Successful Treatment of Gastric Relapse in Multiple Myeloma with Bortezomib after Autologous Hematopoietic Stem Cell Transplantation (autoHSCT)
Serdar Sivgin1, Suleyman Baldane2, Leylagul Kaynar1, Fatih Kurnaz1,Mevlut Baskol3, Mustafa Kula4, Celalettin Eroglu5, Kemal Deniz6,
Bulent Eser1, Ali Unal1 and Mustafa Cetin1
1Dedeman
Stem Cell Transplantation Hospital, Department of Hematology, Faculty
of Medicine, Erciyes University, Kayseri, Turkey,
2Department of Internal Medicine, Faculty of Medicine, Erciyes University, Kayseri,Turkey,
3Department of Gastroenterology, Faculty of Medicine, Erciyes University, Kayseri,Turkey,
4Department of Nuclear Medicine, Faculty of Medicine, Erciyes University, Kayseri, Turkey,
5Department of Radiation Oncology, Faculty of Medicine, Erciyes University, Kayseri, Turkey
6Department of Pathology, Faculty of Medicine, Erciyes University, Kayseri, Turkey.
2Department of Internal Medicine, Faculty of Medicine, Erciyes University, Kayseri,Turkey,
3Department of Gastroenterology, Faculty of Medicine, Erciyes University, Kayseri,Turkey,
4Department of Nuclear Medicine, Faculty of Medicine, Erciyes University, Kayseri, Turkey,
5Department of Radiation Oncology, Faculty of Medicine, Erciyes University, Kayseri, Turkey
6Department of Pathology, Faculty of Medicine, Erciyes University, Kayseri, Turkey.
Correspondence
to:
M.D. Serdar Sivgin. Dedeman Stem Cell Transplantation Hospital,
Department of Hematology, Faculty of Medicine, Erciyes University,
Kayseri, Turkey. Tel: 0 352/2076666/27028, fax: 0
352/4379348.
E-mail: drssivgin@gmail.com
Published: January 2, 2013
Received: August 7, 2012
Accepted: December 3, 2012
Meditter J Hematol Infect Dis 2013, 5(1): e2013006, DOI 10.4084/MJHID.2013.006
This article is available on PDF format at:

