Received: November 30, 2013
Accepted: February 11, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014018, DOI 10.4084/MJHID.2014.018
This article is available on PDF format at:

Dilip Kumar Patel1, Manoj Kumar Mohapatra1, Ancil George Thomas1, Siris Patel2 and Prasanta Purohit2
1
Department of Medicine. Veer Surendra Sai Medical College, Burla,
Odisha, India.
2 Sickle Cell Clinic and Molecular Biology
Laboratory, Veer Surendra Sai Medical College, Burla, Odisha, India.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Sickle
cell anaemia (SCA) patients with vaso-occlusive crisis (VOC) have signs
of inflammation and it is often difficult to diagnose a bacterial
infection in them. This study was undertaken to evaluate the role of
serum procalcitonin (PCT) as a biomarker of bacterial infection in
acute sickle cell vaso-occlusive crisis. Hundred homozygous SCA
patients were studied at Sickle Cell Clinic and Molecular Biology
Laboratory, V.S.S. Medical College, Burla, Odisha, India. All the
patients were divided into three categories namely category-A (VOC/ACS
with SIRS but without evidence of bacterial infection - 66 patients),
category-B (VOC/ACS with SIRS and either proven or suspected bacterial
infection - 24 patients) and category-C (SCA patients in steady state
without VOC/ACS or SIRS - 10 patients). Complete blood count,
C-reactive protein (CRP) estimation and PCT measurement were done in
all the patients. There was no significant difference in TLC and CRP
values between category-A and B. In category-A, the PCT level was
<0.5 ng/mL in 83.3% and 0.5-2 ng/mL in 16.7% of cases. In
category-B, all the patients had PCT value >0.5 ng/mL with 87.5%
of
patients having >2 ng/mL. In category-C, PCT value was
<0.5
ng/mL. PCT had a high sensitivity (100%) and negative predictive value
(100%) for bacterial infection at a cut-off value of 0.5 ng/mL; whereas
the specificity is excellent at a cut-off value of 2 ng/mL. SCA
patients with VOC/ACS and SIRS having a PCT level of <0.5 ng/mL
have
a low probability of bacterial infection whereas PCT value of >2
ng/mL is indicative of bacterial infection necessitating early
antimicrobial therapy.
|
Introduction
Sickle
cell anaemia (SCA) is a genetic disorder resulting in the production of
abnormal sickle haemoglobin. Various factors like hemolysis, chronic
inflammation and endothelial dysfunction culminate in acute
vaso-occlusion which is responsible for much of the morbidity observed
in SCA patients.[1,2]
Vaso-occlusive crisis (VOC) is a common medical
emergency in SCA patients necessitating hospitalization.[3]
Another
complication namely infection is a major cause of morbidity and
mortality in these patients. This is due to relative asplenic state and
abnormal humoral immunity found in these patients.[4,5]
Moreover SCA
patients with VOC may present with features of systemic inflammatory
response syndrome (SIRS) like fever, tachycardia, tachypnea and raised
leukocyte count without associated bacterial infection.[4]
Since untreated bacterial infection may result in serious complications
with an unfavorable outcome, these patient are treated with
antibiotics. However blind and over prescription of antibiotics in such
a situation contributes to development of antimicrobial resistance,
increases cost of management and exposes the patients to various side
effects.[6,7] Early diagnosis of
bacterial infection in SCA patients
presenting with VOC and SIRS is a challenge for emergency department
physicians. Routine laboratory tests including total leukocytes count
(TLC) and biomarkers such as C-reactive protein (CRP) have insufficient
power and sensitivity for correctly identifying bacterial infection.
Diagnosis of bacterial infection by microscopy or radiological
examination may take 12-24 hours. Confirmatory microbial tests are
unavailable for 24-48 hours.[8] So
to guide the use of antibiotics in
the early hours of admission (within 6 hours) there is a need of a
biomarker that could differentiate bacterial infection from nonspecific
inflammation due to VOC.
