Update on the Role of Autologous Hematopoietic Stem Cell Transplantation in Follicular Lymphoma
Mónica Cabrero, Alba Redondo, Alejandro Martin and Dolores Caballero
Servicio
de Hematología: Hospital Universitario e Instituto Biosanitario (IBSAL)
de Salamana . Paseo de San Vicente 37007 Salamanca, Spain
Correspondence
to:
Dra. Dolores Caballero. Hematology Department, Hospital Universitario
de Salamanca. IBSAL (Instituto Biosanitario de Salamanca). Paseo de San
Vicente, 50-180. 37007 Salamanca. Spain. E-mail: cabarri@usal.es
Published: November 7, 2012
Received: October 1, 2012
Accepted: October 26, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012074, DOI 10.4084/MJHID.2012.074
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Follicular
lymphoma (FL) remains incurable despite advances in new strategies of
treatment, including monoclonal antibodies (MoAb). Except for early
stages, FL is characterized by responses to treatments and systematic
relapses. The main objective in this disease is to achieve a better
progression free survival (PFS) and to increase overall survival (OS),
mainly in young patients. In order to improve the results of
conventional chemotherapy, autologous stem cell transplant (ASCT) is a
feasible treatment in these patients. In this moment, ASCT is not
recommended as first line treatment, except for transformed FL, but is
a good strategy as salvage therapy with an improved PFS and OS. New
drugs have been introduced to enhanced responses of ASCT, but nowadays
they are not part of conventional conditioning regimen.
Introduction
Follicular lymphoma (FL) is the most common low-grade lymphoma, and the second in frequency of the totality of non-Hodgkin lymphomas (NHL). FL accounts for 25% of newly diagnosed lymphomas. The natural history of this disease is characterized by responses to treatments and systematic relapses.[1]
Introduction of new drugs, mainly Rituximab and other monoclonal antibodies have improved progression free survival (PFS) and overall survival (OS), but nowadays, FL remains incurable.
Intensified regimens including autologous stem cell transplant (ASCT) are feasible strategies in young patients mainly as salvage therapy.
Conventional treatments include radiotherapy for early stages and immune chemotherapy for advances stages. More than 80% of patients have advanced disease at diagnosis, but only those with related symptoms of lymphoma must be treated. In the Rituximab era, an induction regimen including this drug is the choice of treatment. Moreover, recently, the addiction of Rituximab maintenance has improved PFS in both untreated and relapse/refractory FL patients who did not receive prior Rituximab (PRIMA and EORTC studies).[2,3,4]
Autologous Stem Cell Transplantation in Follicular Lymphoma
FIRST LINE THERAPY: Prior to the widespread use of Rituximab in first line treatment, high dose chemotherapy followed by ASCT has been tested in young patients. There are 5 randomized clinical trials[4-8] that have shown some improvement in PFS but not in OS. Among these trials there are 2 that include patients who received Rituximab during induction therapy.[7,8] Results in terms of PFS and OS are similar independently of prior exposition to anti-CD20, with similar data in all the published trials. (Table 1).
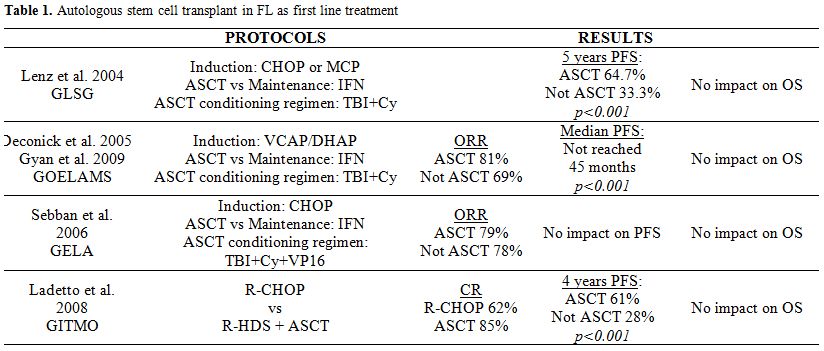
Table 1. Autologous stem cell transplant in FL as first line treatment
Before deciding to perform an ASCT as first line therapy, the potential risk of second malignancies like myelodysplastic syndromes, acute myeloid leukemias or solid neoplasm should be considered.[9]
Finally, two meta-analysis[10,11] have evaluated the role of ASCT as first line treatment in FL, and no benefit in OS compared with conventional therapy despite the prolonged PFS observed has been demonstrated.
Based on these results, we can conclude that, despite the benefit on PFS, ASCT is not recommended as first line treatment for FL because there is no evidence of improvement in OS, and a higher risk of second malignancies has been reported.
Relapse FL
There are many options for relapsed FL patients; we can consider Rituximab alone, combination regimen of chemotherapy and rituximab or radioimmunotherapy. Autologous stem cell transplant has an important role in young relapsed patients.