This is an Open
Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
We
report a case of 59-year-old Turkish man with history of mitral valve
replacement (MVR) and chronic obstructive pulmonary disease (COPD) who
was diagnosed with stage IIIA IgG lambda multiple myeloma (MM) in 1997.
He underwent autologous hematopoietic stem cell transplantation after a
conditioning regimen with melphalan 200mg per body area (m2)
in February 2006. On February 2011, he was admitted to the emergency
service of university hospital with complaints of hematemesis and
melena. Pathological evaluation of gastric biopsy, obtained from a
lesion of small gastric curvature, showed the gastric mucosa
infiltrated by neoplastic plasma cells, monoclonal lambda light chain
positive. The patient was considered as having local gastric relapsed
disease and was treated with 2 cycles of bortezomib. He achieved an
excellent local response after 2 cycles of bortezomib, cyclophosphamide
and prednisone (BEP) regimen, with healing of gastric ulcer and no
recurrence of the hematemesis or melena.
Introduction
Extramedullary accumulation of plasma cells, defined plasmocytoma, occur in up to 20% of patients with multiple myeloma.[1-3] The most common site for extramedullary involvement is the upper aero digestive tract, which includes the oronasal pharynx, nasal cavities, sinuses and larynx.[4-6] Plasma cell infiltration can involve also any segment of the gastro intestinal tract, representing only 5% of patients with extramedullary involvement.[7] The most common site involved in the gastrointestinal tract is the small bowel, the involvement of this region presents intestinal obstruction and malabsorption.[3,4] Other gastrointestinal sites are the stomach, colon and oesophagus, in order of frequency of involvement.[8,9] The involvement of the gastrointestinal tract years after an initial diagnosis of MM is exceptional and, when reported, always associated with a poor prognosis.[10,11] We report a case of 59-year-old man with MM with gastric relapse who had been treated successfully with bortezomib and achieved remission after autologous hematopoietic stem cell transplantation (autoHSCT) .
Case Presentation
We report a case of 59-year-old man with history of MVR and COPD diagnosed with IgG lambda MM (stage IIIA) in 1997. He was initially treated with six cycles of vincristine, doxorubicin and dexamethasone, followed by high-dose melphalan (10mg/m2, 1-4 days, orally) and cyclophosphamide (50mg/day, continuously, orally) which resulted in a complete response with a bone marrow plasma cell lower than <5% for 12 months. He received maintenance therapy with melphalan (10mg/m2) for 2 years. In July 2005; he was admitted to emergency service with complaints of bone ache and fatigue. The patient relapsed with rapidly progressing disease, characterized by an increased paraprotein level (15 g/l), a mildly raised LDH level (295 U/l). In bone marrow aspiration analysis; plasma cell ratio was found > 30% and considered as relapsed status. He was treated with vincristine, doxorubicin and dexamethasone (VAD) for 4 cycles. After achieving complete remission (no finding of the original monoclonal paraprotein in serum and urine, also in bone marrow aspirate plasma cells were found <5%) he underwent autologous hematopoietic stem cell transplantation following melphalan 200mg per body area (m2) in Stem Cell Transplantation Hospital, Department of Hematology, Erciyes University, Kayseri, Turkey in February 2006. The patient was followed up in complete remission in outpatient clinic of the hospital with regular control sessions. On February 2011; he was admitted to the emergency service of university hospital with complaints of hematemesis and melena. The patient has been using warfarin for prophylaxis of thromboembolism after MVR operation. The laboratory parameters were as follows; prothrombin time (PT): 26,8 sec (10.1-14.9), activated partial thromboplastin time (aPTT): 31,3 sec (25-35), INR:2,48 (0.8-1.2) in total blood count, white blood cell (WBC): 6,11 x 103/ÁL, hemoglobin(Hb): 7,7 g/dL (14.0-18.0) and platelet count (PLT): 169 x 103/ÁL (130-400). The biochemical tests were; blood urea nitrogen (BUN): 29 mg/dL (9-23), creatinin:0,85 mg/dL (0.7-1.23), potassium (K): 4,6 mg/dL (3.5-5.5), calcium (Ca): 8,1 mg/dL (8.3-10.6), LDH:284 U/L (120-246), AST: 19 U/L (0-34), ALT: 9 U/L (10-49), ALP: 64 U/L (45-129), GGT: 101U/L (0-73). The upper gastrointestinal endoscopic examination revealed a bleeding ulcerative lesion with a diameter of 2 cm in the small curvature of the stomach (Figure 1).