Procalcitonin (PCT), the pro-hormone of calcitonin is normally produced
by the thyroid gland in physiological condition. Its level increases
thousand fold during acute bacterial infection.[9,10]
The level of PCT
is found to correlate with the severity of bacterial infection and
mortality.[11-14] Several studies
have reported that PCT can be used to
distinguish systemic bacterial and fungal infection from viral and
noninfectious causes of SIRS.[15-19]
Inherited haemoglobin disorders are highly prevalent in the state of
Odisha in eastern India. In a cross-sectional prevalence study, the
observed frequency of sickle cell gene was found to be 21% in this
region.[20] Advanced facility for
diagnostic workup of microbial
infections is unavailable in most of this region. For this reason,
antibiotic overuse tends to be a large problem. Although PCT is a
relatively expensive investigation (Indian Rupees 1000/- or 17 USD per
test), the cost of empirical antibiotic therapy is higher. There are
limited number of studies describing the usefulness of PCT in diagnosis
of bacterial infection in patients with sickle cell disease (SCD) with
conflicting results.[4,19,21]
Moreover, these studies have focused
mostly SCD patients of Europe and USA with a different β-globin gene
cluster haplotype and the results may not be applicable to Indian SCA
patients with Asian-Indian haplotype. In view of this, we undertook
this study with an aim to find the role of PCT as a biomarker of
infection in Indian SCA patients.
Materials and Methods
Study
design and patients.
This prospective non-interventional observational mono-centric study
was undertaken at the Sickle Cell Clinic and Molecular Biology
Laboratory, Veer Surendra Sai Medical College Hospital, Burla in the
state of Odisha, India, from October 2011 to September 2013. It is a
referral centre for SCD patients located in the state of Odisha in
eastern India. During the study period 152 SCA patients were admitted
to the Department of Internal Medicine with VOC/ACS. After exclusion,
90 patients with VOC/ACS and SIRS were included in the study. Written
informed consent was obtained from all the participants. Ten SCA
patients in steady state without VOC/ACS attending to the Sickle Cell
Clinic outdoor were taken as a control. The study was approved by the
Institutional Ethical Committee.
Definitions.
VOC
was defined as an acute painful event that required oral/injectable
analgesics and that lasted for at least 4 hours when no other cause
could explain the symptom.[22,23]
ACS was defined on the basis of the
finding of a new pulmonary infiltrate involving at least one complete
lung segment that was consistent with the presence of alveolar
consolidation, but excluding atelectasis. In addition, the patients had
to have chest pain, a temperature of more than 38.5°C, tachypnea,
wheezing, or cough.[24] SIRS was
diagnosed in a patient with any two of
the four clinical criteria namely hypothermia (<36°C), fever
(>38°C), tachycardia (>90 beats/minute); and tachypnea
(>20
breaths/minute).[25]
Bacterial infection was categorized into ‘proven bacterial infection”
and “suspected bacterial infection”. Proven bacterial infection was
defined when a causative bacterium could be identified by microscopy or
culture of sputum, blood, urine and body fluid, supplemented with
supportive history, clinical signs and symptoms. Suspected bacterial
infection was defined when a causative bacterium could not be
identified by microscopy or culture of sputum, blood, urine and body
fluid, but clinical signs and symptoms were in concordance with
radiological findings.[6] For
instance, a history of upper abdominal
pain and fever with Murphy sign positive and ultrasound abdomen showing
gallstones, pericholecystic fluid, gallbladder wall thickening
(>4
mm) and sonographic Murphy sign was diagnosed as a case of suspected
bacterial infection of gallbladder. When there were no supportive
history, clinical sign and symptoms and a causative bacterium could not
be identified by microscopy or culture of sputum, blood, urine or body
fluid then the patient was categorized as a case of SCA with VOC/ACS
and SIRS without proven or suspected bacterial infection.
Exclusion
criteria.
Patients with the following criteria were excluded from the study: (a)
Patients with other sickle cell syndromes such as HbSβ-thalassemia,
HbSE, HbSC, HbSD-Punjab and others; (b) children under 14 years and
adults above 60 years of age; (c) Patients who were a part of special
program/trial that may have affected their clinical/haematological
status; (d) Patients who could not be followed-up; (e) Patients who
received antibiotics treatment prior to attending our facility; (f)
Patients positive for malarial infection; (g) Patients who refused to
participate in the study;
Categorization
of patients.
Final diagnosis and categorization of the SCA patients was made at
discharge using all of the available data including clinical,
pathological, microbiological and radiological findings. Basing upon
these 100 SCA patients were conveniently divided into three categories:
Category-A. VOC/ACS and SIRS without proven or suspected bacterial
infection. All these cases were admitted to the indoors for a period
varying from 3-7 days (median, 5 days). Category-B. VOC/ACS and SIRS
with either proven or suspected bacterial infection. All these cases
were admitted to the indoors for a period varying from 5-11 days
(median, 7 days).
