Autologous Stem Cell Transplantation for Chronic Lymphocytic Leukemia - Still a Valid Treatment Option, or is the Game Over?
Fabienne McClanahan1,2 and Peter Dreger1
1 Department of Medicine V, University of Heidelberg, Heidelberg, Germany
2 Centre for Haemato-Oncology, Barts Cancer Institute, Queen Mary, University of London, London, UK
2 Centre for Haemato-Oncology, Barts Cancer Institute, Queen Mary, University of London, London, UK
Correspondence
to:
Fabienne McClanahan, Centre for Haemato-Oncology, Barts Cancer
Institute, Queen Mary, University of London, London, UK. E-mail: f.mcclanahan@qmul.ac.uk
Published: November 6, 2012
Received: September 28, 2012
Accepted: October 15, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012071, DOI 10.4084/MJHID.2012.071
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Chemoimmunotherapy
with fludarabine, cyclophosphamide, and rituximab (FCR) has been
established as the current standard of care for young and fit patients
with chronic lymphocytic leukemia (CLL). In the early nineties of the
last century, long before the advent of fludarabine or antibody-based
strategies, there was realistic hope that myeloablative therapy
followed by autologous stem cell transplantation (autoSCT) might be an
effective and potentially curative front-line treatment option for
suitable patients with CLL. Since then, several prospective trials have
disenthralled this hope: although autoSCT can prolong event and
progression-free survival if used as part of early front-line
treatment, it does not improve overall survival, while it is associated
with an increased risk of late adverse events such as secondary
malignancies. In addition, autoSCT lacks the potential to overcome the
negative impact of biomarkers that confer resistance to chemotherapy or
early relapse. The role of autoSCT has also been explored in the
context of FCR, and it was demonstrated that its effect is inferior to
the currently established optimal treatment regimen. In view of ongoing
attempts to improve on FCR, promising clinical activity of new
substances even in relapsed/ refractory CLL patients, exciting novel
cell therapy approaches and advantages in the understanding of the
disease and detection of Minimal Residual Disease (MRD), autoSCT has
lost its place as a standard treatment option for CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is one of the most common lymphoid malignancies,[1] and the most common adult leukemia in Western countries.[1,2] Large multicenter trials have established the combination chemoimmunotherapy of fludarabine, cyclophosphamide, and rituximab (FCR) as the current standard of care for young patients without any concomitant diseases.[3,4] This approach, however, is neither curative nor is it suited for the very elderly and those with comorbidities. In addition, there is a sub-group of high-risk patients which poorly respond to chemoimmunotherapy and suffer from early relapse.[5] Therefore, there is a substantial need to explore alternative therapeutic approaches.
Phase-II Trials on Autologous SCT in CLL
Long before the advent of FCR and other fludarabine or monoclonal antibody-based strategies, several studies suggested that autologous stem cell transplantation (autoSCT) might represent an effective and potentially curative treatment option for suitable patients with CLL.[6-10] The 2005 update from the original Dana-Faber-Cancer-Institute (DFCI) single-center series showed a 6-year progression-free survival (PFS) of 30% and a 6-year overall survival (OS) of 58% after autoSCT.[11] In the UK MRC pilot study, a multicenter phase-II trial on autoSCT as part of first-line CLL treatment, the 5-year OS and PFS rates were 78% and 52%.[12] In 1996, the German CLL Study Group (GCLLSG) designed a large phase-II multicenter trial to assess the feasibility and efficacy of early autoSCT in poor-risk CLL. Compared to the UK study, the CLL3 trial followed a very aggressive treatment approach by including poor-risk patients at an early stage of disease (i.e. patients were lacking conventional treatment indications) and applying Dexa-BEAM as mobilization to improve mobilization efficacy and disease control before autoSCT (dose-identification). After a median follow-up of 8.7 years, median PFS and OS were 5.7 years and 11.3 years respectively.[13] PFS was significantly affected by unfavourable IGHV (p < .001) and 17p- (p < .001) in a multivariate setting. Predictors of a shorter OS in a multivariate setting were 17p- (p < .001), unfavourable IGHV (p = .008) and Binet stage C (p = .03). Partly reflecting the high toxicity of this intense treatment regimen, the cumulative incidence of non-relapse-mortality (NRM) was 6.5% after 5 years and 14% after 10 years. Although these phase II trials indicated that autoSCT could - when used as part of first-line therapy - effectively control the disease in a subgroup of patients, their long-term follow-up data provided little evidence of curative potential in a substantial proportion of patients. Further details and the main findings of these phase II studies are summarised in table 1.
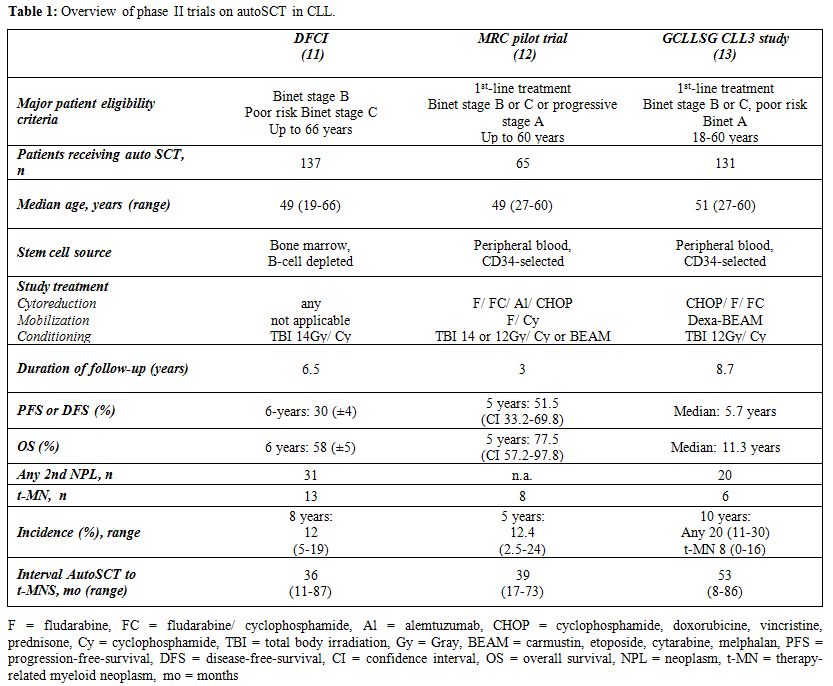
Table 1. Overview of phase II trials on autoSCT in CLL
Phase-III Trials on Autologous SCT in CLL
