivedi HL. Malaria induced acute renal failure: a single
center experience. Saudi J Kidney Dis Transplant. 2010; 21: 1088-91
PMid:21060178

- Ehrich JHH, Eke FU. Malaria-induced renal damage: facts and myths. Pediatr Nephol. 2007; 22: 626-37 http://dx.doi.org/10.1007/s00467-006-0332-y PMid:17205283

- Kaur D, Wasir V, Gulati S, Bagga A.
Unusual presentation of Plasmodium vivax malaria with severe
thrombocytopenia and acute renal failure. J Trop Pediatr. 2007; 53:
210-2 http://dx.doi.org/10.1093/tropej/fml092 PMid:17264156

- Neri S, Pulvirenti D, Patamia I, Zoccolo
A, Castellino P. Acute renal failure in Plasmodium malariae infection.
Neth J Med. 2008, 66: 166-8 PMid:18424865

- Barsoun RS. Malaria nephropathies. Nephrol Dial Transplant. 1998;, 13: 1588-97 http://dx.doi.org/10.1093/ndt/13.6.1588

- Elsheikha HM, Sheashaa HA. Epidemiology,
pathophysiology, management and outcome of renal dysfunction associated
with plasmodia infection. Parasitol Res. 2007, 101: 1183-90 http://dx.doi.org/10.1007/s00436-007-0650-4 PMid:17628830

- Nguansangiam S, Day NP, Hien TT, Mai NT,
Chaisri U, Riganti M, Dondorp AM, Lee SJ, Phu NH, Turner GD, White NJ,
Ferguson DJ, Pongponratn E. A quantitative ultrastructural study of
renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int
Health. 2007; 12: 1037-50 http://dx.doi.org/10.1111/j.1365-3156.2007.01881.x

- Sinniah R, Rui-Mei L, Kara AU.
Up-regulation of cytokine in glomerulonephritis associated with murine
malaria. Int. J. Path. 1999, 80: 87-95 http://dx.doi.org/10.1046/j.1365-2613.1999.00101.x PMCid:2517762

- Mishra SK, Pati SS, Mahanta KC, Mohanty
S. Rhabdomyolysis in falciparum malaria – a series of twelve cases
(five children and seven adults). Trop Doct. 2010, 40: 87-8 http://dx.doi.org/10.1258/td.2009.090387 PMid:20305101

- Siqueira AM, Alexandre MA, Mourao MP,
Santos VS, Nagahashi-Marie SK, Alecrim MG, Lacerda MV. Case report:
severe rhabdomyolysis caused by Plasmodium vivax malaria in the
Brazilian Amazon. Am J Trop Med Hyg. 2010, 83: 271-3 http://dx.doi.org/10.4269/ajtmh.2010.10-0027 PMid:20682866 PMCid:2911169

- Reynaud F, Mallet L, Lyon A, Rodolfo JM.
Rhabdomyolysis and acute renal failure in Plasmodium falciparum
malaria. Nephrol Dial Transplant. 2005; 20: 847-55 http://dx.doi.org/10.1093/ndt/gfh686 PMid:15772274

- Khan FY. An imported case of P.
falciparum malaria presenting as black water fever with acute renal
failure. Travel Med Infect Dis. 2009; 7: 378-80 http://dx.doi.org/10.1016/j.tmaid.2009.11.002 PMid:19945017

- Oumar AA, Poudiougou B, Sylla M, Sall A,
Konate S, Togo B, Diakite M, Keita MM. Black water fever in children
during cerebral malaria: 3 case-reports in Bamako. Arch Pediatr. 2007,
14: 993-5 http://dx.doi.org/10.1016/j.arcped.2007.04.005 PMid:17524629

- Rogier C, Imbert P, Tall A, Sokhna C,
Spiegel A, Trape JF. Epidemiological and clinic aspects of black water
fever among African children suffering frequent malaria attacks. Trans
R Soc Trop Med Hyg. 2003, 97: 193-7 http://dx.doi.org/10.1016/S0035-9203(03)90116-7

- Bardaji A, Sigauque B, Sanz S, Maixenchs
M, Ordi J, Aponte JJ, Mabunda S, Alonso PL, Menendez C. Impact of
malaria at the end of pregnancy on infant mortality and morbidity. J
Infect Dis. 2011, 203: 691-9 http://dx.doi.org/10.1093/infdis/jiq049 PMid:21199881 PMCid:3071276

- Uneke CJ. Impact of Placental Plasmodium
falciparum malaria on pregnancy and perinatal outcome in Sub-Saharan
Africa. Part III: placental malaria, maternal health and public health.
Yale J Biol Med. 2008; 81: 1-7 PMid:18604306 PMCid:2442721

- Aribodor DN, Nwaorgu OC, Eneanya CI,
Okoli I, Pukkila-Worley R, Etaga HO. Association of low birth weight
and placental malarial infection in Nigeria. J Infect Dev Ctries. 2009;
3:620-3 http://dx.doi.org/10.3855/jidc.554

- Adam I, Elhassan EM, Haggaz AE, Ali AA,
Adam GK. A perspective of the epidemiology of malaria and anaemia and
their impact on maternal and perinatal outcomes in Sudan. J Infect Dev
Ctries. 2011, 5:083-7

- Biswajit P, Bijeeyani M, Krishna K.
Maternal deaths in a tertiary health care centre of Odisha: an in-depth
study supplemented by verbal autopsy. Indian J Community Med. 2011; 36:
213-6 http://dx.doi.org/10.4103/0970-0218.86523 PMid:22090676 PMCid:3214447

