Early Application of High Cut-Off Haemodialysis for de-Novo Myeloma Nephropathy is Associated with Long-Term Dialysis-Independency and Renal Recovery
Alhossain A. Khalafallah1,2, Sie Wuong Loi1, Sarah Love3, Muhajir Mohamed1,2, Rose Mace1, Ramy Khalil1,2, Miriam Girgs1,2, Rajesh Raj1 and Mathew Mathew1
1Launceston
General Hospital, Launceston, Tasmania, Australia
2School of Medicine, University of Tasmania, Australia
3Faculty of Health and Life Sciences, Coventry University, Coventry, West Midlands, UK.
2School of Medicine, University of Tasmania, Australia
3Faculty of Health and Life Sciences, Coventry University, Coventry, West Midlands, UK.
Correspondence
to:
Associate Professor A. Khalafallah, Launceston General Hospital,
Charles Street, Launceston, Tasmania, 7250 Australia. Tel: +61363487111
Fax: +613-63353388. E-mail: khalafallah@dhhs.tas.gov.au
Published: January 2, 2013
Received: September 8, 2012
Accepted: December 1, 2012
Meditter J Hematol Infect Dis 2013, 5(1): e2013007, DOI 10.4084/MJHID.2013.007
This article is available on PDF format at:

This is an Open
Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Background:
Multiple myeloma (MM) is a haematological malignancy associated with
kidney injury resulting from cast nephropathy, which can be caused by
monoclonal free light chains (FLC). It has been demonstrated that early
reduction of FLC can lead to a higher proportion of patients recovering
renal function with a better outcome, especially if high cut-off
haemodialysis (HCO-HD) combined with chemotherapy is used.
Patients and Methods: In this study, four cases with MM nephropathy were treated with HCO-HD and chemotherapy at a single institution during the period from August 2009 to August 2011. All of the patients presented with acute renal failure and high serum FLC. All patients underwent a bone marrow biopsy to confirm the diagnosis of MM, according to the WHO criteria. Three patients had de novo MM and one patient had relapsed light chain myeloma disease. All patients underwent HCO-HD concomitantly with specific myeloma therapy once the diagnosis or relapse of MM was established.
Results: After a medial follow up of 26 months, (range, 13-36) our data showed that all patients had a significant decrease in serum FLC through HCO-HD, proving the effectiveness of HCO-HD in managing MM. De-novo MM patients restored their renal function and achieved low-level FLC early in the treatment and became dialysis-independent. One patient with relapsed myeloma remained dialysis-dependent.
Conclusion: In summary, our study suggests that in myeloma nephropathy associated with light-chain MM, HCO-HD should be initiated as early as possible. At the same time a specific MM treatment should be initiated to gain control of the disease and salvage the kidneys in order to achieve dialysis-independency. Further randomized trials to confirm our results are warranted.
Patients and Methods: In this study, four cases with MM nephropathy were treated with HCO-HD and chemotherapy at a single institution during the period from August 2009 to August 2011. All of the patients presented with acute renal failure and high serum FLC. All patients underwent a bone marrow biopsy to confirm the diagnosis of MM, according to the WHO criteria. Three patients had de novo MM and one patient had relapsed light chain myeloma disease. All patients underwent HCO-HD concomitantly with specific myeloma therapy once the diagnosis or relapse of MM was established.
Results: After a medial follow up of 26 months, (range, 13-36) our data showed that all patients had a significant decrease in serum FLC through HCO-HD, proving the effectiveness of HCO-HD in managing MM. De-novo MM patients restored their renal function and achieved low-level FLC early in the treatment and became dialysis-independent. One patient with relapsed myeloma remained dialysis-dependent.
Conclusion: In summary, our study suggests that in myeloma nephropathy associated with light-chain MM, HCO-HD should be initiated as early as possible. At the same time a specific MM treatment should be initiated to gain control of the disease and salvage the kidneys in order to achieve dialysis-independency. Further randomized trials to confirm our results are warranted.
Background
Multiple myeloma (MM) is a haematological malignancy most commonly associated with some degree of renal impairment and possibly acute kidney injury.[1] The kidney injury in MM is more often caused by monoclonal free light chains (FLC) which lead to cast nephropathy.[2-4] In a normal subject the immunoglobulin FLC are the by-products of immunoglobulin synthesis. The FLC are bound to the heavy chains of immunoglobulin, with only a minimal amount of unbound “free” light chains and then released in small quantities into the blood circulation. The FLC are then actively cleared by the kidneys as they are filtered through the glomerulus and reabsorbed in the proximal tubule to be broken down into amino acids which can be removed from the body safely.[5] In myeloma patients, the clonal proliferation of plasma cells can produce FLC in quantities which exceed the normal range by hundreds to thousand fold.[5-8] This overwhelms the proximal tubule’s re-absorption capacity and the excess FLC are transferred to the distal tubules. The FLC bind to a specific peptide on the Tamm-horsfall proteins where they aggregate and form casts causing cast nephropathy.[9-11] Renal failure in myeloma patients can result from tubular obstruction by cast nephropathy, as well as by a severe tubulo-interstitial inflammatory process, as serum FLC can also promote proinflammatory cytokines.[12,13]