Subsequently, prospective randomized trials were opened for enrollment, and despite being initiated in the pre-Rituximab era, the results have been eagerly awaited for. The French intergroup trial randomized patients in complete remission (CR) after mini-CHOP- and fludarabine-based therapy to autoSCT or observation. Those patients who did not achieve CR, received cytarabine-based salvage therapy followed by autoSCT or FC.[14] While autoSCT significantly increased 3-year event-free survival (EFS) in CR patients (80% vs. 36%, p = .003), there was no difference in EFS between the treatment groups in non-CR patients and in OS in all response subgroups. An EBMT phase-III multinational trial randomized patients in CR or PR after first- or second-line treatment to consolidating autoSCT or watchful waiting,[15] including virtually all CR patients from the French phase III trial. While autoSCT almost doubled EFS and time to retreatment (TTRT), it did not have a significant impact on OS. A similar pattern was observed in the small French GOELAMS LLC 98 trial, which randomized patients (n=86) between conventional CHOP chemotherapy and CHOP followed by upfront autoSCT in remission:[16] as observed in the previous randomized trials, autoSCT significantly prolonged PFS but was lacking any survival advantage. Table 2 summarizes the major findings of these phase III trials. However, the results from all phase III trials need to be interpreted with a certain degree of caution, as neither trial applied any upfront therapy that would be in accordance with today’s gold-standard treatment (i.e. FCR).
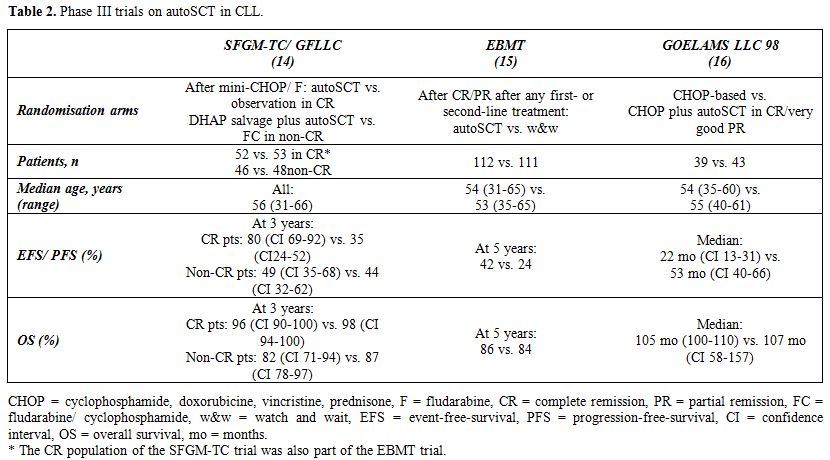
Table 2. Phase III trials on autoSCT in CLL
Comparison autoSCT versus FCR
A direct prospective randomized comparison between Rituximab based therapy and auto-SCT has never been undertaken, and in the view of promising new agents and cellular therapy approaches, it can be assumed that this is very unlikely to happen in the future either. However, in a recent study within this setting, 110 patients from the GCLLSG autoSCT CLL3 trial and 126 patients from the FCR arm of the GCLLSG CLL8 trial were retrospectively compared.[13] Patients were matched for age, time from diagnosis to study entry, serum thymidine kinase levels, cytogenetic risk group by fluorescence-in-situ-hybridisation (FISH) and IGHV mutation status. In this cohort, PFS was significantly longer in the autoSCT group (median 6.2 years) than in the FCR group (median 4.3 years), which however did not translate into prolonged TTRT (median 7.7 years vs. not reached, p=.91) as observed in the EBMT trial. Four-year OS (86% vs. 90%, p=.39) was comparable between autoSCT and FCR. Unfavorable IGHV status was the only factor significantly affecting TTRT in a multivariate setting, whereas OS was adversely influenced by unfavorable IGHV, Binet C stage and age. Specific poor-risk subgroups did not benefit from autoSCT. Although these results are probably the closest to a valid comparison between FCR and autoSCT, they should be interpreted with caution: all findings are based on a retrospective analysis, with the limitations of such an approach being frequently discussed. In addition, there were differences between the two patient populations that could not be eliminated by matching, especially the duration and intensity of follow-up.
AutoSCT in Richter’s Syndrome
A recently published retrospective EBMT study suggested that autoSCT might play a role in the treatment of patients with chemo-sensitive Richter's syndrome.[17] Although autoSCT seemed to lack a curative effect, the estimated probability of surviving 3 years after autoSCT was more than 50%, which compared favourably to the survival of chemotherapy-sensitive patients without autoSCT consolidation in another series.[18]
Secondary Malignancies
In addition to the lack of full curative potential and convincing advantages over conventional chemoimmunotherapy, serious long-term effects such as solid tumours and secondary haematological malignancies need to be taken into consideration after autoSCT. The 2008 WHO-classification of haematopoietic and lymphoid tissues has implemented therapy-related myeloid neoplasms (t-MNs) as a separate category, which includes therapy-related acute myeloid leukaemia (t-AML), myelodysplastic syndrome (t-MDS) and myelodysplastic/ myeloproliferative neoplasms (t-MDS/MPN).[19] T-MNs have emerged as serious long-term complications of cytotoxic therapy for CLL:[20-26] among 2,028 patients with CLL/ small lymphocytic lymphoma (SLL) treated at the MD Anderson Cancer Centre from 1985 to 2005, 11% developed other malignancies during the follow-up period, and the risk of a second cancer was 2.2 times higher than the expected risk calculated from the SEER database.[21] In contrast, a population-based analysis of the SEER database of 1-year survivors with CLL/ SLL (n=15,915), revealed that CLL patients have a significantly elevated risk for lung cancer and melanoma, but not for acute non-lymphocytic leukaemia when compared to other lymphoid malignancies.[27] In a randomized study comparing treatment with chlorambucil, fludarabine, or fludarabine plus chlorambucil (FC), 1.2% developed t-MNs after a median follow-up of 4.2 years, with the majority occurring after combination FC therapy.[28] In another study, 6% of CLL patients developed t-MNs 5 years after treatment with combination fludarabine.[20] In a long-term follow-up study of first-line FCR, there were eight cases of MDS (2.8%) that occurred during first remission.[3] In contrast, no case of t-MN was observed in the CALGB 9712 trial after fludarabine plus rituximab (FR) therapy.[29] Long-term follow-up observations have raised concerns that the incidence of t-MNs might be even more pronounced after autoSCT: in a Finnish analysis of patients being treated with autoSCT from 1990 to 2003, the risk of NRM was highest in patients with CLL (9.5%), with another malignancy being the most common cause of late NRM.[30] The most common forms of fatal secondary malignancies were t-MNs. In the DFCI and MRC series, t-MN occurred in 9% and 8% of patients, translating into a 5- and 8-year incidence-rate of 12%.[11,31] In the CLL3 trial, the 10-year incidence was 19%, with no significant difference of any secondary malignancy among individuals treated with and without autoSCT.[13] However, all cases of t-MN occurred after autoSCT, yielding a 10-year incidence rate of t-MN of 8%, which is in the range of the DFCI and MRC series. Within all series, the outcome after t-MNs was poor, which makes this a very relevant and serious long-term complication and is particularly relevant to patients that may have benefited from less aggressive regimens.