The patient was recommended not taking food and drinks, and He was submitted to gastric decompression with a nasogastric tube. The bleeding was controlled with argon plasma coagulation and sclerotherapy. Biopsy of the gastric lesion showed neoplastic plasma cells, monoclonal lambda light chain positive, infiltrating gastric mucosa (Figure 2).
In immunohistochemistry; staining with CD38 and lambda were positive and kappa was negative (Figures 3 and 4).
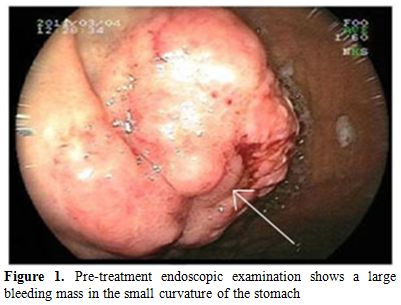
Figure 1. Pre-treatment endoscopic examination shows a large bleeding mass in the small curvature of the stomach.
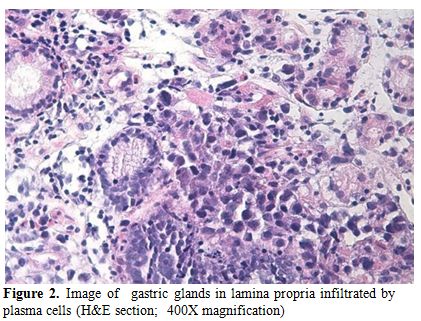
Figure 2. Image of gastric glands in lamina propria infiltrated by plasma cells (H&E section; 400X magnification).
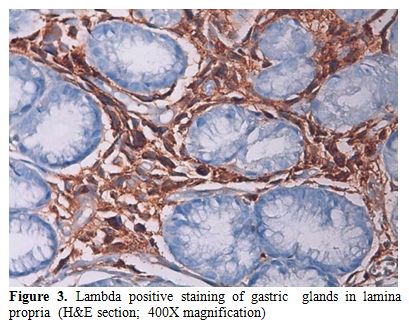
Figure 3. Lambda positive staining of gastric glands in lamina propria (H&E section; 400X magnification).
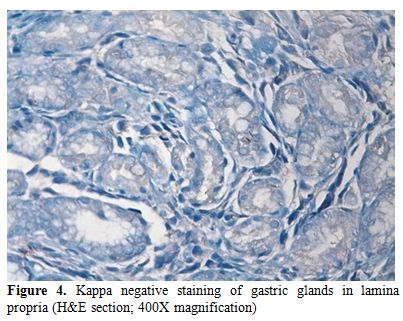
Figure 4. Kappa negative staining of gastric glands in lamina propria (H&E section; 400X magnification).
In laboratory tests; serum IgG was; 1520 mg/dL (650-1600), IgM: 112 mg/dL (50-301), IgA: 342 mg/dL 845-380), kappa: 338 mg/dL (629-1350), lambda: 540 mg/dL (313-723), β2 microglobulin: 2,66 mg/dL (1.16-2.52). A bone marrow biopsy was performed and plasma cell was found below 5%.
A PET-CT scan was performed to determine the gastric involvement of the disease. The scan showed dense FDG uptake (suv max: 26,5) in gastric fundus and corpus with a wall thickness of 35 mm (Figure 5).
Serum immunofixation test showed no monoclonality and total blood count was in normal ranges. Through these findings, the patient was considered as local gastric relapsed disease and was treated with 2 cycles of bortezomib at a dose of bortezomib 1,3 mg/m2, on days 1,4,8 and 11 intravenously, oral cyclophosphamide 50 mg per day continously and oral prednisone 100 mg per day on days 1,4,8 and 11. The cycle was repeated every 3 weeks. After the chemotherapy, control upper gatrointestinal endoscopy was performed and found that the lesion was completely resolved. (Figure 6).
Also, we performed a PET-CT scan to determine the last status of the lesions in the body. PET-CT scan showed no residual lesion in whole body and. showed a dramatic shrinkage of the gastric mass (Figure 7) also shown in PET-CT fusion images (Figures 8 and 9).
An excellent response was achieved after 2 cycles of BEP regimen, the paraprotein level was not detectable and there was no recurrence of the hematemesis or melena. His general condition improved rapidly and he was discharged after the second cycle had commenced.
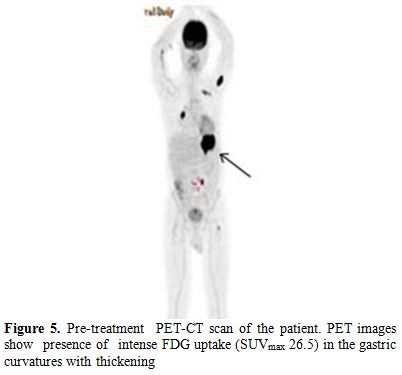
Figure 5. Pre-treatment PET-CT scan of the patient. PET images show presence of intense FDG uptake (SUVmax 26.5) in the gastric curvatures with thickening.
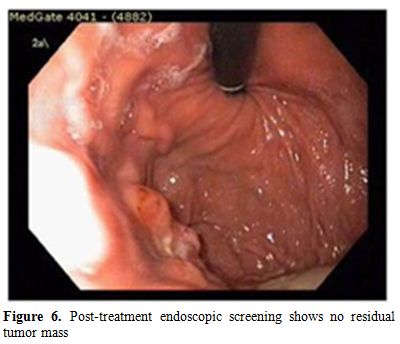
Figure 6. Post-treatment endoscopic screening shows no residual tumor mass.
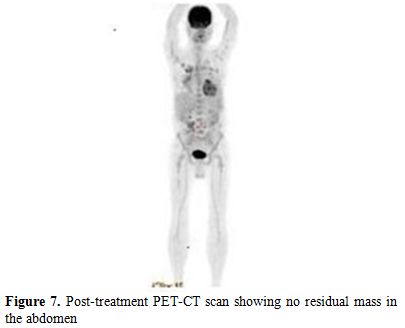
Figure 7. Post-treatment PET-CT scan showing no residual mass in the abdomen.
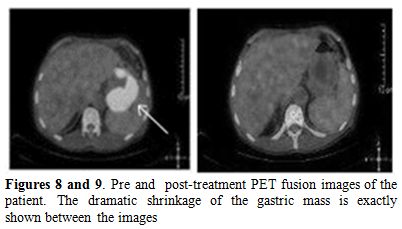
Figure 8 and 9. Pre and post-treatment PET fusion images of the patient. The dramatic shrinkage of the gastric mass is exactly shown between the images.
Discussion
