Received: November 26, 2013
Accepted: November 11, 2013
Meditter J Hematol Infect Dis 2014, 6(1): e2014003, DOI 10.4084/MJHID.2014.003
This article is available on PDF format at:

Massimo Breccia and Giuliana Alimena
Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited. |
|
Abstract With the advent of target therapies, imatinib became the mainstay for treatment of chronic myeloid leukemia. However, despite the brilliant results obtained with this drug, more than 30% of patients discontinue therapy in long-term due to several reasons, including failure and/or intolerance. Second-generation tyrosine kinase inhibitors (TKIs) are more potent drugs and have expanded inhibition against a broad spectrum of mutations resistant to imatinib. Both nilotinib and dasatinib have demonstrated in vitro and in vivo clinical activity against different types of mutations and various forms of resistance. However, patients with T315I mutation do not obtain an advantage from these drugs and a third generation inhibitor ponatinib, a pan-BCR drug, was tested with significant results. In this review, we report the results of second- and third-generation TKIs tested as second or third line therapy in patients resistant and/or intolerant to previous inhibitors. |
Introduction
Although
the use of standard dose imatinib as first therapeutic strategy has
dramatically changed the outcome of chronic myeloid leukemia patients
in chronic phase, one third of patients does not achieve an optimal
outcome and requires alternative therapies due to the emergence of drug
resistance. Eight-year follow-up of the international IRIS study showed
85% overall survival (OS) rate, but 30% of patients had unfavourable
outcome, mostly due to primary (17%) or acquired resistance (15%).[1]
In 2006, the European LeukemiaNet (ELN) group published recommendations
and identified at 3, 6, 12 and 18 months different categories of
patients defined as optimal or suboptimal response or failure to
imatinib given as first line therapy.[2]
In 2013 the recommendations were updated[3]
and the category of suboptimal patients was excluded while resistant
patients were identified also based on early evaluation of molecular
response. Resistance mechanisms were studied first in vitro and then in
vivo, and allowed the development of second-generation tyrosine kinase
inhibitors. These agents were tested on a large series of patients
resistant and/or intolerant to imatinib, providing the basis for
prescription in second line.
In the present paper, we analyse the different options for patients
resistant or intolerant to TKIs through a review of second-generation
drugs trials performed in this category of subjects.
Dasatinib
Dasatinib is a second-generation BCR-ABL inhibitor that has 325-fold
higher potency in vivo with inhibitory activity against the majority of
imatinib-resistant BCR-ABL mutants. Several studies tested the efficacy
and safety of dasatinib as a second line therapy.[4]
Phase II START-C trial 5 evaluated dasatinib as a single agent at the
dose of 70 mg twice daily in 387 patients with resistance (75%) or
intolerance (25%) to imatinib. Fifty-five percent of patients had
received as prior therapy high doses of imatinib, and 10% of patients
were included after failure of bone marrow transplantation. Complete
hematologic remission (CHR) was achieved by 90% of patients; major
cytogenetic response (MCyR) was obtained in 62% of patients with 88% of
these maintaining the response at 24 months. Complete cytogenetic
response (CCyR) rate was 53%, and this response was maintained in 90%
of patients at 24 months. PFS at 2 years was 80%, and 2-year OS was
94%. Mutations of the kinase domain were detected at baseline in 44% of
enrolled patients, with high frequency of G250E and T315I mutations.
The presence of mutations at baseline, even if detected in the p-loop
region, did not influence overall response rate.[5]
A
subsequent sub-analysis was reported by Branford and colleagues on the
development of new detectable mutations in 479 patients treated with
dasatinib after imatinib failure: the results showed that the emergence
of new mutations, such as T315A, F317L, V299L, occurred in only 13% of
treated patients.[6] As regards
hematological adverse
events of grade 3/4 reported in the first 2 years, these consisted
predominantly of neutropenia (50%) and thrombocytopenia (49%).
