Received: December 21, 2013
Accepted: February 9, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014019, DOI 10.4084/MJHID.2014.019
This article is available on PDF format at:

Ayad Ahmed Hussein,1,* Randa M. Bawadi,2 Lubna H. Tahtamouni,2 Haydar Frangoul3 and Ali Z. ElKarmi 2
1
Bone Marrow and Stem Cell Transplantation Program, King Hussein Cancer
Center, Amman, Jordan.
2 Department of Biology and Biotechnology,
Faculty of Science, The Hashemite University, Zarqa, Jordan.
3 Pediatric Stem Cell Transplant Program, Monroe
Carell Jr. Children’s Hospital at Vanderbilt, Nashville, TN, United
States.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Background:
Cord blood transplant is an accepted treatment for many malignant and
non-malignant diseases. We sought to determine the feasibility of
collecting cord blood in Jordan and the effect of maternal and fetal
factors on the quality of the cord blood units.
Methods: A total of 124 cord blood units were collected, and 75 (60%) cord blood units were included in this analysis. Cord blood volume, total nucleated cell (TNC) count, cell viability and CD34+ content were measured, and clonogenic assay was performed. Results: The mean volume of the collected units was 68.9 ml (range 40-115) with mean nucleated cell count of 6.5 x 108 (range 1-23.0). Our results showed a positive correlation between the volume of cord blood and TNC count (p=0.008), cell viability (p=0.001), CD34+ content (p=0.034) and the length of the umbilical cord (p=0.011). In addition, our results showed an inverse relation between the Colony Forming Unit-Granulocyte Macrophage (CFU-GM) concentration and the gestation duration (p=0.038). Conclusion: We conclude that it is feasible to collect cord blood units in Jordan with excellent TNC and CD34+ cell content. The volume of cord blood collected was associated with higher TNC count and CD34+ count. Efforts toward establishing public cord blood banks in our area are warranted. |
Introduction
Hematopoietic
stem cell transplant (HSCT) is a well-established therapy for various
malignant and non-malignant diseases in adult and children. Bone marrow
was the main source of hematopoietic stem cells for decades. Recently,
more commonly used sources include peripheral blood and umbilical cord
blood. Umbilical cord blood (UCB) emerged as a new source for
hematopoietic stem cells (HSC) in the early 1988.[1]
A
major advantage for UCB as a stem cell source for allogeneic HSCT is
its immediate availability. Additionally, the naive nature of its
lymphocytes led to decreased risks of graft versus host disease (GVHD)
and allowed for successful HLA mismatched transplant with low rates of
acute and chronic GVHD.[2-4] This
has resulted in UCB being a widely used source for HSCT to treat many
malignant and non-malignant diseases.
Higher numbers of total nucleated cells (TNC) and CD34+
cells in the UCB units have resulted in faster and more sustained
engraftment and improved survival following cord blood transplant.[3,4]
Several studies from Europe,[5-7]
Japan,[8] Taiwan,[9]
and the United States[10,11]
have examined the various factors that can improve the quality of the
collected UCB units. Some of the variables that were identified
included maternal-related factors such as mother age, race, number of
previous births and smoking status, and fetal-related factors such as
weight, sex, birth order, placental weight and umbilical cord length.
The rational being is that it would be useful to predict UCB cell
content using information of donor-related variables before collection
and cell processing.[7]
None of the above cited studies were performed in any of the Middle
Eastern countries, despite the fact that there are unique demographic
and genetic differences in patients in this region.[12]
In the current study we sought to investigate the feasibility of
collecting UCB and the effect of different maternal and fetal variables
that might have an impact on the hematopoietic parameters of UCB in
Jordan. According to our knowledge, this is the first study to be
conducted particularly in the Arab region and in Jordan.
Materials and Methods
Umbilical
cord blood collection.