Gastrointestinal involvement in MM is very rare. It most often occurs in the context of an isolated, primary, extramedullary plasmacytoma.[12] Patients with newly diagnosed MM rarely present with symptoms which are related to gastrointestinal involvement.[13]
Multiple myeloma is a clonal malignancy of plasma cells characterized by the development of anemia. The malignant plasma cells are usually confined to the bone marrow, relying on the marrow stroma for their growth and survival.[14] Extramedullary involvement is rare, accounting for 14% of relapses following autologous stem-cell transplantation, with fewer than 5% of those with extramedullary disease having gastrointestinal involvement.[7] In our patient both gastric biopsy and PET-CT scan showed gastric involvement of multiple myeloma admitted with upper gastrointestinal bleeding. Benusiglio et al. reported a patient with MM whom had gastrointestinal relapse.[15] The patient was successfully treated with lenalidomide and achieved a long-lasting response to treatment. The clinician should take into account gastric involvement in patients with MM presenting gastrointestinal bleeding. In our case, the gastric involvement - which can be considered as a rare event - was successfully treated with bortezomib. Our data suggest that features and treatment modalities of extramedullary plamocytoma can be different and that the extramedullary localization is most frequent in genomically defined high-risk multiple myeloma; however, in any case, extramedullary disease is associated with shorter progression-free survival and overall survival.[16-20]
The prognosis following an extra medullary relapse of myeloma is generally significantly worse than for medullary relapse, with most patients having few remaining therapeutic options. Some groups, however, have demonstrated that an individualized treatment Schedule following extramedullary relapse could be successful in controlling the disease and could offer survival rates that are comparable to those seen following medullary relapse.[21] In our patient, prognosis was excellent after the treatment with bortezomib and, at present, the patient is under routine control. Traditionally, nuclear medicine scans exploiting the increased metabolic activity of hematological tumors, such as lymphoma, have not been used in the staging and monitoring of myeloma patients; however, recent evidence supports the use of modalities such as FDG-PET for the early diagnosis of relapsed myeloma. The recent data suggests that this technique is especially useful in the diagnosis and monitoring of aggressive extramedullary disease.[22] The data showed that 18F-FDG PET/CT could be used for staging, identifying optimal sites for biopsy, restaging, and monitoring response to treatment for MM and related plasma cell dyscrasias.[23] Gozzetti et al.[24] stated that imaging techniques have long been used to help diagnose patients and determine the stage of the disease, especially PET-CT. In a recent study,[25] the importance and feasibility of imaging techniques like PET-CT, have been demonstrated. This study showed that PET-CT has an important role on diagnosis and staging of solitary plasmacytoma, also determining the response to treatment. In our case, PET-CT scan was used to determine involvemnet of all body sites including gastric mucosa and also after chemotherapy to assess the effect of the treatment. 18F-FDG uptake in the stomach could be considered as a non-specific finding and physicians should differentiate several benign disorders.
Conclusion
In this case, we experienced that bortezomib was very effective in patient with gastric involvement. It reminds us that, in addition to much more common causes (for example, ulcers), the clinician must consider gastrointestinal involvement in patients with MM presenting gastrointestinal hemorrhage. It also shows that patients with MM who have been heavily pre-treated can benefit from novel drugs, like bortezomib even when they are critically ill. Finally, we should emphasized that a response to this drug was obtained despite active bleeding in the upper gastrointestinal system.