Non-hematological adverse events observed in this trial consisted
prevalently of diarrhea, headache, rash and fatigue, but were of grade
3/4 in <5% of patients with a slight increase in the prevalence
between the first and the second year of follow-up. Pleural effusion
rate was 22%, but the majority of cases were grade 1/2 (grade 3 in less
than 10%, with no grade 4) and most occurred during the first two
years, of follow-up 7. Cross-intolerance between imatinib and dasatinib
was not evidenced in this trial.[5,7]
The START-R trial included imatinib-resistant patients that were
randomized, with a ratio of 2:1, to dasatinib 70 mg twice daily or
high-dose imatinib (800 mg daily).[8]
After 2-year
follow-up, CHR was obtained in 93% of patients treated with dasatinib
and in 82% of patients treated with high-dose imatinib. A higher MCyR
rate was observed among patients treated in the dasatinib arm than in
the imatinib arm (53% vs. 33%; p=0.017), with a CCyR rate of 44% and
18%, respectively. MCyR was maintained in 90% of patients in the
dasatinib arm and in 74% of patients in the high-dose imatinib arm.
Major molecular responses (MMR) were also more frequently seen in
patients treated with dasatinib than in those treated with high-dose
imatinib (29% vs. 12%). As in other trials, the most frequent grade 3/4
events with dasatinib was neutropenia, thrombocytopenia and leukopenia,
diarrhea, fatigue and headache.[8]
START-R study
results showed that second-generation drug represents a better choice
for resistant patients compared with dose escalation of imatinib.
START-A trial recruited 174 accelerated phase (AP) CML patients, with
the majority of them being resistant to imatinib. At a minimum
follow-up of 14 months, major hematologic response (MaHR) and CHR were
achieved in 64% and 45% of patients treated with dasatinib 70 mg twice
daily. MCyR and CCyR were obtained in 39% and 32% of patients,
respectively. No significant differences in terms of rate of responses
were observed among resistant or intolerant patients, previous stem
cell transplant or presence of baseline mutations. At a median
follow-up of 1 year, PFS and OS were 66% and 82%. Grade 3/4 neutropenia
and thrombocytopenia occurred in 76% and 82% of patients, respectively.
Diarrhoea occurred in 52% of patients with 8% being of grade 3/4,
whereas pleural effusion occurred in 27% of patients (5% as grade 3/4).[9]
Dasatinib was tested also in CML blast phase (BP) and Ph+ acute
lymphoblastic leukemia (ALL) (START-B and START-L trials); the former
enrolled 74 myeloid blast phase (MBP) and 42 lymphoid blast phase (LBP)
patients.[10] At 8-month follow-up
the rates of MaHR
were 34% and 31% in MBP and LBP, respectively; MCyR rates were 31% and
50%, while CCyR rates were 27% and 43%, respectively. More resistant
mutations (M244V, G250E, Y253H, E255K, E255V, T315I, F359V, H396R) were
associated with lower response rates to dasatinib. Among MBP patients,
the most frequently encountered AEs were diarrhoea (36%), pleural
effusion (28%, 14% as grade 3/4), peripheral oedema (19%), and dyspnoea
(18%). Common side effects observed in LBP patients were diarrhoea
(31%), fatigue (29%), nausea and vomiting (24%). The START-L trial
reported the results obtained in 36 Ph+ acute lymphoblastic leukemia
(ALL).[11] Out of 24 patients
(67%) who had achieved
a MaHR, 5 experienced disease progression and CCyR rate at 8-month
follow-up was 58%. No differences in response rates were revealed for
patients with resistant mutations compared to whole non-mutated
population: T315I mutation was found at baseline in 6 patients and was
associated with a worse response. The most frequently reported AEs of
any grade in ALL were diarrhoea (31%), pyrexia (25%), and nausea (22%),
whereas the most common grade 3/4 events, were febrile neutropenia
(11%), diarrhoea (8%), and asthenia (8%). The results of trials in an
advanced phase of disease showed that dasatinib is a valid option due
to its large spectrum of inhibition, even if in most of the patients
treated in blast crisis the responses were not long lasting.