Prior to the Rituximab era, a phase III randomized clinical trial (The CUP trial)[12] compared conventional chemotherapy to ASCT in 89 patients with relapsed or progressive FL, and showed a benefit on PFS and OS for patients who received an ASCT. Two years PFS was 26% versus 55%, and 4 years OS was 46% versus 71% for chemotherapy and ASCT respectively.
More recently, several retrospective phase II studies (have shown) showed a longer PFS and OS for ASCT versus chemotherapy regimens.[13-18] Sebban et al. published data of a retrospective analysis of 254 patients previously included in 2 prospective randomized trials from GELA/GELF group, who relapsed after first line therapy. Comparing the treatment at relapse, those patients who have received salvage therapy including Rituximab followed by an ASCT exhibit an enhanced PFS and OS comparing to those receiving only chemotherapy. (Five year overall survival after relapse 70% (95% CI, 59% to 79%) vs. 42% (33% to 50%) and 5-year event free survival after relapse 51% (40% to 61%) vs. 24% (17% to 31%), p < .0001 in both cases). Although a benefit from ASCT was observed in all patients who received Rituximab as part of salvage therapy, these results have not been confirmed in another patient cohort, so it is not clear yet if the benefit of ASCT remains after including Rituximab as part of the rescue therapy.
The recent study, by Le Gouill et al, includes 175 patients in first relapse FL, previously treated according to a randomized phase III trial (FL2000). This study reports a significant better 3 years OS in ASCT (92%, CI 78-97%) as compared with conventional chemotherapy treatment (63%, CI 51-72%); otherwise, it also showed the benefit of Rituximab treatment in relapsed FL patients both Rituximab naive and prior treated.[19]
In conclusion, according to the available data, ASCT is recommended in first relapse FL, based mainly in pre-Rituximab studies.
New Drugs in ASCT
Despite that ASCT seems to be the best option for relapse FL, the primary cause of failure of this approach is the disease recurrence. In order to achieve durable remissions and decrease relapse after ASCT with limited toxicity, new drugs have been included as part of conditioning regimen.
Radioinmunotherapy (RIT)
We know that lymphomas are sensitive to irradiation, so radiotherapy has been used as conditioning regimen in ASCT and more recently the association of radioisotopes with tumour-associated antigens such as CD20 has allowed exploring a new way to improve ASCT’s results by the intensification of tumour irradiation without increasing organ toxicity. Yttrium (90) ibritumomab tiuxetan (Zevalin) and I-131 tositumomab have been tested, and the results of many phase I and II trials suggest that this can be a safety and effective strategy combined with high-dose chemotherapy and further stem cell transplantation.[20]
Nademadee A. et al published in 2005 the results of a phase I/II study combining high dose Y-90 ibritumomab tiuxetan with high dose etoposide (40-60 mg/kg) and cyclophosphamide (100 mg/kg) that included 12 patients with relapse FL.[21] At a median follow up of 22 months, the 2-year estimated overall survival (OS) was 92% and the disease-free survival was 78%.
More recently, Decaudin D. et al have reported the results of a GELA phase II study to evaluate the safety and efficacy of a conventional dose of (90)Y ibritumomab tiuxetan combined with carmustine, etoposide, cytarabine and melphalan (BEAM) regimen before autologous stem cell transplantation (ASCT) in B-NHL.[22] Sixty-nine patients with diagnosis of FL were included in the trial. Before ASCT, 77% and 23% of patients were in CR and PR respectively. At day +100, 88% of patients were in CR/CRu, and at a median follow up of 28 months, 2-year event-free survival and OS were 63% and 97%, respectively.
In 2007, Gopal et al. published data from 24 patients older than 60 years who received a myeloablative dose of I-131 tositumomab (monitored by dosimetry) followed by an ASCT.[23] Having been submitted to a median of four prior regimens, these patients were not considered suitable for conventional conditioning regimen because of its potential toxicity. With a median follow-up of 2.9 years, the estimated 3-year OS and PFS rates were 59% and 51%, respectively.