Conclusions and Perspectives
What major conclusions can be drawn from almost two decades of clinical trials on autoSCT in CLL? Firstly, autoSCT indeed has the capacity to provide prolonged disease control at least similar to modern immunochemotherapy regimens, such as FCR. However, like immunochemotherapy, autoSCT has no significant curative potential in CLL. Secondly, autoSCT does not have the potential to overcome the negative impact of biomarkers that confer resistance to chemotherapy. Therefore, patients who have gained the highest benefit from autoSCT would also most likely respond to conventional immunochemotherapy. Thirdly, autoSCT is associated with an increased risk of secondary neoplasms, which is a very serious long-term adverse event with a poor outcome.
Over the past few years, CLL research and treatment have made a huge leap forward: to name a few examples, randomized clinical trials aiming to improve the potential of FCR and to optimize treatment for high-risk and older patients or those with comorbidities are ongoing, and their recruitment has for the most part been exceeding expectations. There are also several exciting new small molecule inhibitors such as the BTK-inhibitor ibrutinib or the bcl-2 inhibitor navitoclax, which show promising activity even in the relapsed/ refractory setting, and in combination with conventional chemoimmunotherapy.[32-34] Several studies have indicated that allogeneic HSCT is currently the only treatment with curative potential on the basis of its capacity to induce graft-versus-leukemia (GVL) activity, even in high-risk CLL patients.[35-37] This approach, however, is never indicated as part of first-line treatment in standard-risk patients and should be restricted to patients who meet the EBMT transplant consensus criteria.[38] Sensitive techniques for MRD quantification have been further optimized and might serve as a surrogate marker to assess treatment efficacy.[39] In addition, experimental treatment approaches such as chimeric-antigen-receptor (CAR) T cells have shown exciting preliminary results which lead us to believe that this might direct us into the future of CLL treatment.[40] In view of these and other developments, autoSCT does currently not play a role in the treatment of CLL; therefore the autoSCT game indeed seems to be over for the time being.[41]
Chronic lymphocytic leukemia (CLL) is one of the most common lymphoid malignancies,[1] and the most common adult leukemia in Western countries.[1,2] Large multicenter trials have established the combination chemoimmunotherapy of fludarabine, cyclophosphamide, and rituximab (FCR) as the current standard of care for young patients without any concomitant diseases.[3,4] This approach, however, is neither curative nor is it suited for the very elderly and those with comorbidities. In addition, there is a sub-group of high-risk patients which poorly respond to chemoimmunotherapy and suffer from early relapse.[5] Therefore, there is a substantial need to explore alternative therapeutic approaches.
Phase-II Trials on Autologous SCT in CLL
Long before the advent of FCR and other fludarabine or monoclonal antibody-based strategies, several studies suggested that autologous stem cell transplantation (autoSCT) might represent an effective and potentially curative treatment option for suitable patients with CLL.[6-10] The 2005 update from the original Dana-Faber-Cancer-Institute (DFCI) single-center series showed a 6-year progression-free survival (PFS) of 30% and a 6-year overall survival (OS) of 58% after autoSCT.[11] In the UK MRC pilot study, a multicenter phase-II trial on autoSCT as part of first-line CLL treatment, the 5-year OS and PFS rates were 78% and 52%.[12] In 1996, the German CLL Study Group (GCLLSG) designed a large phase-II multicenter trial to assess the feasibility and efficacy of early autoSCT in poor-risk CLL. Compared to the UK study, the CLL3 trial followed a very aggressive treatment approach by including poor-risk patients at an early stage of disease (i.e. patients were lacking conventional treatment indications) and applying Dexa-BEAM as mobilization to improve mobilization efficacy and disease control before autoSCT (dose-identification). After a median follow-up of 8.7 years, median PFS and OS were 5.7 years and 11.3 years respectively.[13] PFS was significantly affected by unfavourable IGHV (p < .001) and 17p- (p < .001) in a multivariate setting. Predictors of a shorter OS in a multivariate setting were 17p- (p < .001), unfavourable IGHV (p = .008) and Binet stage C (p = .03). Partly reflecting the high toxicity of this intense treatment regimen, the cumulative incidence of non-relapse-mortality (NRM) was 6.5% after 5 years and 14% after 10 years. Although these phase II trials indicated that autoSCT could - when used as part of first-line therapy - effectively control the disease in a subgroup of patients, their long-term follow-up data provided little evidence of curative potential in a substantial proportion of patients. Further details and the main findings of these phase II studies are summarised in table 1.