CA180-034 study was an international, open-label, four-arm randomized
phase III study, which enrolled 662 patients resistant or intolerant to
prior imatinib therapy. Patients received 140 mg or 100 mg of
dasatinib, both administered in one (QD) or two daily (BID) doses.[12] Baseline features, as well as
outcomes at 72 months, were similar among the different arms.[13]
CHR was achieved in 92% of the 100 mg QD arm, in 88% of the 70 mg BID
arm, in 87% of the 140 mg QD arm and in 92% of the 50 mg BID arm. CCyR
was achieved in 50% and 53% of the 100 mg QD and 70 mg BID arms,
respectively, and in 50% and 49% of the 140 mg QD and 50 mg BID
cohorts, respectively. MMR rate was 45% in the 100 mg QD cohort at the
last follow-up of 72 months. OS was estimated to be 71% in the 100 mg
QD arm with a cumulative incidence of death due to CML of 12.5%. PFS in
the 100 mg QD arm was estimated to be 49% at 6 years. A comprehensive
sub-analysis, including patients treated in phase II/III trials because
resistant to imatinib, showed that the drug was active against several
ABL mutations. The results showed that the drug induced high
cytogenetic and molecular response rates in imatinib-resistant patients
with and without mutations at baseline (including mutations at residues
G250, M351, L248, Y253, E255, F359 and H396). However, mutations with
half maximal inhibitory concentration (IC50) > 3 nM obtained a
CCyR
rate of 32% and MMR rate of 23% compared to CCyR rate of 53% and MMR of
38% of patients with IC50 < 3 nM, with also different PFS, that
was
67% in IC50 > 3 nM group and 80% in IC50 < 3 nM group.[14]
A subsequent phase 3 study assessed safety and efficacy of two
different schedules (140 mg QD or 70 mg BID) of dasatinib in patients
in blast phase. A two-year follow-up showed that MaHRs were similar in
MBP treated with the two different schedules (28%), whereas for LBP the
response rate was 42% for 140 mg QD and 32% for 70 mg BID. MCyR rate
was 25% for 140 mg QD and 28% for 70 mg BID in MBP patients and
respective rates were 50% and 40% for LBP. Two-year OS rate with 140 mg
QD and with 70 mg BID was 24% and 28% in MBP patients, respectively and
21% and 16% in LBP, respectively. In this trial a trend of better
tolerability was reported among patients treated with 140 mg QD.[15]
Nilotinib
Nilotinib (Tasigna) was rationally designed to have enhanced
selectivity and potency toward BCR-ABL, with clinical activity against
the majority of resistant mutations to imatinib and was recommended as
second line therapy for CP and AP patients resistant or intolerant to a
previous imatinib.[16]
Nilotinib was tested in a phase II trial enrolling 321 CP patients with
resistance or intolerance to imatinib that received nilotinib at 400
BID. Results were reported at a median follow-up of 48 months: 94% of
patients reached CHR in a median time of 1 month and 59% of patients
achieved MCyR in a median time of 1.4 months, with 45% of these being
CCyR; 78% of patients achieving MCyR, maintained this response. MMR
rate was 28%, PFS was 57% and OS was 78%.[17]
Grade
3/4 neutropenia and thrombocytopenia occurred in 30% of patients; the
most frequent non haematological side effects reported were biochemical
abnormalities, including an increase of lipase/amylase, total bilirubin
and hyperglycemia; other non-hematologic side effects included skin
rash, nausea and headache. A mutational screening at baseline revealed
that 114 out of 281 tested patients (41%) presented a mutation. The
results of this sub-analysis showed that mutations with IC50 >
150
nM occurred in 14% of resistant patients and affected prevalently 3
amino acid residues (Y253H, E255K/V, F359C/V); 15% of patients had a
mutation with unknown IC50. After 12 months of therapy, MCyR rates of
mutated patients (both with IC50 < 150 nM and with unknown IC50)
were comparable to those of non-mutated patients (MCyR 49% vs 60%,
respectively). MCyR in mutated patients with IC50 > 150 nM was
less
favourable (19%). Disease progression rate was higher in mutated
patients when compared to that of patients without mutations (46% vs
26%); in patients with IC50 > 150 nM the rate was 69%.[18]
An expanded access characterized safety of nilotinib in a large series
of patients (1,422). In CP subjects, the CHR and CCyR rates were 43%
and 34%, respectively. Faster responses were detected in particular in
patients with sub-optimal response to imatinib, who reached 50% of
CCyR. The 18-month PFS was 80%. Non-hematologic side effects reported
were mild to moderate and included rash, headache, nausea and
elevations in serum bilirubin and lipase that occurred in 45 and 7% of
patients, respectively.[19] In the
same series of
patients, 181 were in accelerated phase and 190 in blast phase (133
myeloid, 50 lymphoid and 7 unknown). As regards toxicity,
myelosuppression was usually manageable with drug dose reductions or
temporary interruptions, and grade 3/4 increase in serum bilirubin and
lipase were infrequent.[20]
Nilotinib at the dose of 400 mg BID was tested in 105 MBP patients and
31 LBP: at 2-year follow-up, 60% of MBP and 59% of LBP had achieved a
MaHR and 38% and 52% had obtained an MCyR, respectively. CCyR rate was
30% and 32%, respectively. Two-year OS was 32% for MBP and 10% for LBP
patients. Fourteen patients underwent allogeneic stem cell transplant.