Category-C. SCA patients in steady state without VOC/ACS or SIRS
attending to the Sickle Cell Clinic outdoor for routine checkup.
Laboratory
investigation.
A sickling slide test and alkaline agarose gel haemoglobin
electrophoresis (pH-8.6) were carried out as the initial screening
procedures and those samples found positive in these two results were
subjected to cation exchange high-performance liquid chromatography
(CE-HPLC) using the VARIANT II Haemoglobin testing system; Bio-Rad
Laboratories, Hercules, CA, USA as per the manufacturer’s guideline.
Confirmation of SCA [codon 6 β(GAG>GTG) mutation] was done by
amplification refractory mutation system-polymerase chain reaction
(ARMS-PCR) using established protocols.[26]
A complete blood count (CBC) was carried out on an automated
haematology analyzer (Sysmex KX-21; Sysmex Corporation, Kobe, Japan).
CRP was done by latex turbidometry (SPINREACT, S.A./S.A.U. Ctra.Santa
Coloma, 7 E-17176 SANT ESTEVE DE BAS (GI) Spain). Since the area under
our study was a malaria endemic area, all the patients were screened
for the same using peripheral smear by microscopy or
Immunochromatographic test (ICT). Relevant investigations like plain
radiography, sputum examination including microscopy and culture,
culture of exudates and blood, ultrasonography were done as and when
required. Urine examination including microscopy and culture was done
for all the cases.
PCT was estimated semi-quantitatively within 6 hours of admission by
using the BRAHMS PCT-Q kit (BRAHMS Aktiengesellschaft Neuendorfstrasse
25 D-16761 Hennigsdorf, Germany). As per the manufacturer’s guideline
the semi-quantitative kit was able to detect PCT in the range of
<0.5 ng/mL , 0.5-2.0 ng/mL, 2.0-10.0 ng/mL and ≥10 ng/mL.
Follow-up.
Following discharge from the hospital, each patient was followed up at
the end of one week and one month to determine the course and fate of
the illness. This was done at the outpatient department during the
regular follow up sessions. Those who could not attend the same were
followed up by telephonic conversation. At each visit during follow-up,
detailed clinical, relevant microbiological and radiological
investigations were performed in all the cases.
Statistical
analysis.
Statistical analysis was done using GraphPad InStat Version 3.00 for
Windows. For comparison between groups, the Mann-Whitney U test or
Chi-square test were used as appropriate. TLC and CRP values in
different PCT ranges were compared with Tukey-Kramer multiple
comparison tests in patients with category-A and B. The diagnostic
performance of PCT was reported as sensitivity, specificity, positive
and negative predictive value for bacterial infection. p value of
<0.05 was considered to be statistically significant.
Result
Patients.
The study included 100 patients of SCA in the three categories of A, B
and C, of whom, 52 patients were males. Category-A had 66 patients (VOC
in 54, and ACS in 12 patients). Category-B included 24 patients of whom
22 had proven bacterial infection and rest 2 patients had suspected
bacterial infection. Category-C had 10 patients of SCA in steady state
without VOC/ACS or SIRS. The mean age of the study participants in
category-A, B and C were 24.12±3.0, 23.04±5.04 and 26.3±2.2 years
respectively. There was no difference in the age and sex distribution
of cases in three categories. The recruitment procedure,
categorization, treatment and outcome of SCA patients in the study are
presented in a flowchart (Figure
1).