Between August 2010 and July 2011, 177 mothers delivering their babies
at Al-Isra'a hospital, Amman, Jordan, were approached to participate in
this prospective study. One hundred and twenty-four mothers (70%)
agreed to participate and signed a consent form. The UCB was collected
exclusively from term (gestation period 37-42 weeks) single-birth
babies born through normal vaginal delivery. Cord blood was collected
after the baby was delivered but before the delivery of the placenta. A
regular blood-donor set was used for UCB collection containing 28 ml
citrate phosphate dextrose-adenine (CPD-A) anticoagulant. The
collection was performed by the obstetrician delivering the baby and
not by a trained technician. The umbilical cord (UC) was sterilized
with povidone iodine in a unidirectional move, and 16-gauge needle of
the prepared blood-donor set was inserted into the umbilical vein.
Blood was allowed to flow by gravity, and the needle was removed when
blood flow ceased as has been previously described.[6,10]
The study design and UCB collection procedure was approved by the
Hashemite University, and Al-Isra'a general hospital Institute Review
Boards.
Evaluation
of umbilical cord blood parameters.
For the current study, UCB units were deemed unacceptable if the total
volume collected was less than 30 ml and/or if the unit was delivered
for analysis past 24 hours of collection. The UCB units were processed
and analyzed in the biology laboratory at the Hashemite University,
Jordan. The UCB was incubated with FITC-conjugated anti-CD45
fluorescein (MACS, Germany) and PE-conjugated anti-CD34 PE (MACS,
Germany) for 30 min at room temperature in the dark. After incubation,
RBCs were lysed with the lysis solution (Coulter, France) and then
washed twice with 10% bovine serum albumin (BSA) in phosphate buffer
saline (PBS). For each tube, 20,000 live events were counted in a
flowcytometer counter (Partec, Germany). CD34+
cells were selected based on their forward- and 90o-scatter
properties and dim CD45 expression.[13]
The clonogenic assay (CFU-GM assay) was performed as described
previously.[14] Briefly,
mononucleated cells (MNCs) were cultured at 1.0 X 105/ml
in RBMI-1640 medium (MACS, Germany) containing 0.8% methylcellulose
(Sigma Aldrich, USA), 20% fetal bovine serum (FBS; Lonza, Belgium), 450
µg/ml human transferrin, 10 ng/ml GM-CSF, 10 ng/ml IL-3 (Stem Cell
Technologies), and 1% BSA. Cells were incubated at 37oC
in a humidified atmosphere of 5% CO2
for 14 days. Colonies (clusters containing at least 50 cells) were
counted using an inverted microscope (Leica, Germany). Viability was
determined using trypan blue dye exclusion method, where the non-viable
cells stain deep blue.
Maternal
and neonatal data collection.
Data regarding maternal age, the number of previous pregnancies and
live births were collected from the medical files. Neonatal data such
as the weight of the baby and the placenta, baby’s gender, and UC
length were collected from the obstetric staff clinical notes at
Al-Isra'a general hospital. A standard questionnaire was prepared and
used for data collection.
Statistical
analysis.
Statistical analysis was carried out using STATISTICA 7 analysis
program (StatSoft Inc., OK, USA). Results were expressed as mean ±
standard deviation (SD). One-way analysis of variance (ANOVA) was used
to test for a significant difference between mean values of all.
Spearman's correlation was used to assess the association between the
different variables. A p value of ≤ 0.05 was considered statistically
significant.
Results
Characteristics
of the study population.
A total of 177 prospective mothers were approached to participate in
the current study, 53 (30%) of them refused to participate due to
cultural and/or lack of knowledge regarding benefits of UCB
and
safety of the collection procedure. 124 units were prospectively
collected for this study. In 17 (13.7%) UCB units the net volume of
cord blood was less than 30 ml, in 23 (18.5%) units some maternal
and/or neonatal data were missing, and in 9 (7.3%) units the samples
were not delivered for the laboratory within 24 hour of collection. A
total of 75 UCB units (60.5% of the total collected units) were
included and analyzed in this study. The characteristics of the
donating mothers and babies are shown in Table 1.