Hematological toxicity was frequent with grade 3/4 neutropenia,
thrombocytopenia and anemia occurring in 68%, 63% and 47%,
respectively. Laboratory abnormalities were common, with grade 3/4
hypophosphatemia being detected in 15%, hyperbilirubinemia in 11% and
lipase elevation in 11% of patients.[21]
Bosutinib
Bosutinib (or SKI-606) is an oral once-daily dual Src/ABL inhibitor
approved by FDA for the treatment of adult patients with CP, AP or BP
Ph+CML, resistant or intolerant to imatinib or second-generation TKIs.
It was tested in a phase I/II study, which enrolled 288 patients (200
resistant and 88 intolerant to imatinib) and the final dose of 500 mg
QD was found. After a median follow-up of 24 months, 86% of patients
achieved a CHR, 53% an MCyR (of them 41% had a CCyR). Among patients
with CCyR, 64% of imatinib-resistant and 65% of imatinib-intolerant
patients achieved MMR with CMR being obtained in 49% of
imatinib-resistant and 61% of imatinib-intolerant patients. Two-year OS
was 92%. Responses were seen across all types of mutants, with the
exception of T315I. Most frequent side effects reported were diarrhea,
rash and vomiting.[22]
Bosutinib was tested also in third line after imatinib and dasatinib
and/or nilotinib failure (37 patients resistant and 50 intolerant to
dasatinib; 27 patients resistant and 1 intolerant to nilotinib). The
study included 118 patients that, after a median follow-up of 28
months, achieved CHR with a rate of 73% and CCyR with a rate of 24%. An
MCyR was reported for 31% and 30% of dasatinib resistant and intolerant
patients, with 14% and 28% achieving a CCyR, respectively. An MCyR and
CCyR were observed in 35% and 27% of patients resistant to nilotinib.
After 2 years, PFS was 73% with a median OS estimated to be 83%. Also
in third line, bosutinib was able to overcome all types of mutations
(including mutations resistant to dasatinib and nilotinib, with the
most frequent being F317L, T351I, G250E and Y253H), except T315I.[23]
A recent sub-analysis of 119 patients aged over 65 years treated with
bosutinib was reported in comparison with 451 younger patients.[24]
Bosutinib was administered at the dose of 500 mg/day in 3 cohorts
consisting of CP patients after imatinib failure, CP patients after
imatinib and dasatinib or nilotinib failure and patients in advanced
phases of disease. Bosutinib was discontinued in 80% of patients aged
over 65 years compared to 67% of younger patients, in 32% of cases
owing to adverse events, mostly thrombocytopenia. Similar response
rates were obtained in older patients in terms of CHR in the first two
cohorts of patients (81% and 72%) and in terms of CCyR (38% and 23%).
Rate of disease transformation was similar between older and younger
patients. Two-year OS was 87% and 80% respectively in the two
categories of patients, whereas 2-year PFS was 76% and 70%. Incidence
of haematological side effects as well as of diarrhea was similar
between older and younger patients.