Table 1. Overview of phase II trials on autoSCT in CLL
Phase-III Trials on Autologous SCT in CLL
Subsequently, prospective randomized trials were opened for enrollment, and despite being initiated in the pre-Rituximab era, the results have been eagerly awaited for. The French intergroup trial randomized patients in complete remission (CR) after mini-CHOP- and fludarabine-based therapy to autoSCT or observation. Those patients who did not achieve CR, received cytarabine-based salvage therapy followed by autoSCT or FC.[14] While autoSCT significantly increased 3-year event-free survival (EFS) in CR patients (80% vs. 36%, p = .003), there was no difference in EFS between the treatment groups in non-CR patients and in OS in all response subgroups. An EBMT phase-III multinational trial randomized patients in CR or PR after first- or second-line treatment to consolidating autoSCT or watchful waiting,[15] including virtually all CR patients from the French phase III trial. While autoSCT almost doubled EFS and time to retreatment (TTRT), it did not have a significant impact on OS. A similar pattern was observed in the small French GOELAMS LLC 98 trial, which randomized patients (n=86) between conventional CHOP chemotherapy and CHOP followed by upfront autoSCT in remission:[16] as observed in the previous randomized trials, autoSCT significantly prolonged PFS but was lacking any survival advantage. Table 2 summarizes the major findings of these phase III trials. However, the results from all phase III trials need to be interpreted with a certain degree of caution, as neither trial applied any upfront therapy that would be in accordance with today’s gold-standard treatment (i.e. FCR).