At diagnosis approximately 50% of patients with MM have already developed renal failure. Approximately 10% of these patients will either be dialysis-dependent or require dialysis.9 Patients with multiple myeloma associated with renal failure generally have a worse prognosis due to a more aggressive disease and a reduced quality of life caused by increased complications.[1,14] Therefore, it is especially important in myeloma patients to find methods to reduce FLC levels in the body.
In various trials it has been demonstrated that achieving an early reduction in serum FLC levels allows renal recovery in a high proportion of MM patients and improves patient outcome.[15,16] Different strategies have been tried to achieve an early reduction of serum FLC levels in MM patients. These include; plasma exchange, conventional dialysis and extended haemodialysis. Both plasma exchange and conventional dialysis may not be sufficient enough to achieve sustained removal of FLC. Studies have found that they might only remove intravascular FLC, whereas it is necessary for both intra and extra vascular FLC to be removed in order to prevent the levels of FLC re-balancing.[11,17,18] However, extended haemodialysis using high cut-off haemodialysis (HCO-HD) in combination with chemotherapy can provide good FLC reduction. HCO membranes have large pores, with a cut off of 45kD. This provides good filtration of the kappa and lambda light chains which have molecular weights of 22kD and 45kD respectively.[19,20] It is also theorized that HCO-HD removes both intra and extravascular FLC and as a result is able to remove a much greater proportion of FLC than other methods.[11,21]
The aim of this research was to observe the effectiveness of HCO-HD in addition to chemotherapy, in managing myeloma nephropathy in an acute setting in patients with multiple myeloma.
This study was approved by the Tasmanian Human Ethics Committee, Australia. It was registered in the Australian and New Zealand Clinical Trials Registry at http://www.ANZCTR.org.au under ACTRN 12612000024842.
Case Series
In our study, four cases of MM nephropathy were treated with HCO-HD and chemotherapy at a single institution during the period from August 2009 to August 2011. All of the patients presented with acute renal failure and high serum FLC. All patients underwent a bone marrow biopsy to confirm the diagnosis of MM, according to the WHO criteria. Three of the cases were de-novo MM; the fourth case was relapsed MM. The patients were followed up for a median period of 26 months (range, 13-36). Male to female ratio was 1:1.
The first case (patient 1) was a 65 year old female, initially admitted with acute renal impairment and hypocalcaemia. Upon admission her creatinine was 610 μmol/L (Table 1), with a corrected calcium level of 2.99mmol/L. Her urine Bence Jones protein (BJP) showed positive and a skeletal survey showed lytic lesions at the proximal humerus and scalp. A serum electrophoresis was completed and showed elevated IgA levels of 10.3g/L and serum FLC of 7220 mg/L (Lambda), (Table 1). A bone marrow biopsy revealed a high plasma infiltration of >80%.
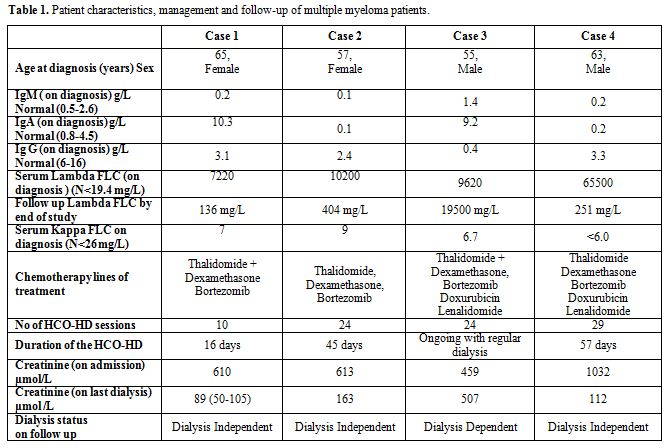
Table 1. Patient characteristics, management and follow-up of multiple myeloma patients.
Patient 1 was commenced on thalidomide and dexamethasone, however when this did not achieve significant improvement of renal function, thalidomide was replaced by lenalidomide, and the patient was commenced on HCO-HD with baseline serum FLC of 1100 mg/L. As a result of the treatment the patient’s serum free light chain level (lambda) decreased (Figure 1.a) and her renal function significantly improved upon completion of HCO-HD. Patient 1 became thereafter dialysis-independent.
Patient 2 was a 57 year old female who presented with acute oliguric renal failure. The patient had a creatinine of 613μmol/L and her urine BJP tested positive with lambda light chains detected.
The patient’s serum paraprotein concentration by electrophoresis was 2g/L however her serum Lambda FLC by free-lite assay was very high at 10200 mg/L (Table 1). A bone marrow aspiration revealed 90% plasma cell infiltration.
Patient 2 started treatment with bortezomib (1.3mg on days 1, 4, 8 and 11), dexamethasone (40mg on days 1-4 fortnightly). She was then commenced on HCO-HD and showed improved renal function, urine output and serum FLC (Figure 1.b). Patient 2 became dialysis-independent on discharge from hospital.
Patient 3 was a 55 year old male who was diagnosed with MM in 2005 and presented to Launceston General Hospital with acute renal failure in 2010. The patient had a creatinine level of 459 μmol/L and a serum lambda FLC level of 9620 mg/L. A repeated bone marrow biopsy confirmed progressive MM. Initially the patient’s myeloma treatment was thalidomide and dexamethasone but was changed to modified VAD (Velcade, Adriamycin and Dexamethasone) upon admission. Patient 3 was also commenced on HCO-HD but his Lambda FLC levels never dropped below 1000 mg/L (Figure 1.c). Despite several sessions of HCO-HD and a change in his chemotherapy regimen the patient remained dialysis dependant. Patient 3 was subsequently discharged from hospital with a cessation of HCO-HD and with maintenance of FLC levels with conventional dialysis in the community.
Patient 4 was a 63 year old male who presented with acute renal failure. The patient’s creatinine was 1032 μmol/L. On his second day of admission the patient was commenced on conventional haemodialysis, as his serum lambda FLC level was found to be 65500 mg/L and a bone marrow aspiration showed 40% plasma infiltration.
The patient was commenced on a treatment with thalidomide and dexamethasone in addition to the HCO-HD for up to 57 days post presentation. After two weeks, the chemotherapy was changed to modified VAD chemotherapy as the patient had a poor response to the original treatment. Patient 4 achieved dialysis-independency with a combination of chemotherapy and HCO-HD and he is now on maintenance therapy with lenalidomide.