Extramedullary accumulation of plasma cells, defined plasmocytoma, occur in up to 20% of patients with multiple myeloma.[1-3] The most common site for extramedullary involvement is the upper aero digestive tract, which includes the oronasal pharynx, nasal cavities, sinuses and larynx.[4-6] Plasma cell infiltration can involve also any segment of the gastro intestinal tract, representing only 5% of patients with extramedullary involvement.[7] The most common site involved in the gastrointestinal tract is the small bowel, the involvement of this region presents intestinal obstruction and malabsorption.[3,4] Other gastrointestinal sites are the stomach, colon and oesophagus, in order of frequency of involvement.[8,9] The involvement of the gastrointestinal tract years after an initial diagnosis of MM is exceptional and, when reported, always associated with a poor prognosis.[10,11] We report a case of 59-year-old man with MM with gastric relapse who had been treated successfully with bortezomib and achieved remission after autologous hematopoietic stem cell transplantation (autoHSCT) .
Case Presentation
We report a case of 59-year-old man with history of MVR and COPD diagnosed with IgG lambda MM (stage IIIA) in 1997. He was initially treated with six cycles of vincristine, doxorubicin and dexamethasone, followed by high-dose melphalan (10mg/m2, 1-4 days, orally) and cyclophosphamide (50mg/day, continuously, orally) which resulted in a complete response with a bone marrow plasma cell lower than <5% for 12 months. He received maintenance therapy with melphalan (10mg/m2) for 2 years. In July 2005; he was admitted to emergency service with complaints of bone ache and fatigue. The patient relapsed with rapidly progressing disease, characterized by an increased paraprotein level (15 g/l), a mildly raised LDH level (295 U/l). In bone marrow aspiration analysis; plasma cell ratio was found > 30% and considered as relapsed status. He was treated with vincristine, doxorubicin and dexamethasone (VAD) for 4 cycles. After achieving complete remission (no finding of the original monoclonal paraprotein in serum and urine, also in bone marrow aspirate plasma cells were found <5%) he underwent autologous hematopoietic stem cell transplantation following melphalan 200mg per body area (m2) in Stem Cell Transplantation Hospital, Department of Hematology, Erciyes University, Kayseri, Turkey in February 2006. The patient was followed up in complete remission in outpatient clinic of the hospital with regular control sessions. On February 2011; he was admitted to the emergency service of university hospital with complaints of hematemesis and melena. The patient has been using warfarin for prophylaxis of thromboembolism after MVR operation. The laboratory parameters were as follows; prothrombin time (PT): 26,8 sec (10.1-14.9), activated partial thromboplastin time (aPTT): 31,3 sec (25-35), INR:2,48 (0.8-1.2) in total blood count, white blood cell (WBC): 6,11 x 103/ÁL, hemoglobin(Hb): 7,7 g/dL (14.0-18.0) and platelet count (PLT): 169 x 103/ÁL (130-400). The biochemical tests were; blood urea nitrogen (BUN): 29 mg/dL (9-23), creatinin:0,85 mg/dL (0.7-1.23), potassium (K): 4,6 mg/dL (3.5-5.5), calcium (Ca): 8,1 mg/dL (8.3-10.6), LDH:284 U/L (120-246), AST: 19 U/L (0-34), ALT: 9 U/L (10-49), ALP: 64 U/L (45-129), GGT: 101U/L (0-73). The upper gastrointestinal endoscopic examination revealed a bleeding ulcerative lesion with a diameter of 2 cm in the small curvature of the stomach (Figure 1).
The patient was recommended not taking food and drinks, and He was submitted to gastric decompression with a nasogastric tube. The bleeding was controlled with argon plasma coagulation and sclerotherapy. Biopsy of the gastric lesion showed neoplastic plasma cells, monoclonal lambda light chain positive, infiltrating gastric mucosa (Figure 2).
In immunohistochemistry; staining with CD38 and lambda were positive and kappa was negative (Figures 3 and 4).