Although phase III trials comparing radioimmunotherapy with conventional conditioning regimens are not available in this moment, Gopal et al. compared data from FL patients included in phase I/II trials of I-131-tositumomab with 98 historical controls treated with conventional high- dose therapy followed by infusion of autologous stem cell.[24] Historical controls received total body irradiation plus chemotherapy or chemotherapy alone. After a multivariate analysis, patients who receive radioimmunotherapy had a significant reduction in the risk of the disease progression or death (HR=0.5, p=0.03) compared to the control group. The estimated OS was 67% vs. 53%, and estimated PFS was 48% vs. 29%, for patients receiving radioimmunotherapy and conventional ASCT respectively.
Based on these data we can conclude that RIT is a promising option to improve results of ASCT in FL, but randomized trials are needed to establish the role of these new drugs in salvage therapy.
Transformed FL
Histologic transformation of follicular lymphoma into an aggressive lymphoma, like diffuse large B-cell lymphoma, implies a worsening of prognosis, including worse response to conventional treatment. Approximately 3% of patients with follicular lymphoma develop transformation annually, clinically characterised by a rapid lymph node growth with increased levels of lactate dehydrogenase or an intensive uptake in positron emission tomography.
Most studies published about ASCT in transformed FL are based on pre-rituximab data, and they show that we can obtain sustained complete remission with this strategy. In the more recent study published in 2011, a prospective phase II by the Norwegian group[25] including patients who did not receive Rituximab in first line treatment, 60% of patients obtained CR after ASCT. With a median follow-up of 75 months, PFS and OS were 26 and 47 months respectively, compared with a median OS of 10 months for patients not eligible for ASCT.
The impact of rituximab has not been established yet, but ASCT seems to remain an effective option for transformed FL.[26]
Conclusions
Recently, an evidence-based review has been published by a panel of follicular lymphoma experts with the following recommendations:[27]
- ASCT is recommended as salvage therapy based on pre-rituximab data, with a significant improvement in overall survival (OS) and progression-free (PFS) survival. After Rituximab containing salvage therapy, there is no sufficient evidence-based data to recommend ASCT, so more studies are needed to establish the actual role of ASCT in FL.
- ASCT is not recommended as first-line treatment. Despite the prolongation in PFS there is no improvement in OS.
- ASCT is recommended for transformed follicular lymphoma patients.
Follicular lymphoma (FL) is the most common low-grade lymphoma, and the second in frequency of the totality of non-Hodgkin lymphomas (NHL). FL accounts for 25% of newly diagnosed lymphomas. The natural history of this disease is characterized by responses to treatments and systematic relapses.[1]
Introduction of new drugs, mainly Rituximab and other monoclonal antibodies have improved progression free survival (PFS) and overall survival (OS), but nowadays, FL remains incurable.
Intensified regimens including autologous stem cell transplant (ASCT) are feasible strategies in young patients mainly as salvage therapy.
Conventional treatments include radiotherapy for early stages and immune chemotherapy for advances stages. More than 80% of patients have advanced disease at diagnosis, but only those with related symptoms of lymphoma must be treated. In the Rituximab era, an induction regimen including this drug is the choice of treatment. Moreover, recently, the addiction of Rituximab maintenance has improved PFS in both untreated and relapse/refractory FL patients who did not receive prior Rituximab (PRIMA and EORTC studies).[2,3,4]
Autologous Stem Cell Transplantation in Follicular Lymphoma
FIRST LINE THERAPY: Prior to the widespread use of Rituximab in first line treatment, high dose chemotherapy followed by ASCT has been tested in young patients. There are 5 randomized clinical trials[4-8] that have shown some improvement in PFS but not in OS. Among these trials there are 2 that include patients who received Rituximab during induction therapy.[7,8] Results in terms of PFS and OS are similar independently of prior exposition to anti-CD20, with similar data in all the published trials. (Table 1).