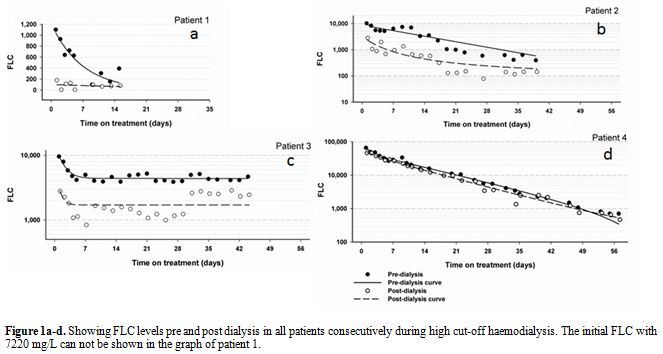
Figure 1a-d. Showing FLC levels pre and post dialysis in all patients consecutively during high cut-off haemodialysis. The initial FLC with 7220 mg/L can not be shown in the graph of patient 1.
Discussion
The concept of HCO-HD is based on a new technology of protein permeable dialyzers which efficiently remove FLC molecules.[18-21] The new haemodialysis membrane has a molecular weight cut-off similar to that of the normal kidney (approximately 65 kD).[18] On the other hand, conventional dialysis is limited to remove only large uraemic toxins by their specific molecular weight cut-off. The recent introduction of the HCO-HD technology allows clinicians to use these membranes for the treatment of MM nephropathy with efficient elimination of high cut-off FLC molecular weight.[18-21] In contrast, previously described plasma exchange (PE) removes only intravascular FLC in significant quantities; however, shortly thereafter, there is a rapid accumulation of FLC from the extra-vascular compartment into the intra-vascular compartment.[24] Accordingly, the FLC levels return to their pre-treatment baseline without change.[24] In contrast, HCO-HD allows an extended treatment, with subsequent redistribution of extra-vascular FLC into the intravascular compartment. By using this method, there are no significant additional side effects recorded in our cohort beyond the conventional haemodialysis. However, at least, in an ex-vivo study, there might be an increased plasma protein loss including albumin, coagulation factors, growth factors and hormones.[25] Therefore, specific trials to examine this theory are warranted.
Our study was conducted under the assumption that; HCO-HD was an efficient way to reduce FLC concentration in MM patients; HCO-HD is more effective when the patients are diagnosed and treated early; and that chemotherapy is a necessary part of the treatment therapy.[2,11]
Our study showed that HCO-HD is an effective means of reducing FLC concentration in MM patients, as all of the patients, except for patient 3, showed a large decrease in their FLC concentration and improved renal function as a result of the HCO-HD. Patient 3 may not have shown the same results because he had relapsed MM and therefore HCO-HD and salvage treatment did not achieve a favorable outcome. From this a further point can be drawn that patients who achieved a low FLC level and restored their renal function early on during the treatment of MM had a better chance of becoming dialysis-independent.[2,11]
Patients 1, 2 and 4 had de-novo myeloma and when treated they became dialysis-independent. Patient 3 had relapsed MM and showed little response to the treatment. Patient 3 remained dialysis-dependent. This may be due to established myeloma nephropathy and possibly more renal damage, such as scarring and possible fibrosis to the kidneys and in particular the nephrons. This highlights that early treatment provides a greater likelihood of the patient becoming dialysis-independent.
This study demonstrated that the FLC concentration would rebound on successive days unless the chemotherapy was effective. Consequently whilst HCO-HD is effective in managing myeloma nephropathy, chemotherapy is also an important component of the treatment as it treats the actual disease. The chemotherapy treatment must be suited to the individual or it will not work as well. Patient 2 initially started with thalidomide and dexamethasone but showed little to no results, the chemotherapy was changed to a bortezomib containing regimen and thereafter the patient’s response was dramatically improved. This may be explained by the fact that different therapies have different effects on the myeloma kidney disease. For example bortezomib inhibits the 26S proteasome inhibitor and works down that pathway.[14,22] Studies have also found that bortezomib is ideal for patients with renal injury because it reduces the serum cystatin-C level and also interferes with active nuclear factor kappa-B in renal epithelial cells and hence improves renal disease.[11,14] Other drugs may not work in this way. Thus it is important to use the most appropriate course of therapy for the individual patient.
Our study also showed that HCO-HD is effective when combined with specific myeloma therapy, especially bortezomib. Without long-term effective control of MM with proper chemotherapy, patients with renal failure will inevitably have a progression of their renal nephropathy because the underlying disease has not been addressed. Thus, mechanical removal of serum FLC through HCO-HD only, is not enough without specific myeloma therapy. When HCO-HD is coupled with other therapies it can be used to manage the disease and its effects; hence improving patient’s survival. Other studies have also found that overall survival increased, from no less than 2 years to 7 years with the introduction of novel therapies.[11] An increased efficiency of HCO-HD could further increase the overall survival while improving quality of life, as recent studies emphasise the importance of this aspect of the treatment.[23]
Multiple myeloma associated with some degree of renal impairment is responsible for approximately 1% of malignancies, 13% of these malignancies being haematological malignancies.[11] Therefore, it is important that patients with unexplained renal failure undergo specific investigations to exclude MM, especially with the easily performed serum FLC test among other tests. If the nephropathy can be linked to MM, specific treatment for MM with HCO-HD and chemotherapy should start without delay. This would result in a favourable outcome as we demonstrated in our study.