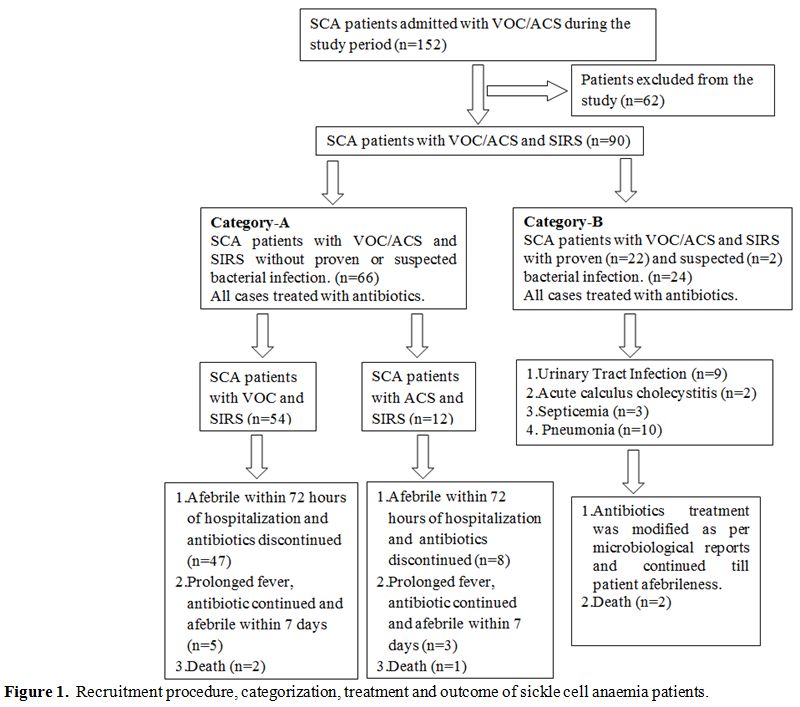 | Figure 1. Recruitment procedure, categorization, treatment and outcome of sickle cell anaemia patients. |
Of the 22 patients
with proven bacterial infection, nine had urinary tract infection (Escherichia coli
was isolated in seven cases, and two cases had Klebsiella oxytoca).
Three patients of septicemia were diagnosed by positive blood culture
(Pseudomonas aeruginosa
was isolated in two cases, and one case had Escherichia coli
infection). The rest ten patients in category-B had
community acquired pneumonia (CAP) diagnosed radiologically. In these
cases Streptococcus
pneumoniae was isolated in five cases from sputum
culture, three cases from blood culture and two cases from pleural
fluid examination. Two patients with suspected bacterial infection had
acute calculous cholecystitis diagnosed by clinical and
ultrasonographic criteria. In these two patients blood culture was
negative.
Clinical
parameters.
Temperature and heart rate were significantly lower in category-C
compared to both the category-A and B (p<0.001). Both these
parameters were found to be significantly high in category-B compared
to category-A (p<0.01).
Laboratory
findings.
TLC and CRP were significantly lower in category-C compared to both the
category-A and B (p<0.001). But there was no difference in
values of
these parameters between category-A and B. The baseline investigations
and other parameters are provided in table 1.
In category-A the PCT level was <0.5 ng/mL in 55 cases (83.3%)
and
0.5-2 ng/mL in 11 (16.7%) cases. In category-B all the cases had PCT
value >0.5 ng/mL. PCT level in various patients group in
category-B
is depicted in table 2.
All
the patients in category-C had PCT value of <0.5 ng/mL. The PCT
value differed significantly (χ2, 89.17; p <0.0001) in the three
categories of SCA patients.
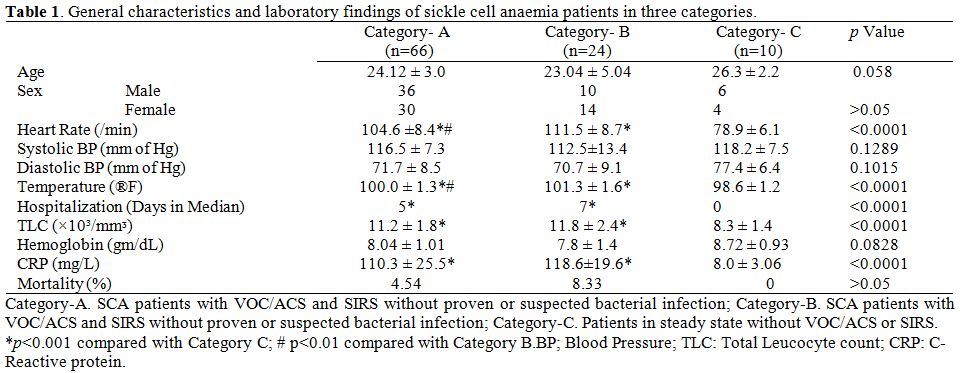 | Table 1. General characteristics and laboratory findings of sickle cell anaemia patients in three categories. |
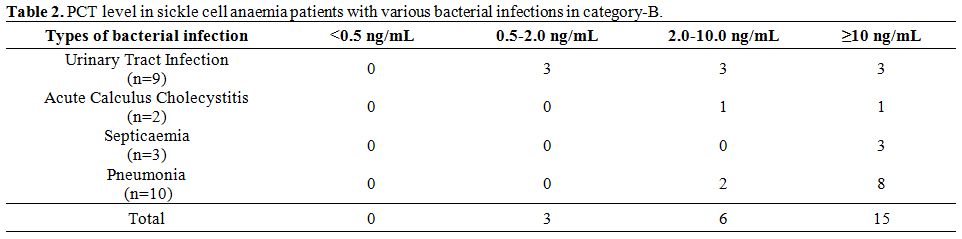 | Table 2. PCT level in sickle cell anaemia patients with various bacterial infections in category-B. |
A total of 14 patients (both in category-A and B) had a PCT value of 0.5-2 ng/mL of which majority belonged to category-A (78.5%). The TLC and CRP values were similar (p>0.05) when compared in four different ranges of PCT (< 0.5 ng/mL, 0.5-2.0 ng/mL, 2.0-10.0 ng/mL and ≥10 ng/mL) in category-A and B (Figure 2 and 3). PCT was evaluated as a potential biomarker of bacterial infection at various cut-off points. It was found that the test had a high sensitivity (100%) and negative predictive value (100%) at a cut-off value of 0.5 ng/mL; whereas the specificity is excellent at a cut-off value of 2 ng/mL (Table 3).