Table 1. Autologous stem cell transplant in FL as first line treatment
Before deciding to perform an ASCT as first line therapy, the potential risk of second malignancies like myelodysplastic syndromes, acute myeloid leukemias or solid neoplasm should be considered.[9]
Finally, two meta-analysis[10,11] have evaluated the role of ASCT as first line treatment in FL, and no benefit in OS compared with conventional therapy despite the prolonged PFS observed has been demonstrated.
Based on these results, we can conclude that, despite the benefit on PFS, ASCT is not recommended as first line treatment for FL because there is no evidence of improvement in OS, and a higher risk of second malignancies has been reported.
Relapse FL
There are many options for relapsed FL patients; we can consider Rituximab alone, combination regimen of chemotherapy and rituximab or radioimmunotherapy. Autologous stem cell transplant has an important role in young relapsed patients.
Prior to the Rituximab era, a phase III randomized clinical trial (The CUP trial)[12] compared conventional chemotherapy to ASCT in 89 patients with relapsed or progressive FL, and showed a benefit on PFS and OS for patients who received an ASCT. Two years PFS was 26% versus 55%, and 4 years OS was 46% versus 71% for chemotherapy and ASCT respectively.
More recently, several retrospective phase II studies (have shown) showed a longer PFS and OS for ASCT versus chemotherapy regimens.[13-18] Sebban et al. published data of a retrospective analysis of 254 patients previously included in 2 prospective randomized trials from GELA/GELF group, who relapsed after first line therapy. Comparing the treatment at relapse, those patients who have received salvage therapy including Rituximab followed by an ASCT exhibit an enhanced PFS and OS comparing to those receiving only chemotherapy. (Five year overall survival after relapse 70% (95% CI, 59% to 79%) vs. 42% (33% to 50%) and 5-year event free survival after relapse 51% (40% to 61%) vs. 24% (17% to 31%), p < .0001 in both cases). Although a benefit from ASCT was observed in all patients who received Rituximab as part of salvage therapy, these results have not been confirmed in another patient cohort, so it is not clear yet if the benefit of ASCT remains after including Rituximab as part of the rescue therapy.
The recent study, by Le Gouill et al, includes 175 patients in first relapse FL, previously treated according to a randomized phase III trial (FL2000). This study reports a significant better 3 years OS in ASCT (92%, CI 78-97%) as compared with conventional chemotherapy treatment (63%, CI 51-72%); otherwise, it also showed the benefit of Rituximab treatment in relapsed FL patients both Rituximab naive and prior treated.[19]
In conclusion, according to the available data, ASCT is recommended in first relapse FL, based mainly in pre-Rituximab studies.
New Drugs in ASCT
Despite that ASCT seems to be the best option for relapse FL, the primary cause of failure of this approach is the disease recurrence. In order to achieve durable remissions and decrease relapse after ASCT with limited toxicity, new drugs have been included as part of conditioning regimen.
Radioinmunotherapy (RIT)
We know that lymphomas are sensitive to irradiation, so radiotherapy has been used as conditioning regimen in ASCT and more recently the association of radioisotopes with tumour-associated antigens such as CD20 has allowed exploring a new way to improve ASCT’s results by the intensification of tumour irradiation without increasing organ toxicity. Yttrium (90) ibritumomab tiuxetan (Zevalin) and I-131 tositumomab have been tested, and the results of many phase I and II trials suggest that this can be a safety and effective strategy combined with high-dose chemotherapy and further stem cell transplantation.[20]
Nademadee A. et al published in 2005 the results of a phase I/II study combining high dose Y-90 ibritumomab tiuxetan with high dose etoposide (40-60 mg/kg) and cyclophosphamide (100 mg/kg) that included 12 patients with relapse FL.[21] At a median follow up of 22 months, the 2-year estimated overall survival (OS) was 92% and the disease-free survival was 78%.