The outcome of our study is consistent with findings from other studies.[14-16] However, in our cohort of patients we found that early reduction of serum FLC led to an increased likelihood of the patient becoming dialysis-independent, with HCO-HD combined with chemotherapy being most effective. The favourable results demonstrated in cases 1, 2 and 4 should be interpreted carefully, as employing bortezomib is associated with favourable outcome in cases of myeloma nephropathy.[11] However this was not the case in patient 3. It was most likely the combination of HCO-HD and bortezomib, which produced the favourable outcomes in patients 1, 2 and 4.
Conclusion
Application of HCO-HD should be considered as early as possible once the diagnosis of myeloma nephropathy has been established. At the same time, a specific myeloma treatment should be initiated for each patient in order to salvage kidney function, achieve dialysis-independency and to gain good control of the disease. This may increase the chances of kidney injury being reversed and the patient becoming dialysis-independent. Accordingly, this should improve the patient’s survival and quality of life. Perhaps prospective randomised controlled trials with a larger number of patients addressing the efficacy of HCO-HD in conjunction with various treatments of MM are required.
Multiple myeloma (MM) is a haematological malignancy most commonly associated with some degree of renal impairment and possibly acute kidney injury.[1] The kidney injury in MM is more often caused by monoclonal free light chains (FLC) which lead to cast nephropathy.[2-4] In a normal subject the immunoglobulin FLC are the by-products of immunoglobulin synthesis. The FLC are bound to the heavy chains of immunoglobulin, with only a minimal amount of unbound “free” light chains and then released in small quantities into the blood circulation. The FLC are then actively cleared by the kidneys as they are filtered through the glomerulus and reabsorbed in the proximal tubule to be broken down into amino acids which can be removed from the body safely.[5] In myeloma patients, the clonal proliferation of plasma cells can produce FLC in quantities which exceed the normal range by hundreds to thousand fold.[5-8] This overwhelms the proximal tubule’s re-absorption capacity and the excess FLC are transferred to the distal tubules. The FLC bind to a specific peptide on the Tamm-horsfall proteins where they aggregate and form casts causing cast nephropathy.[9-11] Renal failure in myeloma patients can result from tubular obstruction by cast nephropathy, as well as by a severe tubulo-interstitial inflammatory process, as serum FLC can also promote proinflammatory cytokines.[12,13]
At diagnosis approximately 50% of patients with MM have already developed renal failure. Approximately 10% of these patients will either be dialysis-dependent or require dialysis.9 Patients with multiple myeloma associated with renal failure generally have a worse prognosis due to a more aggressive disease and a reduced quality of life caused by increased complications.[1,14] Therefore, it is especially important in myeloma patients to find methods to reduce FLC levels in the body.
In various trials it has been demonstrated that achieving an early reduction in serum FLC levels allows renal recovery in a high proportion of MM patients and improves patient outcome.[15,16] Different strategies have been tried to achieve an early reduction of serum FLC levels in MM patients. These include; plasma exchange, conventional dialysis and extended haemodialysis. Both plasma exchange and conventional dialysis may not be sufficient enough to achieve sustained removal of FLC. Studies have found that they might only remove intravascular FLC, whereas it is necessary for both intra and extra vascular FLC to be removed in order to prevent the levels of FLC re-balancing.[11,17,18] However, extended haemodialysis using high cut-off haemodialysis (HCO-HD) in combination with chemotherapy can provide good FLC reduction. HCO membranes have large pores, with a cut off of 45kD. This provides good filtration of the kappa and lambda light chains which have molecular weights of 22kD and 45kD respectively.[19,20] It is also theorized that HCO-HD removes both intra and extravascular FLC and as a result is able to remove a much greater proportion of FLC than other methods.[11,21]
The aim of this research was to observe the effectiveness of HCO-HD in addition to chemotherapy, in managing myeloma nephropathy in an acute setting in patients with multiple myeloma.
This study was approved by the Tasmanian Human Ethics Committee, Australia. It was registered in the Australian and New Zealand Clinical Trials Registry at http://www.ANZCTR.org.au under ACTRN 12612000024842.
Case Series
In our study, four cases of MM nephropathy were treated with HCO-HD and chemotherapy at a single institution during the period from August 2009 to August 2011. All of the patients presented with acute renal failure and high serum FLC. All patients underwent a bone marrow biopsy to confirm the diagnosis of MM, according to the WHO criteria. Three of the cases were de-novo MM; the fourth case was relapsed MM. The patients were followed up for a median period of 26 months (range, 13-36). Male to female ratio was 1:1.
The first case (patient 1) was a 65 year old female, initially admitted with acute renal impairment and hypocalcaemia. Upon admission her creatinine was 610 μmol/L (Table 1), with a corrected calcium level of 2.99mmol/L. Her urine Bence Jones protein (BJP) showed positive and a skeletal survey showed lytic lesions at the proximal humerus and scalp. A serum electrophoresis was completed and showed elevated IgA levels of 10.3g/L and serum FLC of 7220 mg/L (Lambda), (Table 1). A bone marrow biopsy revealed a high plasma infiltration of >80%.