Figure 1. Pre-treatment endoscopic examination shows a large bleeding mass in the small curvature of the stomach.

Figure 2. Image of gastric glands in lamina propria infiltrated by plasma cells (H&E section; 400X magnification).

Figure 3. Lambda positive staining of gastric glands in lamina propria (H&E section; 400X magnification).

Figure 4. Kappa negative staining of gastric glands in lamina propria (H&E section; 400X magnification).
In laboratory tests; serum IgG was; 1520 mg/dL (650-1600), IgM: 112 mg/dL (50-301), IgA: 342 mg/dL 845-380), kappa: 338 mg/dL (629-1350), lambda: 540 mg/dL (313-723), β2 microglobulin: 2,66 mg/dL (1.16-2.52). A bone marrow biopsy was performed and plasma cell was found below 5%.
A PET-CT scan was performed to determine the gastric involvement of the disease. The scan showed dense FDG uptake (suv max: 26,5) in gastric fundus and corpus with a wall thickness of 35 mm (Figure 5).
Serum immunofixation test showed no monoclonality and total blood count was in normal ranges. Through these findings, the patient was considered as local gastric relapsed disease and was treated with 2 cycles of bortezomib at a dose of bortezomib 1,3 mg/m2, on days 1,4,8 and 11 intravenously, oral cyclophosphamide 50 mg per day continously and oral prednisone 100 mg per day on days 1,4,8 and 11. The cycle was repeated every 3 weeks. After the chemotherapy, control upper gatrointestinal endoscopy was performed and found that the lesion was completely resolved. (Figure 6).
Also, we performed a PET-CT scan to determine the last status of the lesions in the body. PET-CT scan showed no residual lesion in whole body and. showed a dramatic shrinkage of the gastric mass (Figure 7) also shown in PET-CT fusion images (Figures 8 and 9).
An excellent response was achieved after 2 cycles of BEP regimen, the paraprotein level was not detectable and there was no recurrence of the hematemesis or melena. His general condition improved rapidly and he was discharged after the second cycle had commenced.

Figure 5. Pre-treatment PET-CT scan of the patient. PET images show presence of intense FDG uptake (SUVmax 26.5) in the gastric curvatures with thickening.

Figure 6. Post-treatment endoscopic screening shows no residual tumor mass.

Figure 7. Post-treatment PET-CT scan showing no residual mass in the abdomen.

Figure 8 and 9. Pre and post-treatment PET fusion images of the patient. The dramatic shrinkage of the gastric mass is exactly shown between the images.
Discussion
Gastrointestinal involvement in MM is very rare. It most often occurs in the context of an isolated, primary, extramedullary plasmacytoma.[12] Patients with newly diagnosed MM rarely present with symptoms which are related to gastrointestinal involvement.[13]
Multiple myeloma is a clonal malignancy of plasma cells characterized by the development of anemia. The malignant plasma cells are usually confined to the bone marrow, relying on the marrow stroma for their growth and survival.[14] Extramedullary involvement is rare, accounting for 14% of relapses following autologous stem-cell transplantation, with fewer than 5% of those with extramedullary disease having gastrointestinal involvement.[7] In our patient both gastric biopsy and PET-CT scan showed gastric involvement of multiple myeloma admitted with upper gastrointestinal bleeding. Benusiglio et al. reported a patient with MM whom had gastrointestinal relapse.[15] The patient was successfully treated with lenalidomide and achieved a long-lasting response to treatment. The clinician should take into account gastric involvement in patients with MM presenting gastrointestinal bleeding. In our case, the gastric involvement - which can be considered as a rare event - was successfully treated with bortezomib. Our data suggest that features and treatment modalities of extramedullary plamocytoma can be different and that the extramedullary localization is most frequent in genomically defined high-risk multiple myeloma; however, in any case, extramedullary disease is associated with shorter progression-free survival and overall survival.[16-20]
The prognosis following an extra medullary relapse of myeloma is generally significantly worse than for medullary relapse, with most patients having few remaining therapeutic options. Some groups, however, have demonstrated that an individualized treatment Schedule following extramedullary relapse could be successful in controlling the disease and could offer survival rates that are comparable to those seen following medullary relapse.[21] In our patient, prognosis was excellent after the treatment with bortezomib and, at present, the patient is under routine control. Traditionally, nuclear medicine scans exploiting the increased metabolic activity of hematological tumors, such as lymphoma, have not been used in the staging and monitoring of myeloma patients; however, recent evidence supports the use of modalities such as FDG-PET for the early diagnosis of relapsed myeloma. The recent data suggests that this technique is especially useful in the diagnosis and monitoring of aggressive extramedullary disease.[22] The data showed that 18F-FDG PET/CT could be used for staging, identifying optimal sites for biopsy, restaging, and monitoring response to treatment for MM and related plasma cell dyscrasias.[23] Gozzetti et al.[24] stated that imaging techniques have long been used to help diagnose patients and determine the stage of the disease, especially PET-CT. In a recent study,[25] the importance and feasibility of imaging techniques like PET-CT, have been demonstrated. This study showed that PET-CT has an important role on diagnosis and staging of solitary plasmacytoma, also determining the response to treatment. In our case, PET-CT scan was used to determine involvemnet of all body sites including gastric mucosa and also after chemotherapy to assess the effect of the treatment. 18F-FDG uptake in the stomach could be considered as a non-specific finding and physicians should differentiate several benign disorders.
Conclusion
In this case, we experienced that bortezomib was very effective in patient with gastric involvement. It reminds us that, in addition to much more common causes (for example, ulcers), the clinician must consider gastrointestinal involvement in patients with MM presenting gastrointestinal hemorrhage. It also shows that patients with MM who have been heavily pre-treated can benefit from novel drugs, like bortezomib even when they are critically ill. Finally, we should emphasized that a response to this drug was obtained despite active bleeding in the upper gastrointestinal system.
References
- Zeiser R, Deschler
B, Bertz H, Engelhardt
M. Extramedullary vs medullary relapse after autologous or allogeneic
hematopoietic stem cell transplantation (HSCT) in multiple myeloma (MM)
and its correlation to clinical outcome. Bone Marrow
Transplant.2004;34:1057-65. http://dx.doi.org/10.1038/sj.bmt.1704713
PMid:15516937