More recently, Decaudin D. et al have reported the results of a GELA phase II study to evaluate the safety and efficacy of a conventional dose of (90)Y ibritumomab tiuxetan combined with carmustine, etoposide, cytarabine and melphalan (BEAM) regimen before autologous stem cell transplantation (ASCT) in B-NHL.[22] Sixty-nine patients with diagnosis of FL were included in the trial. Before ASCT, 77% and 23% of patients were in CR and PR respectively. At day +100, 88% of patients were in CR/CRu, and at a median follow up of 28 months, 2-year event-free survival and OS were 63% and 97%, respectively.
In 2007, Gopal et al. published data from 24 patients older than 60 years who received a myeloablative dose of I-131 tositumomab (monitored by dosimetry) followed by an ASCT.[23] Having been submitted to a median of four prior regimens, these patients were not considered suitable for conventional conditioning regimen because of its potential toxicity. With a median follow-up of 2.9 years, the estimated 3-year OS and PFS rates were 59% and 51%, respectively.
Although phase III trials comparing radioimmunotherapy with conventional conditioning regimens are not available in this moment, Gopal et al. compared data from FL patients included in phase I/II trials of I-131-tositumomab with 98 historical controls treated with conventional high- dose therapy followed by infusion of autologous stem cell.[24] Historical controls received total body irradiation plus chemotherapy or chemotherapy alone. After a multivariate analysis, patients who receive radioimmunotherapy had a significant reduction in the risk of the disease progression or death (HR=0.5, p=0.03) compared to the control group. The estimated OS was 67% vs. 53%, and estimated PFS was 48% vs. 29%, for patients receiving radioimmunotherapy and conventional ASCT respectively.
Based on these data we can conclude that RIT is a promising option to improve results of ASCT in FL, but randomized trials are needed to establish the role of these new drugs in salvage therapy.
Transformed FL
Histologic transformation of follicular lymphoma into an aggressive lymphoma, like diffuse large B-cell lymphoma, implies a worsening of prognosis, including worse response to conventional treatment. Approximately 3% of patients with follicular lymphoma develop transformation annually, clinically characterised by a rapid lymph node growth with increased levels of lactate dehydrogenase or an intensive uptake in positron emission tomography.
Most studies published about ASCT in transformed FL are based on pre-rituximab data, and they show that we can obtain sustained complete remission with this strategy. In the more recent study published in 2011, a prospective phase II by the Norwegian group[25] including patients who did not receive Rituximab in first line treatment, 60% of patients obtained CR after ASCT. With a median follow-up of 75 months, PFS and OS were 26 and 47 months respectively, compared with a median OS of 10 months for patients not eligible for ASCT.
The impact of rituximab has not been established yet, but ASCT seems to remain an effective option for transformed FL.[26]
Conclusions
Recently, an evidence-based review has been published by a panel of follicular lymphoma experts with the following recommendations:[27]
- ASCT is recommended as salvage therapy based on pre-rituximab data, with a significant improvement in overall survival (OS) and progression-free (PFS) survival. After Rituximab containing salvage therapy, there is no sufficient evidence-based data to recommend ASCT, so more studies are needed to establish the actual role of ASCT in FL.
- ASCT is not recommended as first-line treatment. Despite the prolongation in PFS there is no improvement in OS.
- ASCT is recommended for transformed follicular lymphoma patients.
References
- Armitage JO, and Weisenburger D. New
approach to classifying non-Hodgkin's lymphomas: clinical features of
the major histologic subtypes. Non-Hodgkin's Lymphoma Classification
Project. J Clin Oncol 1998;16: 2780-95. PMid:9704731