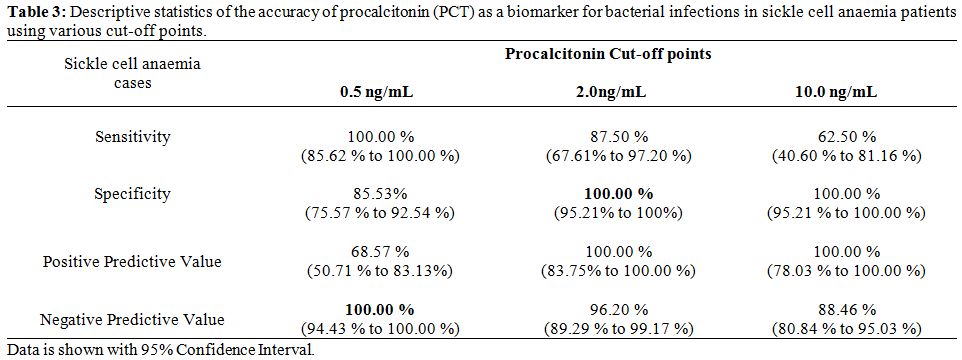 | Table 3. Descriptive statistics of the accuracy of procalcitonin (PCT) as a biomarker for bacterial infections in sickle cell anaemia patients using various cut-off points. |
Treatment
and outcome. Empirical antibiotic treatment with 3rd generation
cephalosporin (ceftriaxone) was initiated in all 66 patients of
category-A. 55 patients became afebrile in 3rd
day of hospital admission and had no evidences of bacterial infection
for which antibiotic treatment was discontinued. In the rest,
antibiotic was continued in view of persistent fever even though there
was no documented bacterial infection. There were 3 deaths in this
category; two cases died due to suspected pulmonary embolism on 4th and 5th day respectively and one case with ACS died on 5th day of admission to the hospital.
In category-B, suitable empirical antibiotic treatment was started in
all the patients. The antibiotic regimen was changed following the
availability of a microbiological report. Two patients of acute
calculous cholecystitis were treated with 3rd
generation cephalosporin (ceftriaxone) and metronidazole for a period
of seven days; subsequently they were referred to Department of Surgery
for cholecystectomy. In category-B, there were two deaths (one case had
pneumonia with multiorgan dysfunction syndrome (MODS) who died on 4th day and
another case of septicemia died on 5th
day of hospital admission).
Follow-up.
All the 63 patients of category-A were clinically normal at the end of
follow-up. In category-B, all the 9 patients with urinary tract
infection were asymptomatic and afebrile at follow-up and urine
examination was normal. Two patients of acute calculous cholecystitis
underwent cholecystectomy in the second week and were better by
symptoms at the end of one month. Both the patients of septicemia were
afebrile and well. Nine patients of pneumonia improved clinically and
radiologically at the end of follow-up and all except one had complete
radiological resolution.