- Dawson MA,
Polizzotto MN, Gordon A, Roberts
SK, Spencer A. Extramedullary relapse of multiple myeloma presenting as
hematemesis and melena. Nat Clin Pract Oncol.2006;3:223-6. http://dx.doi.org/10.1038/ncponc0454
PMid:16596146

- Damaj G, Mohty M,
Vey N, Dincan E,
Bouabdallah R, Faucher C, Stoppa AM, Gastaut JA. Features of
extramedullary and extraosseous multiple myeloma. a report of 19
patients from a single center. Eur J Haematol.2004;73:402-6. http://dx.doi.org/10.1111/j.1600-0609.2004.00331.x
PMid:15522061

- Richardson PG,
Barlogie B, Berenson JR, et
al. A phase II study of bortezomib in relapsed, refractory myeloma. N
Engl J Med. 2003;348:2609-17.

- Jagannath S,
Barlogie B, Berenson J, et al.
A phase 2 study of two doses of bortezomib in relapsed or refractory
multiple myeloma. Br J Haematol.2004;127:165-72.

- Richardson PG,
Sonneveld P, Schuster MW et
al. Bortezomib demonstrates superior efficacy to high-dose
dexamethasone in relapsed multiple myeloma: final report of the APEX
study. N Engl J Med.2005; 352:2487-98.

- Alegre A, Granda
A, Martinez-Chamorro C,
Draz-Mediavilla J, Martinez R, Garcia-Laraca J, Lahuerta JJ, Sureda A,
Blady J, de la Rubia J, Fernandez-Racada JM, San Miguel J. Different
patterns of relapse after autologous peripheral blood stem cell
transplantation in multiple myeloma: clinical results of 280 cases from
the Spanish Registry. Haematologica.2002; 87: 609-14.

- Nicholl D and
Jones T. Intestinal
pseudoobstruction due to amyloidosis of the colon in association with
an intestinal plasmacytoma. PostgradMed J.1991;67: 1075-77.

- Goldstein WB and
Poker N. Multiple myeloma involving the gastrointestinal tract.
Gastroenterology.1966; 51: 87-93.

- Cerny J, Fadare
O, Hutchinson L, Wang SA.
Clinicopathological features of extramedullary recurrence/relapse of
multiple myeloma. Eur J Haematol.2008; 81:65-69. http://dx.doi.org/10.1111/j.1600-0609.2008.01087.x
PMid:18462256

- Umeno Y, Kogawa
K, Matsuishi E et al. A
case of multiple myeloma which developed into multiple extramedullary
involvement in the terminal stage. Fukuoka Igaku Zasshi. 2000; 91:
55-61.

- Alexiou C, Kau
RJ, Dietzfelbinger H,
Kremer M, Spiess JC, Schratzenstaller B, Arnold W. Extramedullary
plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;
85:2305-14. http://dx.doi.org/10.1002/(SICI)1097-0142(19990601)85:11<2305::AID-CNCR2>3.3.CO;2-V

- Herbst A, Renner
SW, Ringenberg QS, Fass
R, Krouse RS. Multiple myeloma presenting with a colonic obstruction
and bony lesions: a clinical dilemma. J Clin
Oncol.2008;26:5645-47. http://dx.doi.org/10.1200/JCO.2008.18.6239
PMid:18981461

- Tricot GJ. New
insights into role of microenvironment in multiple myeloma. Int J
Hematol. 2002;76:334-36.