- Salles G, Seymour JF, Off ner F, et al.
Rituximab maintenance for 2 years in patients with high tumour burden
follicular lymphoma responding to rituximab plus chemotherapy (PRIMA):
a phase 3, randomised controlled trial. Lancet 2011;377:42 - 51.

- Van Oers MH, Van Glabbeke M, Giurgea L et
al. Rituximab maintenance treatment of relapsed/resistant follicular
non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III
randomized intergroup study. J Clin Oncol. 2010 10;28:2853-8. http://dx.doi.org/10.1200/JCO.2009.26.5827 PMid:20439641 PMCid:2903319

- Deconinck E, Foussard C, Milpied N, et al.
High-dose therapy followed by autologous purged stem-cell
transplantation and doxorubicin-based chemotherapy in patients with
advanced follicular lymphoma: A randomized multicenter study by
GOELAMS. Blood 2005;105:3817-3823.

- Lenz G, Dreyling M, Schiegnitz E, et al.
Myeloablative radiochemotherapy followed by autologous stem cell
transplantation in first remission prolongs progression-free survival
in follicular lymphoma: Results of a prospective, randomized trial of
the German Low-Grade Lymphoma Study Group. Blood 2004;104:2667-2674.

- Sebban C, Mounier N, Brousse N, et al.
Standard chemotherapy with interferon compared with CHOP followed by
high-dose therapy with autologous stem cell transplantation in
untreated patients with advanced follicular lymphoma: The GELF-94
randomized study from the Groupe d’Etude des Lymphomes de l’Adulte
(GELA). Blood 2006;108:2540-2544.

- Ladetto M, De Marco F, Benedetti F, et al.
Prospective, multicenter randomized GITMO/IIL trial comparing intensive
(R-HDS) versus conventional (CHOP-R) chemoimmunotherapy in high-risk
follicular lymphoma at diagnosis: The superior disease control of R-HDS
does not translate into an overall survival advantage. Blood
2008;111:4004-4013.

- Lenz G, Dreyling M, Schiegnitz E, et al.
Moderate increase of secondary hematologic malignancies after
myeloablative radiochemotherapy and autologous stem-cell
transplantation in patients with indolent lymphoma: results of a
prospective randomized trial of the German Low Grade Lymphoma Study
Group. J Clin Oncol. 2004;22:4926-4933. http://dx.doi.org/10.1200/JCO.2004.06.016 PMid:15611507

- Gyan E, Foussard C, Bertrand P, et al.
High-dose therapy followed by autologous purged stem cell
transplantation and doxorubicin-based chemotherapy in patients with
advanced follicular lymphoma: A randomized multicenter study by the
GOELAMS with final results after a median follow-up of 9 years. Blood
2009;113:995-1001.

- Al Khabori M, de Almeida JR, Guyatt GH et
al. Autologous stem cell transplantation in follicular lymphoma: a
systematic review and meta-analysis. J Natl Cancer Inst. 2012
4;104:18-28. http://dx.doi.org/10.1093/jnci/djr450 PMid:22190633

- Wang B, Ren C, Zhang W et al. Intensified
therapy followed by autologous stem-cell transplantation (ASCT) versus
conventional therapy as first-line treatment of follicular lymphoma: a
meta-analysis. Hematol Oncol. 2012 4. doi: 10.1002/hon.2015.

- Schouten HC, Qian W, Kvaloy S, et al.
High-dose therapy improves progression-free survival and survival in
relapsed follicular non-Hodgkin's lymphoma: results from the randomized
European CUP trial. J Clin Oncol 21:3918-27.

- Brice P, Simon D, Bouabdallah R, et al.
High-dose therapy with autologous stem-cell transplantation (ASCT)
after first progression prolonged survival of follicular lymphoma
patients included in the prospective GELF 86 protocol. Ann Oncol.
2000;11: 1585-1590. http://dx.doi.org/10.1023/A:1008399623564 PMid:11205467

- Montoto S, Canals C, Rohatiner AZS et al.
Long-term follow-up of high-dose treatment with autologous
haematopoietic progenitor cell support in 693 patients with follicular
lymphoma: an EBMT registry study. Leukemia 2007; 21: 2324-2331.