Discussion
Diagnosis of bacterial infection is challenging because of the
ambiguous clinical sign and limitation of microbial techniques.[27] The
causative microorganism cannot be detected in up to 80% of patients
with suspected blood stream infections.[14,28] Because of these facts
there is a lack of a gold standard for invasive bacterial
infection.[19] Because of these
diagnostic uncertainty several studies
have analyzed the role of surrogate biomarkers like PCT to estimate the
likelihood for presence of a bacterial infection in various clinical
situations.[27]
It is interesting to learn that PCT belongs to the family of calcitonin
gene related peptides and forms a functional entity during infection
and inflammation.[29] Multiple
studies have demonstrated that serum
levels of PCT are markedly increased in humans with sepsis, severe
infection and severe inflammation.10 The serum values of PCT has been
found to correlate with the severity of infection. Moreover, it has
been demonstrated that PCT is a harmful biomarker and a therapeutic
target for bacterial infection in humans. PCT has been found to be more
useful and superior to other biomarkers of inflammation like CRP and
TLC.[14,30,31]
As a diagnostic marker, PCT has several advantages over
CRP because it increases in an earlier stage of infection followed by
rapid decline when the infection is controlled by immune system or
antibiotic treatment. Further unlikely CRP, production of PCT is not
attenuated by steroidal and non-steroidal anti-inflammatory drugs.[9]
In the present study we validated the role of PCT for triage for
selective and early institution (within 6 hours of admission) of
antimicrobials in SCA patients presenting with VOC/ACS and SIRS.
In this study, clinical parameters like temperature and heart rate were
significantly high in patients with proven or suspected bacterial
infection. However laboratory parameters of inflammation like TLC and
CRP were similar in both category-A and B. Reliance on these markers
would have led to overtreatment, undesired exposure to antibiotics with
antecedent consequences.[4] In the
study by Stojanovic et al.,[19]
the
TLC could not differentiate SCA patients with invasive bacterial
infection from those without it. Similarly, CRP level was non
discriminative in both the groups of patients. In another study, the
CRP level was found to be significantly high in SCA patients with VOC
associated with fever in comparison to sickle vaso-occlusive crisis
patients without fever. However this study is less informative as the
authors did not take into account the possibility of bacterial
infection as a cause of fever in SCA patients.[21]
In the present study, the PCT was significantly high in category-A (SCA
patients with VOC/ACS and SIRS without bacterial infection) and
category-B (SCA patients with VOC and SIRS with either proven or
suspected bacterial infection) in comparison to category-C (SCA
patients without VOC/ACS or bacterial infection). In a study of 24
cases of SCA, Scott et al.,[4]
reported that all the 5 patients with
documented bacterial infection at presentation had PCT ≥2 ng/mL. They
estimated the PCT in semi-quantitative kit method similar to that of
ours. In another recent study PCT level was estimated by an
ultrasensitive method in 6 SCA patients with fever, VOC and documented
invasive bacterial infection. The mean PCT value was 1.98 µg/L with a
range from 0.10 to 5.99 µg/L. Four of these patients had a PCT value of
≥1 µg/L whereas two had <1 µg/L (0.09 and 0.10 µg/L,
respectively).
The authors concluded that high level PCT (≥1 µg/L) indicate invasive
bacterial infection.[19]
The probability of bacterial infection was very low in SCA patients
with a PCT value of <0.5 ng/ml. At this level serum PCT had a
negative predictive value (100%) in excluding bacterial infection. In
the study by Scott et al.,[4] a
serum PCT level <2 ng/mL had a
strong negative predictive value in excluding bacterial infection.
However, Stojanovic et al.,[19]
concluded that a single low PCT level
without follow-up measurement does not rule out invasive bacterial
infection as 33% of their patients had low PCT value. We found that all
but 3 of the patients with proven and suspected bacterial infection
(n=24) had a PCT value of >2 ng/mL. At this level PCT had 100%
specificity and positive predictive value (100%) for presence of
bacterial infection in SCA with VOC and SIRS. In view of high
probability of bacterial infection in this group antimicrobial therapy
may be continued until clinical recovery. In the Parisian study, PCT
was estimated by an ultra sensitive ELISA method, and both the
specificity and positive predictive value were 100% at PCT value of ≥1
µg/L.[19]
In the present study, 14 cases (11/66 cases from category-A and 3/24
cases from category-B) had a PCT value in the range of 0.5-2.0 ng/mL.
This was an indeterminate value and there was overlap of both
category-A and B patients. In view of this it would be justifiable to
repeat this test and antimicrobial treatment may be instituted basing
upon the clinical condition of the patient. Majority of the patients
(62.5%) in the bacterial infection category had a PCT level
>10.0
ng/mL. However in view of small number of deaths in our study both in
category-A and B, we could not correlate the high PCT level with
adverse outcome like death. Basing upon the result of PCT value in our
patients we have proposed a clinical algorithm for guidance of
anti-microbial treatment (Figure
4).