Table 1. Patient characteristics, management and follow-up of multiple myeloma patients.
Patient 1 was commenced on thalidomide and dexamethasone, however when this did not achieve significant improvement of renal function, thalidomide was replaced by lenalidomide, and the patient was commenced on HCO-HD with baseline serum FLC of 1100 mg/L. As a result of the treatment the patient’s serum free light chain level (lambda) decreased (Figure 1.a) and her renal function significantly improved upon completion of HCO-HD. Patient 1 became thereafter dialysis-independent.
Patient 2 was a 57 year old female who presented with acute oliguric renal failure. The patient had a creatinine of 613μmol/L and her urine BJP tested positive with lambda light chains detected.
The patient’s serum paraprotein concentration by electrophoresis was 2g/L however her serum Lambda FLC by free-lite assay was very high at 10200 mg/L (Table 1). A bone marrow aspiration revealed 90% plasma cell infiltration.
Patient 2 started treatment with bortezomib (1.3mg on days 1, 4, 8 and 11), dexamethasone (40mg on days 1-4 fortnightly). She was then commenced on HCO-HD and showed improved renal function, urine output and serum FLC (Figure 1.b). Patient 2 became dialysis-independent on discharge from hospital.
Patient 3 was a 55 year old male who was diagnosed with MM in 2005 and presented to Launceston General Hospital with acute renal failure in 2010. The patient had a creatinine level of 459 μmol/L and a serum lambda FLC level of 9620 mg/L. A repeated bone marrow biopsy confirmed progressive MM. Initially the patient’s myeloma treatment was thalidomide and dexamethasone but was changed to modified VAD (Velcade, Adriamycin and Dexamethasone) upon admission. Patient 3 was also commenced on HCO-HD but his Lambda FLC levels never dropped below 1000 mg/L (Figure 1.c). Despite several sessions of HCO-HD and a change in his chemotherapy regimen the patient remained dialysis dependant. Patient 3 was subsequently discharged from hospital with a cessation of HCO-HD and with maintenance of FLC levels with conventional dialysis in the community.
Patient 4 was a 63 year old male who presented with acute renal failure. The patient’s creatinine was 1032 μmol/L. On his second day of admission the patient was commenced on conventional haemodialysis, as his serum lambda FLC level was found to be 65500 mg/L and a bone marrow aspiration showed 40% plasma infiltration.
The patient was commenced on a treatment with thalidomide and dexamethasone in addition to the HCO-HD for up to 57 days post presentation. After two weeks, the chemotherapy was changed to modified VAD chemotherapy as the patient had a poor response to the original treatment. Patient 4 achieved dialysis-independency with a combination of chemotherapy and HCO-HD and he is now on maintenance therapy with lenalidomide.