- Benusiglio PR,
McKee TA, Montet X,
Dumonceau JM, Favet L, George AC Dietrich PY. Gastrointestinal relapse
of multiple myeloma and sustained response to lenalidomide: a case
report Journal of Medical Case Reports. 2011; 5:110. http://dx.doi.org/10.1186/1752-1947-5-110
PMid:21418590 PMCid:3076251

- Rasche L, Bernard
C, Topp MS, Kapp M,
Duell J, Wesemeier C, Haralambieva E, Maeder U, Einsele H, Knop S.
Features of extramedullary myeloma relapse: high proliferation, minimal
marrow involvement, adverse cytogenetics: a retrospective,
single-center study of 24 cases. Ann Hematol. 2012; 91:1031-7. http://dx.doi.org/10.1007/s00277-012-1414-5
PMid:22286070

- Bladi J,
Fernandez de Larrea C, Rosivol L,
Cibeira MT, Jimanez R, Powles R.Soft-tissue plasmacytomas in multiple
myeloma: incidence, mechanisms of extramedullary spread, and treatment
approach. J Clin Oncol. 2011;29:3805-12. PMid:21900099

- Usmani SZ, Heuck
C, Mitchell A, Szymonifka
J, Nair B, Hoering A, Alsayed Y, Waheed S, Haider S, Restrepo A, van
Rhee F, Crowley J, Barlogie B. Extramedullary disease portends poor
prognosis in multiple myeloma and is overrepresented in high risk
disease even in era of novel agents. Haematologica. 2012;97:1761-7. http://dx.doi.org/10.3324/haematol.2012.065698
PMid:22689675 PMCid:3487453

- Terpos E, Rezvani
K, Basu S, Milne AE,
Rose PE, Scott GL, Rahemtulla A, Samson D, Apperley JF. Plasmacytoma
relapses in the absence of systemic progression post-high-dose therapy
for multiple myeloma. Eur J Haematol. 2005;75:376-83. http://dx.doi.org/10.1111/j.1600-0609.2005.00531.x
PMid:16191086

- Madan S, Kumar S.
Review: extramedullary disease in multiple myeloma. Clin Adv Hematol
Oncol. 2009;7:802-4. PMid:20332751

- Zeiser R,
Deschler B, Bertz H, Finke J,
Engelhardt M. Extramedullary vs medullary relapse after autologous or
allogeneic hematopoietic stem cell transplantation (HSCT) in multiple
myeloma (MM) and its correlation to clinical outcome. Bone Marrow
Transplant.2004;34: 1057-65.

- Durie BG,Waxman
AD, D'Agnolo A, Williams CM. Whole-body 8F-FDG PET identifies high-risk
myeloma. J Nucl Med.2002;43:1457-63.

- Makis W, Ciarallo
A, Hickeson M, Lisbona
R.Gastric recurrence of a primary colon plasmacytoma: staging and
evaluating response to therapy with 18F-FDG PET/CT.Br J Radiol.
2012;.doi: 10.1259/bjr/37953406. http://dx.doi.org/10.1259/bjr/37953406
PMid:22190759

- Gozzetti A, Rossi
V, Cerase A, Papini G,
Defina M, Bocchia M. Single Agent Lenalidomide Activity in Multiple
Myeloma Relapse Evidenced Uniquely by CT/PET. Mediterr J Hematol Infect
Dis. 2012;4(1):e2012041. doi:10.4084/MJHID.2012.041. Epub 2012 Jun 18.

- Warsame R, Gertz
MA, Lacy MQ, Kyle RA,
Buadi F, Dingli D, Greipp PR, Hayman SR, Kumar SK, Lust JA, Russell SJ,
Witzig TE, Mikhael J, Leung N, Zeldenrust SR, Rajkumar SV, Dispenzieri
A. Trends and outcomes of modern staging of solitary plasmacytoma of
bone. Am J Hematol. 2012;87:647-51. http://dx.doi.org/10.1002/ajh.23201
PMid:22549792