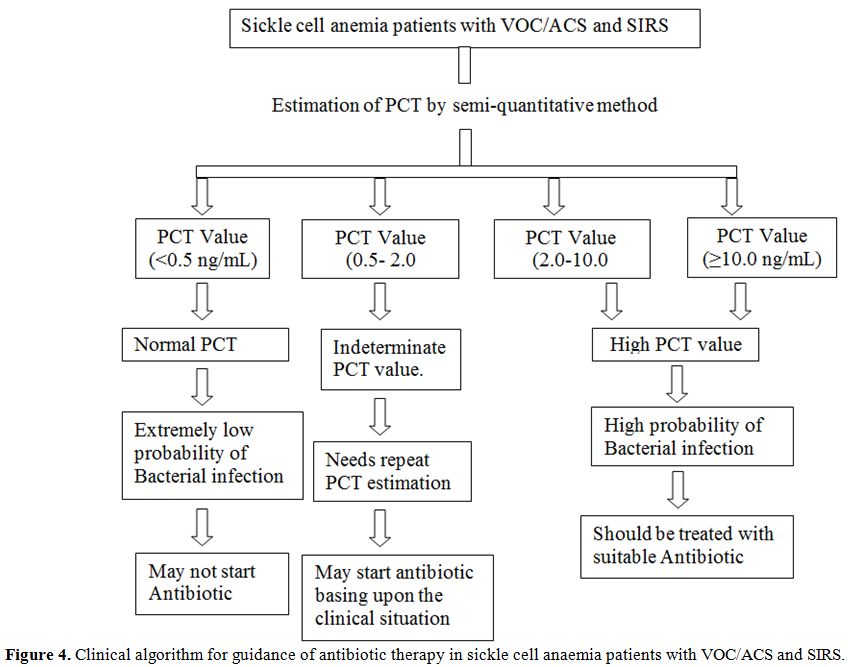 | Figure 4. Clinical algorithm for guidance of antibiotic therapy in sickle cell anaemia patients with VOC/ACS and SIRS. |
PCT has not been
studied as a biomarker for prognostic assessment of
bacterial infection in SCA patients with VOC and SIRS. Several studies
have been undertaken in lower respiratory tract infection (LRTI),
community acquired pneumonia (CAP) and sepsis to study the prognostic
implication of PCT.[27] In the ICU
setting elevated PCT level was an
independent predictor for 90 days all cause mortality in patients with
sepsis. In this situation high CRP level and TLC did not predict
mortality.[32] In CAP, PCT seems
to be a useful diagnostic marker but
is not an ideal prognostic tool.[27]
There are some limitations of this observational study. We evaluated
the role of PCT in only one tertiary hospital and the numbers of SCA
patients with suspected or proven bacterial infection were modest.
Therefore the clinical algorithm proposed by us needs prospective
evaluation and external validation in multicentre study with more
number of patients. Microbial culture is insensitive and there is no
gold standard for bacterial infection. So some SCA patients with
bacterial infection with negative microbial culture may have been
included in category-A. We employed a semi quantitative
immunochromatographic method for estimation of PCT. An ultra-sensitive
ELISA test if employed could have given more credibility. However, we
did not use this ultra-sensitive method in view of financial constrain.
Conclusions
From the above findings it can be presumed that SCA patients with VOC/ACS with SIRS presenting to the emergency department with a PCT level of <0.5ng/mL have a low probability of bacterial infection. These patients may not be administered antibiotics and can be managed with supportive therapy. Those patients with PCT >2 ng/mL have a high probability of bacterial infection and it is prudent to initiate antibacterial therapy without waiting for results of bacteriological tests. In patients with indeterminate PCT value of 0.5 to 2ng/mL, there is a need for repeat PCT estimation. Empirical antibiotic therapy may be initiated in these patients awaiting the availability of microbiological report. In developing countries with limited resources, PCT enhanced triage will support the choice of starting antibiotic therapy, reduce the overall cost of patient management and shorten unnecessary hospital stay while achieving similar quality of life and patient outcome.
Acknowledgements
This study was supported by research funding from Department of Science and Technology (DST), New Delhi, Government of India; Indian Council of Medical Research, (ICMR), New Delhi; and National Health Mission (NHM), Odisha.
References
































[TOP]