Bosutinib was evaluated also in 63 patients with AP, in 48 with BP and
in 23 with Ph+ALL. After a median follow-up of 8 months, 61% of AP
patients and 32% of BP achieved a CHR. MCyR and CCyR rates were 48% and
33% in AP patients and 52% and 29% in BP patients, respectively. MMR
and CMR rates were 15% and 4% in AP patients and 28% and 12% in BP
patients, respectively. Also in advanced phase of disease, bosutinib
was found to be active in mutated patients.[25]
Ponatinib
A third generation inhibitor was recently tested in resistant/intolerant CML patients: ponatinib is a potent, synthetic, oral multi-target pan-BCR/ABL inhibitor able to block native and mutated BCR/ABL, including T315I mutation, resistant to dasatinib and nilotinib. The results of a phase 1 dose-escalation study, which enrolled 81 patients (60 with CML), showed dose-limiting side effects, which included elevated lipase and amylase and pancreatitis. Of 43 chronic phase CML patients, 72% achieved an MCyR and 44% a MMR. Similar responses were obtained in patients without mutations and with T315I.[26] The PACE trial (Ponatinib Ph+ ALL and CML Evaluation), a phase 2 open-label trial, tested ponatinib 45 mg QD in 449 patients resistant or intolerant to dasatinib or nilotinib or with T315I mutation in different phases of the disease. Of the 444 patients included in the efficacy analysis, 267 were in CP, 83 in AP, 62 in MBP and 32 had Ph+ALL. A total of 128 patients were positive for T315I mutation. Primary endpoint was the achievement of MCyR. Overall rate of MCyR in CP patients was 54% with 44% of CCyR. Among these patients, 64 had a T315I mutation and 70% achieved an MCyR with 66% of CCyR. In AP and BP patients MaHR was evaluated as primary endpoint: overall, in AP patients the rate was 52% and of these 44% were CHR (39% in T315I mutated patients), whereas, in BP patients, the rate was 31% (29% in T315I patients). Safety evaluation revealed that the most common adverse events were