Figure 1a-d. Showing FLC levels pre and post dialysis in all patients consecutively during high cut-off haemodialysis. The initial FLC with 7220 mg/L can not be shown in the graph of patient 1.
Discussion
The concept of HCO-HD is based on a new technology of protein permeable dialyzers which efficiently remove FLC molecules.[18-21] The new haemodialysis membrane has a molecular weight cut-off similar to that of the normal kidney (approximately 65 kD).[18] On the other hand, conventional dialysis is limited to remove only large uraemic toxins by their specific molecular weight cut-off. The recent introduction of the HCO-HD technology allows clinicians to use these membranes for the treatment of MM nephropathy with efficient elimination of high cut-off FLC molecular weight.[18-21] In contrast, previously described plasma exchange (PE) removes only intravascular FLC in significant quantities; however, shortly thereafter, there is a rapid accumulation of FLC from the extra-vascular compartment into the intra-vascular compartment.[24] Accordingly, the FLC levels return to their pre-treatment baseline without change.[24] In contrast, HCO-HD allows an extended treatment, with subsequent redistribution of extra-vascular FLC into the intravascular compartment. By using this method, there are no significant additional side effects recorded in our cohort beyond the conventional haemodialysis. However, at least, in an ex-vivo study, there might be an increased plasma protein loss including albumin, coagulation factors, growth factors and hormones.[25] Therefore, specific trials to examine this theory are warranted.
Our study was conducted under the assumption that; HCO-HD was an efficient way to reduce FLC concentration in MM patients; HCO-HD is more effective when the patients are diagnosed and treated early; and that chemotherapy is a necessary part of the treatment therapy.[2,11]
Our study showed that HCO-HD is an effective means of reducing FLC concentration in MM patients, as all of the patients, except for patient 3, showed a large decrease in their FLC concentration and improved renal function as a result of the HCO-HD. Patient 3 may not have shown the same results because he had relapsed MM and therefore HCO-HD and salvage treatment did not achieve a favorable outcome. From this a further point can be drawn that patients who achieved a low FLC level and restored their renal function early on during the treatment of MM had a better chance of becoming dialysis-independent.[2,11]
Patients 1, 2 and 4 had de-novo myeloma and when treated they became dialysis-independent. Patient 3 had relapsed MM and showed little response to the treatment. Patient 3 remained dialysis-dependent. This may be due to established myeloma nephropathy and possibly more renal damage, such as scarring and possible fibrosis to the kidneys and in particular the nephrons. This highlights that early treatment provides a greater likelihood of the patient becoming dialysis-independent.
This study demonstrated that the FLC concentration would rebound on successive days unless the chemotherapy was effective. Consequently whilst HCO-HD is effective in managing myeloma nephropathy, chemotherapy is also an important component of the treatment as it treats the actual disease. The chemotherapy treatment must be suited to the individual or it will not work as well. Patient 2 initially started with thalidomide and dexamethasone but showed little to no results, the chemotherapy was changed to a bortezomib containing regimen and thereafter the patient’s response was dramatically improved. This may be explained by the fact that different therapies have different effects on the myeloma kidney disease. For example bortezomib inhibits the 26S proteasome inhibitor and works down that pathway.[14,22] Studies have also found that bortezomib is ideal for patients with renal injury because it reduces the serum cystatin-C level and also interferes with active nuclear factor kappa-B in renal epithelial cells and hence improves renal disease.[11,14] Other drugs may not work in this way. Thus it is important to use the most appropriate course of therapy for the individual patient.
Our study also showed that HCO-HD is effective when combined with specific myeloma therapy, especially bortezomib. Without long-term effective control of MM with proper chemotherapy, patients with renal failure will inevitably have a progression of their renal nephropathy because the underlying disease has not been addressed. Thus, mechanical removal of serum FLC through HCO-HD only, is not enough without specific myeloma therapy. When HCO-HD is coupled with other therapies it can be used to manage the disease and its effects; hence improving patient’s survival. Other studies have also found that overall survival increased, from no less than 2 years to 7 years with the introduction of novel therapies.[11] An increased efficiency of HCO-HD could further increase the overall survival while improving quality of life, as recent studies emphasise the importance of this aspect of the treatment.[23]
Multiple myeloma associated with some degree of renal impairment is responsible for approximately 1% of malignancies, 13% of these malignancies being haematological malignancies.[11] Therefore, it is important that patients with unexplained renal failure undergo specific investigations to exclude MM, especially with the easily performed serum FLC test among other tests. If the nephropathy can be linked to MM, specific treatment for MM with HCO-HD and chemotherapy should start without delay. This would result in a favourable outcome as we demonstrated in our study.
The outcome of our study is consistent with findings from other studies.[14-16] However, in our cohort of patients we found that early reduction of serum FLC led to an increased likelihood of the patient becoming dialysis-independent, with HCO-HD combined with chemotherapy being most effective. The favourable results demonstrated in cases 1, 2 and 4 should be interpreted carefully, as employing bortezomib is associated with favourable outcome in cases of myeloma nephropathy.[11] However this was not the case in patient 3. It was most likely the combination of HCO-HD and bortezomib, which produced the favourable outcomes in patients 1, 2 and 4.
Conclusion
Application of HCO-HD should be considered as early as possible once the diagnosis of myeloma nephropathy has been established. At the same time, a specific myeloma treatment should be initiated for each patient in order to salvage kidney function, achieve dialysis-independency and to gain good control of the disease. This may increase the chances of kidney injury being reversed and the patient becoming dialysis-independent. Accordingly, this should improve the patient’s survival and quality of life. Perhaps prospective randomised controlled trials with a larger number of patients addressing the efficacy of HCO-HD in conjunction with various treatments of MM are required.
References
- Lameire NH,
Flombaum CD, Moreau D, Ronco C. Acute renal failure in cancer patients.
Ann Med. 2005; 37:13-25. http://dx.doi.org/10.1080/07853890510007205
PMid:15902843