- Rohatiner AZ, Nadler L, Davies AJ et al.
Myeloablative therapy with autologous bone marrow transplantation for
follicular lymphoma at the time of second or subsequent remission:
long-term follow-up. J Clin Oncol. 2007;25:2554-9 http://dx.doi.org/10.1200/JCO.2006.09.8327 PMid:17515573

- Sebban C, Brice P, Delarue R et al. Impact
of rituximab and/or high-dose therapy with autotransplant at time of
relapse in patients with follicular lymphoma: a GELA study. J Clin
Oncol. 2008. 26:3614-20.

- Arcaini L, Montanari F, Alessandrino EP et
al. Immunochemotherapy with in vivo purging and autotransplant induces
long clinical and molecular remission in advanced relapsed and
refractory follicular lymphoma. Ann Oncol 2008; 19: 1331-1335.

- Schaaf M, Reiser M, Borchmann et al.
High-dose therapy with autologous stem cell transplantation versus
chemotherapy or immuno-chemotherapy for follicular lymphoma in adults.
Cochrane Database Syst Rev. 2012 Jan 18;1

- Le Gouill S, De Guibert S, Planche L et
al. Impact of the use of autologous stem cell transplantation at first
relapse both in naive and previously rituximab exposed follicular
lymphoma patients treated in the GELA/GOELAMS FL2000 study.
Haematologica. 2011.96:1128-35

- Cilley J, Winter JN. Radioimmunotherapy
and autologous stem cell transplantation for the treatment of B-cell
lymphomas. Haematologica. 2006 Jan;91:114-20. PMid:16434379

- Nademanee A, Forman S, Molina A et al. A
phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in
combination with high-dose etoposide and cyclophosphamide followed by
autologous stem cell transplantation in patients with poor-risk or
relapsed non-Hodgkin lymphoma. Blood. 2005 15;106:2896-902. http://dx.doi.org/10.1182/blood-2005-03-1310 PMid:16002426 PMCid:1895300

- Decaudin D, Mounier N, Tilly H et al.
(90)Y ibritumomab tiuxetan (Zevalin) combined with BEAM (Z -BEAM)
conditioning regimen plus autologous stem cell transplantation in
relapsed or refractory low-grade CD20-positive B-cell lymphoma. A GELA
phase II prospective study. Clin Lymphoma Myeloma Leuk. 2011;11:212-8. http://dx.doi.org/10.1016/j.clml.2011.03.007 PMid:21575926

- Gopal AK, Rajendran JG, Gooley TA et al.
High-dose [131I]tositumomab (anti-CD20) radioimmunotherapy and
autologous hematopoietic stem-cell transplantation for adults > or =
60 years old with relapsed or refractory B-cell lymphoma. J Clin Oncol.
2007 10;25:1396-402. http://dx.doi.org/10.1200/JCO.2006.09.1215 PMid:17312330

- Gopal AK, Gooley TA, Maloney DG et al.
High-dose radioimmunotherapy versus conventional high-dose therapy and
autologous hematopoietic stem cell transplantation for relapsed
follicular non-Hodgkin lymphoma: a multivariable cohort analysis. Blood
2003; 102:2351-7. http://dx.doi.org/10.1182/blood-2003-02-0622 PMid:12750161

- Eide MB, Lauritzsen GF, Kvalheim G et al.
High dose chemotherapy with autologous stem cell support for patients
with histologically transformed B-cell non-Hodgkin lymphomas. A
Norwegian multi centre phase II study. Br J Haematol. 2011;152:600-10. http://dx.doi.org/10.1111/j.1365-2141.2010.08519.x PMid:21241276

- Ban-Hoefen M, Kelly JL, Bernstein SH et
al. High-dose therapy and autologous stem cell transplant for
transformed non-Hodgkin lymphoma in the rituximab era. Leuk Lymphoma.
2012;53:830-5 http://dx.doi.org/10.3109/10428194.2011.631637 PMid:23090186 PMCid:3484130

- Oliansky DM, Gordon LI, King J et al. The
role of cytotoxic therapy with hematopoietic stem cell transplantation
in the treatment of follicular lymphoma: an evidence-based review. Biol
Blood Marrow Transplant. 2010;16:443-68. http://dx.doi.org/10.1016/j.bbmt.2010.01.008 PMid:20114084