hypertension, rash, abdominal pain, fatigue, headache, arterial thrombosis and hepatotoxicity. In particular 11% of patients experienced arterial thrombosis that was serious in 8% of instances. Congestive heart failure was recorded in 7% of patients, and fluid retention occurred in 23%, most commonly as peripheral edema and pleural/pericardial effusions. Other less common side effects recorded were pancreatitis (28 patients, 6%) and hemorrhagic events (24% of patients, in 5% as serious event).[27,28]
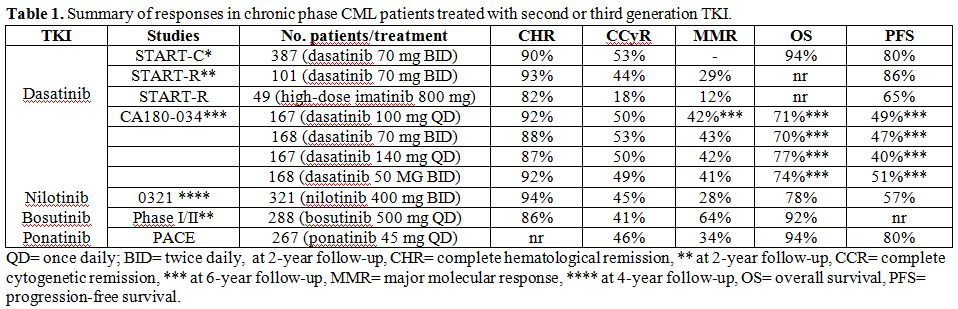 |
Table 1. Summary of responses in chronic phase CML patients treated with second or third generation TKI. |
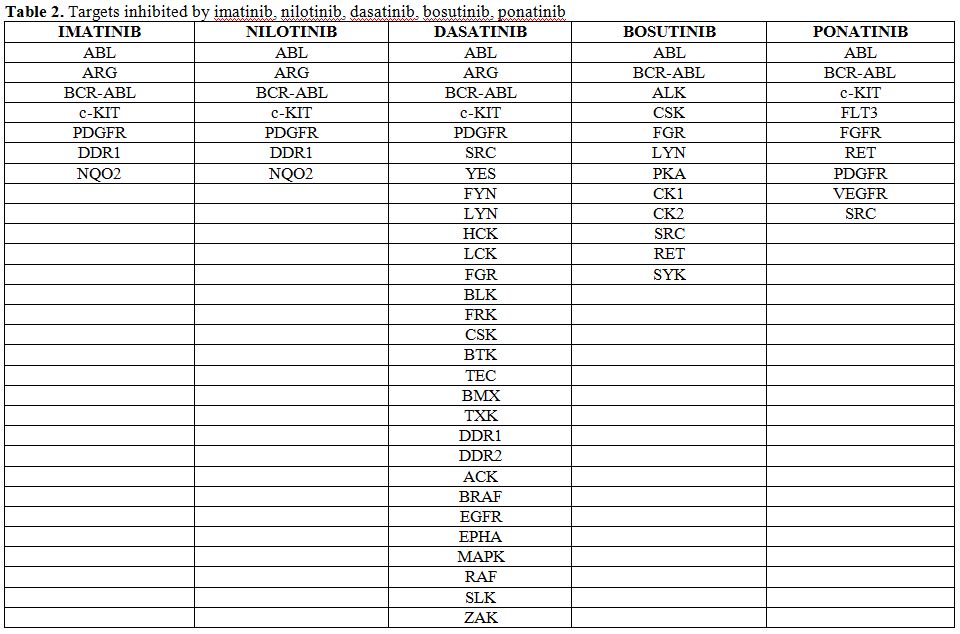 |
Table 2. Targets inhibited by imatinib, nilotinib, dasatinib, bosutinib, ponatinib. |
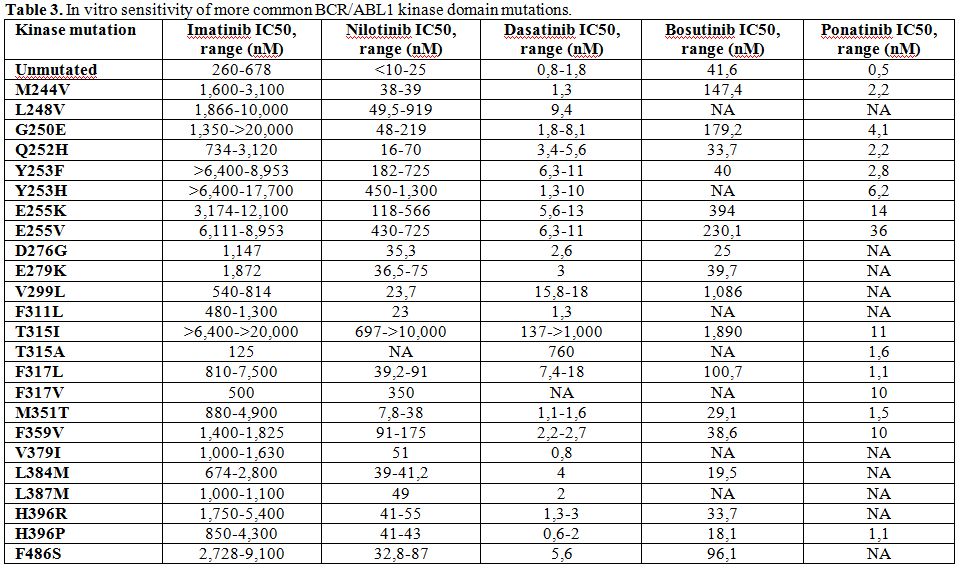 |
Table 3. In vitro sensitivity of more common BCR/ABL1 kinase domain mutations. |
Conclusion
Nilotinib and dasatinib tested as a second line after imatinib failure
and/or intolerance have been proven to be effective and safe drugs,
with the possibility of rescuing about 50% of patients with different
forms of resistance. Bosutinib was proven effective not only in the
second line, but also in patients resistant to previous therapy with
imatinib and nilotinib and/or dasatinib. All these drugs are less or
completely ineffective in patients carrying the T315I mutation. In this
latter subset a third-generation inhibitor, ponatinib, was tested with
brilliant results, and recently approved for patients with resistance
to previous TKIs lines. This agent allowed the rescue of the majority
of patients with T315I mutation with most of responses being
maintained. In the last edition of 2013 ELN recommendations, allogeneic
bone marrow transplant should be considered at the time of
starting second line after failure of any type of TKIs, whereas
it is recommended for patients after failure of two previous TKIs.
References























[TOP]