- Irish AB, Winearls
CG, Littlewood T.
Presentation and survival of patients with severe renal failure and
myeloma. QJM . 1997; 90:773-80. http://dx.doi.org/10.1093/qjmed/90.12.773
PMid:9536342

- Johnson WJ, Kyle
RA, Pineda AA, O'Brien PC,
Holley KE. Treatment of renal failure associated with multiple myeloma.
Plasmapheresis, haemodialysis, and chemotherapy. Arch Intern Med. 1990;
150:863-9. http://dx.doi.org/10.1001/archinte.1990.00390160111022
PMid:2183734

- Pozzi C, Pasquali
S, Donini U, Casanova S,
Banfi G, Tiraboschi G, Furci L, Porri MT, Ravelli M, Lupo A, et al.
Prognostic factors and effectiveness of treatment in acute renal
failure due to multiple myeloma: a review of 50 cases. Report of the
Italian Renal Immunopathology Group. Clin Nephrol. 1987;
28:1-9.
PMid:3621685

- Clark AD, Shetty
A, Soutar R. Renal failure
and multiple myeloma: pathogenesis and treatment of renal failure and
management of underlying myeloma. Blood Rev. 1999; 13:79-90. http://dx.doi.org/10.1016/S0268-960X(99)90014-0

- Bradwell AR,
Carr-Smith HD, Mead GP, Harvey
TC, Drayson MT. Serum test for assessment of patients with Bence Jones
myeloma. Lancet. 2003; 361:489-91. http://dx.doi.org/10.1016/S0140-6736(03)12457-9

- Kaplan B, Livneh
A, Sela BA. Immunoglobulin
free light chain dimers in human diseases. TheScientificWorldJournal.
2011; 11:726-35. http://dx.doi.org/10.1100/tsw.2011.65
PMid:21442150

- Solomon A. Light
chains of human immunoglobulins. Meth Enzymol. 1985; 116:101-21. http://dx.doi.org/10.1016/S0076-6879(85)16008-8

- Darmon M, Ciroldi
M, Thiery G, Schlemmer B,
Azoulay E. Clinical Review: Specific aspects of acute renal failure in
cancer patients. Critical Care. 2006; 10:211. http://dx.doi.org/10.1186/cc4907
PMid:16677413 PMCid:1550893

- Hayne N, Denecke
B, Guthoff M, Oehrlein K,
Kanz L, Haring HU, Weisel KC. Extracorporeal light chain elimination.
Ann Hematol. 2012; 91:729-35. PMid:22170517

- Gaballa MR,
laubach JP, Schlossman RL,
Redman K, Noonan K, Mitsiades CS, Ghobrial IM, Munshi N, Anderson KC,
Richardson PG. Management of myeloma-associated renal
dysfunction
in the era of novel therapies. Expert Reviews Haematology. 2012;
5:51-68. http://dx.doi.org/10.1586/ehm.11.72
PMid:22272706

- Li M,
Hering-Smith KS, Simon EE, et al:
Myeloma light chains induce epithelial-mesenchymal transition in human
renal proximal tubule epithelial cells. Nephrol Dial Transplant. 2008;
23:860-70. http://dx.doi.org/10.1093/ndt/gfm670
PMid:17933841

- Arimura A, Li M,
Batuman V. Potential
protective action of pituitary adenylate cyclase-activating polypeptide
(PACAP38) on in vitro and in vivo models of myeloma kidney injury.
Blood. 2006; 107:661-8. http://dx.doi.org/10.1182/blood-2005-03-1186
PMid:16204306