Table 2. Phase III trials on autoSCT in CLL
Comparison autoSCT versus FCR
A direct prospective randomized comparison between Rituximab based therapy and auto-SCT has never been undertaken, and in the view of promising new agents and cellular therapy approaches, it can be assumed that this is very unlikely to happen in the future either. However, in a recent study within this setting, 110 patients from the GCLLSG autoSCT CLL3 trial and 126 patients from the FCR arm of the GCLLSG CLL8 trial were retrospectively compared.[13] Patients were matched for age, time from diagnosis to study entry, serum thymidine kinase levels, cytogenetic risk group by fluorescence-in-situ-hybridisation (FISH) and IGHV mutation status. In this cohort, PFS was significantly longer in the autoSCT group (median 6.2 years) than in the FCR group (median 4.3 years), which however did not translate into prolonged TTRT (median 7.7 years vs. not reached, p=.91) as observed in the EBMT trial. Four-year OS (86% vs. 90%, p=.39) was comparable between autoSCT and FCR. Unfavorable IGHV status was the only factor significantly affecting TTRT in a multivariate setting, whereas OS was adversely influenced by unfavorable IGHV, Binet C stage and age. Specific poor-risk subgroups did not benefit from autoSCT. Although these results are probably the closest to a valid comparison between FCR and autoSCT, they should be interpreted with caution: all findings are based on a retrospective analysis, with the limitations of such an approach being frequently discussed. In addition, there were differences between the two patient populations that could not be eliminated by matching, especially the duration and intensity of follow-up.
AutoSCT in Richter’s Syndrome
A recently published retrospective EBMT study suggested that autoSCT might play a role in the treatment of patients with chemo-sensitive Richter's syndrome.[17] Although autoSCT seemed to lack a curative effect, the estimated probability of surviving 3 years after autoSCT was more than 50%, which compared favourably to the survival of chemotherapy-sensitive patients without autoSCT consolidation in another series.[18]
Secondary Malignancies
In addition to the lack of full curative potential and convincing advantages over conventional chemoimmunotherapy, serious long-term effects such as solid tumours and secondary haematological malignancies need to be taken into consideration after autoSCT. The 2008 WHO-classification of haematopoietic and lymphoid tissues has implemented therapy-related myeloid neoplasms (t-MNs) as a separate category, which includes therapy-related acute myeloid leukaemia (t-AML), myelodysplastic syndrome (t-MDS) and myelodysplastic/ myeloproliferative neoplasms (t-MDS/MPN).[19] T-MNs have emerged as serious long-term complications of cytotoxic therapy for CLL:[20-26] among 2,028 patients with CLL/ small lymphocytic lymphoma (SLL) treated at the MD Anderson Cancer Centre from 1985 to 2005, 11% developed other malignancies during the follow-up period, and the risk of a second cancer was 2.2 times higher than the expected risk calculated from the SEER database.[21] In contrast, a population-based analysis of the SEER database of 1-year survivors with CLL/ SLL (n=15,915), revealed that CLL patients have a significantly elevated risk for lung cancer and melanoma, but not for acute non-lymphocytic leukaemia when compared to other lymphoid malignancies.[27] In a randomized study comparing treatment with chlorambucil, fludarabine, or fludarabine plus chlorambucil (FC), 1.2% developed t-MNs after a median follow-up of 4.2 years, with the majority occurring after combination FC therapy.[28] In another study, 6% of CLL patients developed t-MNs 5 years after treatment with combination fludarabine.[20] In a long-term follow-up study of first-line FCR, there were eight cases of MDS (2.8%) that occurred during first remission.[3] In contrast, no case of t-MN was observed in the CALGB 9712 trial after fludarabine plus rituximab (FR) therapy.[29] Long-term follow-up observations have raised concerns that the incidence of t-MNs might be even more pronounced after autoSCT: in a Finnish analysis of patients being treated with autoSCT from 1990 to 2003, the risk of NRM was highest in patients with CLL (9.5%), with another malignancy being the most common cause of late NRM.[30] The most common forms of fatal secondary malignancies were t-MNs. In the DFCI and MRC series, t-MN occurred in 9% and 8% of patients, translating into a 5- and 8-year incidence-rate of 12%.[11,31] In the CLL3 trial, the 10-year incidence was 19%, with no significant difference of any secondary malignancy among individuals treated with and without autoSCT.[13] However, all cases of t-MN occurred after autoSCT, yielding a 10-year incidence rate of t-MN of 8%, which is in the range of the DFCI and MRC series. Within all series, the outcome after t-MNs was poor, which makes this a very relevant and serious long-term complication and is particularly relevant to patients that may have benefited from less aggressive regimens.
Conclusions and Perspectives
What major conclusions can be drawn from almost two decades of clinical trials on autoSCT in CLL? Firstly, autoSCT indeed has the capacity to provide prolonged disease control at least similar to modern immunochemotherapy regimens, such as FCR. However, like immunochemotherapy, autoSCT has no significant curative potential in CLL. Secondly, autoSCT does not have the potential to overcome the negative impact of biomarkers that confer resistance to chemotherapy. Therefore, patients who have gained the highest benefit from autoSCT would also most likely respond to conventional immunochemotherapy. Thirdly, autoSCT is associated with an increased risk of secondary neoplasms, which is a very serious long-term adverse event with a poor outcome.
Over the past few years, CLL research and treatment have made a huge leap forward: to name a few examples, randomized clinical trials aiming to improve the potential of FCR and to optimize treatment for high-risk and older patients or those with comorbidities are ongoing, and their recruitment has for the most part been exceeding expectations. There are also several exciting new small molecule inhibitors such as the BTK-inhibitor ibrutinib or the bcl-2 inhibitor navitoclax, which show promising activity even in the relapsed/ refractory setting, and in combination with conventional chemoimmunotherapy.[32-34] Several studies have indicated that allogeneic HSCT is currently the only treatment with curative potential on the basis of its capacity to induce graft-versus-leukemia (GVL) activity, even in high-risk CLL patients.[35-37] This approach, however, is never indicated as part of first-line treatment in standard-risk patients and should be restricted to patients who meet the EBMT transplant consensus criteria.[38] Sensitive techniques for MRD quantification have been further optimized and might serve as a surrogate marker to assess treatment efficacy.[39] In addition, experimental treatment approaches such as chimeric-antigen-receptor (CAR) T cells have shown exciting preliminary results which lead us to believe that this might direct us into the future of CLL treatment.[40] In view of these and other developments, autoSCT does currently not play a role in the treatment of CLL; therefore the autoSCT game indeed seems to be over for the time being.[41]
References
- Sant M, Allemani C, Tereanu C, De Angelis
R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies
in Europe by morphologic subtype: results of the HAEMACARE project.
Blood. 2010 November 11, 2010;116(19):3724-34. PMid:20664057

- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK (eds). SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. 2010.
- Tam CS, O'Brien S, Wierda W, Kantarjian H,
Wen S, Do K-A, et al. Long-term results of the fludarabine,
cyclophosphamide, and rituximab regimen as initial therapy of chronic
lymphocytic leukemia. Blood. 2008 August 15, 2008;112(4):975-80.

- Hallek M, Fischer K, Fingerle-Rowson G,
Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fl udarabine
and cyclophosphamide in patients with chronic lymphocytic leukaemia: a
randomised, open-label, phase 3 trial. Lancet. 2010;376:1164-74. http://dx.doi.org/10.1016/S0140-6736(10)61381-5

- Zenz T, Gribben JG, Hallek M, Doehner H,
Keating MJ, Stilgenbauer S. Risk categories and refractory CLL in the
era of chemoimmunotherapy. Blood. 2012 May 3, 2012;119(18):4101-7.

- Rabinowe SN, Soiffer RJ, Gribben JG, Daley
H, Freedman AS, Daley J, et al. Autologous and allogeneic bone marrow
transplantation for poor prognosis patients with B-cell chronic
lymphocytic leukemia. Blood. 1993 August 15, 1993;82(4):1366-76.
PMid:8151318

- Khouri IF, Keating MJ, Vriesendorp HM,
Reading CL, Przepiorka D, Huh YO. Autologous and allogeneic bone marrow
transplantation for chronic lymphocytic leukemia: preliminary results.
J Clin Oncol. 1994;12:748-58. PMid:9156240

- Itala M, Pelliniemi TT, Rajamaki A, Remes
K. Autologous blood cell transplantation in B-CLL: response to
chemotherapy prior to mobilization predicts the stem cell yield. Bone
marrow transplantation. 1997 Apr;19(7):647-51. http://dx.doi.org/10.1038/sj.bmt.1700730 PMid:9823944

- Sutton L, Maloum K, Gonzalez H, Zouabi H,
Azar N, Boccaccio C, et al. Autologous hematopoietic stem cell
transplantation as salvage treatment for advanced B cell chronic
lymphocytic leukemia. Leukemia: official journal of the Leukemia
Society of America, Leukemia Research Fund, UK. 1998
Nov;12(11):1699-707. http://dx.doi.org/10.1038/sj.leu.2401201

- Dreger P, Von Neuhoff N, Kuse R, Sonnen R,
Glass B, Uharek L, et al. Early stem cell transplantation for chronic
lymphocytic leukaemia: a chance for cure? British Journal of
Haematology. 1998 Jun 1998;77(12):2291-97.

- Gribben JG, Zahrieh D, Stephans K,
Bartlett-Pandite L, Alyea EP, Fisher DC, et al. Autologous and
allogeneic stem cell transplantations for poor-risk chronic lymphocytic
leukemia. Blood. 2005 December 15, 2005;106(13):4389-96.

- Milligan DW, Fernandes S, Dasgupta R,
Davies FE, Matutes E, Fegan CD, et al. Results of the MRC pilot study
show autografting for younger patients with chronic lymphocytic
leukemia is safe and achieves a high percentage of molecular responses.
Blood. 2005 January 1, 2005;105(1):397-404. PMid:22490331

- Dreger P, Doehner H, McClanahan F, Busch
R, Ritgen M, Greinix H, et al. Early autologous stem cell
transplantation for chronic lymphocytic leukemia: long-term follow-up
of the German CLL Study Group CLL3 trial. Blood. 2012 May 24,
2012;119(21):4851-9. PMid:21406717

- Sutton L, Chevret S, Tournilhac O, Divine
M, Leblond V, Corront B, et al. Autologous stem cell transplantation as
a first-line treatment strategy for chronic lymphocytic leukemia: a
multicenter, randomized, controlled trial from the SFGM-TC and GFLLC.
Blood. 2011 Jun 9;117(23):6109-19. http://dx.doi.org/10.1182/blood-2010-11-317073 PMid:21106985