- Agan TU, Ekabua JE, Udoh AE, Ekanem EI,
Efiok EE, Mgbekem MA. Prevalence of anemia in women with asymptomatic
malaria parasitemia at first antenatal care visit at the University of
Calabar Teaching Hospital, Calabar, Nigeria. Int J Womens Health. 2010;
2: 229-33 http://dx.doi.org/10.2147/IJWH.S11887 PMid:21072315 PMCid:2971745

- Falade CO, Tongo OO, Ogunkunle OO,
Orimadegun AE. Effects of malaria in pregnancy on newborn
anthropometry. J Infect Dev Ctries. 2010; 4: 448-53 PMid:20818093

- Tobón-Castaño A, Arismendi Solano M,
Sánchez LG, Trujillo SB. Intrauterine growth retardation, low birth
weight and prematurity in neonates of pregnant women with malaria in
Colombia. Rev Soc Bras Med Trop. 2011; 44: 364-70 PMid:21625805

- Carvalho BO, Matsuda JS, Luz SL,
Martinez-Espinosa FE, Leite JA, Franzin F, Orlandi PP, Gregoracci GG,
Lacerda MV, Nogueira PA, Costa FT. Gestational malaria associated to
Plasmodium vivax and Plasmodium falciparum placental mixed-infection
followed by foetal loss: a case report from an unstable transmission
area in Brazil. Malar J. 2011; 10: 178 http://dx.doi.org/10.1186/1475-2875-10-178 PMid:21708032 PMCid:3141593

- Nosten F, McGready R, Simpson JA, Thwai
KL, Balkan S, Cho T, Hkirijaroen L, Looareesuwan S, White NJ. Effects
of Plasmodium vivax malaria in pregnancy. Lancet. 1999; 354: 546-9 http://dx.doi.org/10.1016/S0140-6736(98)09247-2

- Costa FT, Lopes SC, Ferrer M, Leite JA,
Martin-Jaular L, Barnabeu M, Nogueira PA, Mourão MP, Fernandez-Becerra
C, Lacerda MV, del Portillo H. On cytoadhesion of Plasmodium vivax:
raison d’être? Mem Inst Oswaldo Cruz. 2011, 106: 79-84 PMid:21881760

- Adegnika AA, Luty AJ, Grobusch MP,
Ramharter M, Yazdanbakhsh M, Kremsner PG, Schwarz NG. AB0 blood group
and the risk of placental malaria in sub-Saharan Africa. Malar J. 2011,
10: 101 http://dx.doi.org/10.1186/1475-2875-10-101 PMid:21513504 PMCid:3098819

- Uneke CJ. Impact of Placental Plasmodium
falciparum malaria on pregnancy and perinatal outcome in Sub-Saharan
Africa. I: Introduction to placental malaria. Yale J Biol Med. 2007;
80: 39-50 PMid:18160989 PMCid:2140183

- Sander AF, Salanti A, Lavstses T, Nielsen
MA, Theander TG, Lehe RG, Lo YY, Bobbili N, Arnot DE, Taylor DW.
Positive selection of Plasmodium falciparum parasites with multiple
var2csa-type PfEMP1 genes during the course of infection in pregnant
women. J Infect Dis. 2011; 203: 1679-85 http://dx.doi.org/10.1093/infdis/jir168 PMid:21592998 PMCid:3096795

- Boeuf P, Hasang W, Hanssen E, Glazier JD,
Rogerson SJ. Relevant assay to study the adhesion of Plasmodium
falciparum-infected erythrocytes to the placental epithelium. PLoS One.
2011; 6: e21126 http://dx.doi.org/10.1371/journal.pone.0021126 PMid:21731654 PMCid:3123321

- Rovira-Vallbona E, Dobaño C, Bardajì A,
Cisterò P, Romagosa C, Serra-Casass E, Quintò L, Bassat Q, Sigauque B,
Alonso PL, Ordi J, Menéndez C, Mayor A. Transcription of var genes
other than var2csa in Plasmodium falciparum parasites infecting
Mozambican pregnant women. J Infect Dis. 2011; 204: 27-35 http://dx.doi.org/10.1093/infdis/jir217 PMid:21628655 PMCid:3307158

- Conroy A, Serghides L, Finney C, Owino
SO, Kumar S, Gowda DC, Liles WC, Moore J, Kain KC. C5a enhances
dysregulated inflammatory and angiogenic responses to malaria in vitro:
potential implications for placental malaria. PLoS One. 2009; 4: e4953 http://dx.doi.org/10.1371/journal.pone.0004953 PMid:19308263 PMCid:2655724

- Conroy AL, McDonald CR, Silver KL, Liles
WC, Kain KC. Complement activation: a critical mediator of adverse
fetal outcomes in placental malaria? Trends Parasitol. 2011; 27: 294-9 http://dx.doi.org/10.1016/j.pt.2011.02.005 PMid:21493146

- Muehlenbachs A, Fried M, Lachowitzer J,
Mutabingwa TK, Duffy PE. Natural selection of FLT1 alleles and their
association with malaria resistance in utero. PNAS. 2008; 105: 14488-91
http://dx.doi.org/10.1073/pnas.0803657105 PMid:18779584 PMCid:2567167

- Haldar K, and Mohandas N. Erythrocyte remodeling by malaria parasites. Curr Opin Hematol 2007; 14: 203-209 http://dx.doi.org/10.1097/MOH.0b013e3280f31b2d PMid:17414208

- Kraemer SM, and Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol 2006; 9: 374-380 http://dx.doi.org/10.1016/j.mib.2006.06.006 PMid:16814594

- Kyes SA, Kraemer SM, Smith JD. Antigenic
variation in Plasmodium falciparum: gene organization and regulation of
the var multigene family. Eukaryotic Cell 2007; 6:1511-1520 http://dx.doi.org/10.1128/EC.00173-07 PMid:17644655 PMCid:2043368