- Ying WZ, Wang PX,
Aaron KJ, Batuman V.
Immunoglobulin light chains activate nuclear factor-{kappa} B in renal
epithelial cells through a Src-dependent mechanism. Blood. 2011;
117:1301-7. http://dx.doi.org/10.1182/blood-2010-08-302505
PMid:21098396 PMCid:3056473

- Leung N, Gertz
MA, Zeldenrust SR, Rajkumar
SV, Dispenzieri A, Fervenza FC, Kumar S, Lacy MQ, Lust JA, Greipp PR,
Witzig TE, Hayman SR, Russell SJ, Kyle RA, Winters JL. Improvement of
cast nephropathy with plasma exchange depends on the diagnosis and on
reduction of serum free light chains. Kidney Int. 2008; 73:1282-8.
http://dx.doi.org/10.1038/ki.2008.108 PMid:18385667

- Hutchison CA,
Bradwell AR, Cook M,
Basnayake K, Basu S, Harding S, Hattersley J, Evans ND, Chappel MJ,
Sampson P, Foggensteiner L, Adu D, Cockwell P. Treatment of acute renal
failure secondary to multiple myeloma with chemotherapy and extended
high cut-off Haemodialysis. Clin J Am Soc Nephrol. 2009; 4:745-54. http://dx.doi.org/10.2215/CJN.04590908
PMid:19339414 PMCid:2666427

- Cserti C, Haspel
R, Stowell C, Dzik W.
Light-chain removal by plasmapheresis in myeloma-associated renal
failure. Transfusion. 2007; 47:511-4. http://dx.doi.org/10.1111/j.1537-2995.2006.01143.x
PMid:17319833

- Hutchison CA,
Cockwell P, Reid S, Chandler
K, Mead GP, Harrison J, Hattersley J, Evans ND, Chappell MJ, Cook M,
Goehl H, Storr M, Bradwell AR. Efficient removal of immunoglobulin free
light chains by haemodialysis for multiple myeloma: in vitro and in
vivo studies. J Am Soc Nephrol. 2007; 18:886-95. http://dx.doi.org/10.1681/ASN.2006080821
PMid:17229909

- Basnayake K,
Hutchison C, Kamel D, Sheaff
M, Ashman N, Cook M, Oakervee H, Bradwell A, Cockwell P. Resolution of
cast nephropathy following free light chain removal by haemodialysis in
a patient with multiple myeloma: a case report. J Med Case Reports.
2008; 2:380. http://dx.doi.org/10.1186/1752-1947-2-380
PMid:19068112 PMCid:2630327

- Hutchison CA,
Harding S, Mead G, Goehl H,
Storr M, Bradwell A, Cockwell P. Serum free-light chain removal by high
cut-off haemodialysis: optimizing removal and supportive care. Artif
Organs. 2008; 32:910-7. http://dx.doi.org/10.1111/j.1525-1594.2008.00653.x
PMid:19133018

- Gakhar B, Kobrin
S, Berns JS. Extracorporeal treatment of cast nephropathy. Seminars in
dialysis. 2011; 24:9-11. http://dx.doi.org/10.1111/j.1525-139X.2010.00814.x
PMid:21324000

- Khalafallah A,
Maiwald M, Cox A, Burns D,
Bates G, Hannan T, Seaton D, Fernandopulle B, Meagher D, Brain T.Effect
of immunoglobulin therapy on the rate of infections in multiple myeloma
patients undergoing autologous stem cell transplantation or treated
with immunomodulatory agents. Mediterr J Hematol Infect Dis. 2010 Apr
20;2(1):e2010005. PMid:21415947 PMCid:3033112. http://dx.doi.org/10.4084/mjhid.2010.005

- Khalafallah A,
McDonnell K, Dawar HU,
Robertson I, Woods D. Quality of life assessment in multiple myeloma
patients undergoing dose-reduced tandem autologous stem cell
transplantation. Mediterr J Hematol Infect Dis. 2011; 3(1):e2011057.
Epub 2011 Nov 28. http://dx.doi.org/10.4084/mjhid.2011.057
PMid: 22220254 PMCid:3248334

- Clark WF, Stewart
AK, Rock GA, Sternbach
M, Sutton DM, Barrett BJ, Heidenheim AP, Garg AX, Churchill DN;
Canadian Apheresis Group. Plasma exchange when myeloma presents as
acute renal failure: a randomized, controlled trial. Ann Intern Med.
2005; 143:777-84.

- Lee WCR, Uchino
S, Fealy N, Baldwin I,
Panagiotopoulos S, Goehl H, Morgera S, Neumayer HH, Bellomo R. Super
high-flux hemodialysis at high dialysate flows: an ex vivo assessment.
J Artif Organs2004;27:24-28. PMid:22697381