- Michallet M, Dreger P, Sutton L, Brand R,
Richards S, van Os M, et al. Autologous hematopoietic stem cell
transplantation in chronic lymphocytic leukemia: results of European
intergroup randomized trial comparing autografting versus observation.
Blood. 2010 November 24, 2010;117(5):1516-21. PMid:21725374

- Brion A, Mahe B, Kolb B, Audhuy B,
Colombat P, Maisonneuve H, et al. Autologous transplantation in CLL
patients with B and C Binet stages: final results of the prospective
randomized GOELAMS LLC 98 trial. Bone Marrow Transplant.
2012;47(4):542-8. http://dx.doi.org/10.1038/bmt.2011.117 PMid:22547610

- Cwynarski K, van Biezen A, de Wreede L,
Stilgenbauer S, Bunjes D, Metzner B, et al. Autologous and Allogeneic
Stem-Cell Transplantation for Transformed Chronic Lymphocytic Leukemia
(Richter's Syndrome): A Retrospective Analysis From the Chronic
Lymphocytic Leukemia Subcommittee of the Chronic Leukemia Working Party
and Lymphoma Working Party of the European Group for Blood and Marrow
Transplantation. Journal of Clinical Oncology. 2012 April 30, 2012.

- Tsimberidou AM, O'Brien S, Khouri I, Giles
FJ, Kantarjian HM, Champlin R, et al. Clinical Outcomes and Prognostic
Factors in Patients With Richter's Syndrome Treated With Chemotherapy
or Chemoimmunotherapy With or Without Stem-Cell Transplantation.
Journal of Clinical Oncology. 2006 May 20, 2006;24(15):2343-51.

- Vardiman JW, Arber DA, Brunning RD, Larson
RA, Matutes E, Baumann I, et al. Therapy-related myeloid neoplasms. In:
Swerdlow SH, Campo E, Harris NL, E.S. J, Pileri S, Stein H, et al.,
editors. WHO Classification of haematopoietic and lymphoid tissues. 4th
Edition ed. Lyon: International Agency for Research on Cancer (IARC);
2008. PMid:20962860

- Carney DA, Westerman DA, Tam CS, Milner A,
Prince HM, Kenealy M, et al. Therapy-related myelodysplastic syndrome
and acute myeloid leukemia following fludarabine combination
chemotherapy. Leukemia. 2010 Dec;24(12):2056-62. http://dx.doi.org/10.1038/leu.2010.218 PMid:19114699

- Tsimberidou AM, Wen S, McLaughlin P,
O'Brien S, Wierda WG, Lerner S, et al. Other malignancies in chronic
lymphocytic leukemia/small lymphocytic lymphoma. Journal of clinical
oncology. 2009 Feb 20;27(6):904-10. http://dx.doi.org/10.1200/JCO.2008.17.5398 PMid:17910629

- Maddocks-Christianson K, Slager SL, Zent
CS, Reinalda M, Call TG, Habermann TM, et al. Risk factors for
development of a second lymphoid malignancy in patients with chronic
lymphocytic leukaemia. British Journal of Haematology. 2007
Nov;139(3):398-404. http://dx.doi.org/10.1111/j.1365-2141.2007.06801.x PMid:17351903

- Schollkopf C, Rosendahl D, Rostgaard K,
Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic
leukemia. Int J Cancer. 2007 Jul 1;121(1):151-6. http://dx.doi.org/10.1002/ijc.22672 PMid:23090186 PMCid:3478266

- Callea V, Brugiatelli M, Stelitano
C, Gentile M, Nobile F, Morabito F. Incidence of second neoplasia in
patients with B-cell chronic lymphocytic leukemia treated with
chlorambucil maintenance chemotherapy. Leukemia & Lymphoma. 2006
Nov;47(11):2314-20. http://dx.doi.org/10.1080/10428190600880977 PMid:14746857

- Robak T. Second malignancies and Richter's
syndrome in patients with chronic lymphocytic leukaemia treated with
cladribine. European Journal of Cancer. 2004;40(3):383-9. http://dx.doi.org/10.1016/j.ejca.2003.09.031 PMid:10561309

- Cheson BD, Vena DA, Barrett J, Freidlin B.
Second Malignancies as a Consequence of Nucleoside Analog Therapy for
Chronic Lymphoid Leukemias. Journal of Clinical Oncology.
1999;17(8):2454-60. PMid:20940199 PMCid:3020697

- Morton LM, Curtis RE, Linet MS, Bluhm EC,
Tucker MA, Caporaso N, et al. Second malignancy risks after
non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by
lymphoma subtype. Journal of clinical oncology. 2010 Nov
20;28(33):4935-44. http://dx.doi.org/10.1200/JCO.2010.29.1112 PMid:12228208

- Morrison VA, Rai KR, Peterson BL, Kolitz
JE, Elias L, Appelbaum FR, et al. Therapy-related myeloid leukemias are
observed in patients with chronic lymphocytic leukemia after treatment
with fludarabine and chlorambucil: results of an intergroup study,
cancer and leukemia group B 9011. Journal of clinical oncology. 2002
Sep 15;20(18):3878-84. http://dx.doi.org/10.1200/JCO.2002.08.128 PMid:21321292 PMCid:3084002

- Woyach JA, Ruppert AS, Heerema NA,
Peterson BL, Gribben JG, Morrison VA, et al. Chemoimmunotherapy With
Fludarabine and Rituximab Produces Extended Overall Survival and
Progression-Free Survival in Chronic Lymphocytic Leukemia: Long-Term
Follow-Up of CALGB Study 9712. Journal of Clinical Oncology. 2011 April
1, 2011;29(10):1349-55. PMid:16856906

- Jantunen E, Itala M, Siitonen T, Koivunen
E, Leppa S, Juvonen E, et al. Late non-relapse mortality among adult
autologous stem cell transplant recipients: a nation-wide analysis of
1,482 patients transplanted in 1990-2003. European Journal of
Haematology. 2006 Aug;77(2):114-9. http://dx.doi.org/10.1111/j.1600-0609.2006.00685.x PMid:16611308

- Milligan DW, Kochethu G, Dearden C,
Matutes E, MacConkey C, Catovsky D, et al. High incidence of
myelodysplasia and secondary leukaemia in the UK Medical Research
Council Pilot of autografting in chronic lymphocytic leukaemia. British
Journal of Haematology. 2006;133(2):173-5. http://dx.doi.org/10.1111/j.1365-2141.2006.05982.x

- O'Brien S, Furman R, Coutre S, Burger J, Blum K, Sharman J, et al. The bruton's tyrosine kinase inhibitor ibrutinib is highly active and tolerable in relapses or refractory (R/R) and treatment naive (TN) CLL patients, updated results of a phase Ib/II study. Haematologica EHA Meeting Abstracts. 2012;Vol 97(e-Supplement 1):Abstract 542.
- Brown J, Barrientos J, Flinn I, Barr P, Burger J, Navarro T, et al. The bruton's tyrosine kinase (BTK) inhibitor ibrutinib combined with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL, interim results of a phase IB/II study. Haematologica EHA Meeting Abstracts. 2012;Vol 97(e-Supplement 1):Abstract 543.
- Kipps T, Swinnen L, Wierda W, Jones J,
Coutre S, Smith M, et al. Navitoclax (ABT 263) plus
fludarabine/cyclophosphamide/rituximab (FCR) or bendamustine/rituximab
(BR): a phase I study in patients with relapsed/ refractory CLL.
Haematologica EHA Meeting Abstracts. 2012;Vol 97(e-Supplement
1):Abstract 545. PMid:20595516

- Dreger P, Doehner H, Ritgen M, Boettcher
S, Busch R, Dietrich S, et al. Allogeneic stem cell transplantation
provides durable disease control in poor-risk chronic lymphocytic
leukemia: long-term clinical and MRD results of the German CLL Study
Group CLL3X trial. Blood. 2010;116(14):2438-47. http://dx.doi.org/10.1182/blood-2010-03-275420 PMid:18794548 PMCid:2652085

- Sorror ML, Storer BE, Sandmaier BM, Maris
M, Shizuru J, Maziarz R, et al. Five-year follow-up of patients with
advanced chronic lymphocytic leukemia treated with allogeneic
hematopoietic cell transplantation after nonmyeloablative conditioning.
Journal of Clinical Oncology. 2008;26(40):4912-20. http://dx.doi.org/10.1200/JCO.2007.15.4757 PMid:18711173

- Schetelig J, van Biezen A, Brand R,
Caballero D, Martino R, Itala M, et al. Allogeneic hematopoietic cell
transplantation for chronic lymphocytic leukemia with 17p- deletion: A
retrospective EBMT analysis. Journal of Clinical Oncology.
2008;26(31):5094-100. http://dx.doi.org/10.1200/JCO.2008.16.2982 PMid:17109028

- Dreger P, Corradini P, Kimby E, Michallet
M, Milligan D, Schetelig J. Indications for allogeneic stem cell
transplantation in chronic lymphocytic leukemia: The EBMT transplant
consensus. Leukemia. 2007;21:12-7. http://dx.doi.org/10.1038/sj.leu.2404441 PMid:22331940

- Boettcher S, Ritgen M, Fischer K,
Stilgenbauer S, Busch RM, Fingerle-Rowson Gn, et al. Minimal Residual
Disease Quantification Is an Independent Predictor of Progression-Free
and Overall Survival in Chronic Lymphocytic Leukemia: A Multivariate
Analysis From the Randomized GCLLSG CLL8 Trial. Journal of Clinical
Oncology. 2012 March 20, 2012;30(9):980-8. PMid:21837241

- Koehler P, Schmidt P, Hombach AA, Hallek
M. Engineered T cells for the adoptive therapy of B-cell chronic
lymphocytic leukaemia. Advances in hematology. 2012;595060.

- Montserrat E, Gribben JG. Autografting CLL: the game is over! Blood. 2011 June 9, 2011;117(23):6057-8.