The mean maternal age was 28 years (range 19-43). Thirty three percent
of the donating mothers were delivering their first babies, 21.3%
second, 20% third and 25.3% fourth or more. Fifty one percent of the
delivered babies were males, and 49% were females. The mean weight of
the delivered babies was 3178 gm (range 220-4160), and mean placenta
weight was 526 gm (range 400-655). Seven percent of the donating
mothers reported that they were current smokers.
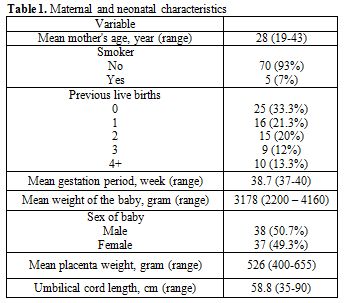 | Table 1. Maternal and neonatal characteristics |
Analysis
of umbilical cord blood samples.
Cord blood cell counts were analyzed within 24 hours of collection. The
mean volume of UCB collected (not including the 28 ml of anticoagulant)
was 68.9 ml (range 40-115 ml). The mean viability was 94.9% (range
80-99%), the mean total nucleated cell (TNC) count was 6.5 x 108 (range
1-32), with 10.6% have TNC of more than 1 x 109
and 4% of more than 1.2 x 109.
The mean total mononuclear cell count (MNC) was 3.4 x 108 (range
0.5-14.9), the mean total CD34+
cell count was 3.8 x 106
(range 0.2-11.8), and the mean total CFU-GM was 9.9 x105 (range
2-25). The UCB unit’s data are summarized in Table 2.
The results of the univariate analysis correlation are presented in Table 3. The volume
of UCB units collected was positively correlated with TNC (p=0.008),
cell viability (p=0.001), MNC (p=0.018), CD34+
cell count (p=0.034) and with the umbilical cord length (p=0.011).
There was also a trend towards obtaining higher UCB volume from mothers
with increasing number of prior live births (p=0.086). Our results
showed that higher TNC is correlated with MNC (p=0.001), CD34+
cell count (p=0.009), and increased viability (p=0.001). Finally, our
study demonstrated an inverse correlation between CFU-GM concentration
and the gestation duration (P = 0.038). There was no significant effect
of gestational age on TNC or CD34+
cell count of the collected UCB.
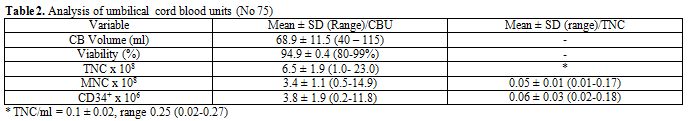 | Table 2. Analysis of umbilical cord blood units (No 75) |
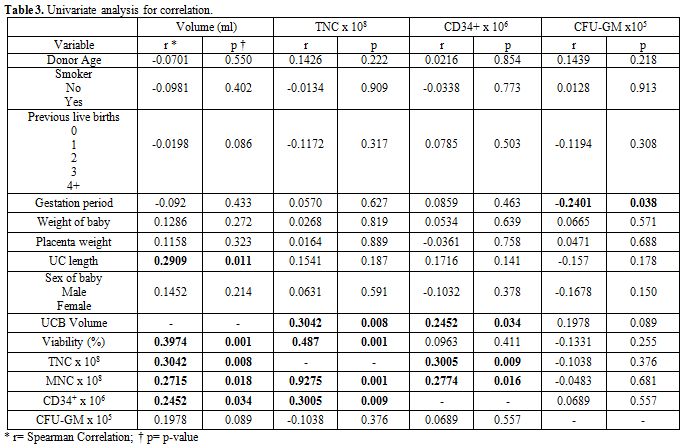 | Table 3. Univariate analysis for correlation |
Discussion
This is the first study to show that collection of cord blood is
feasible and can result in adequate TNC collection and viability in a
developing country in the Middle East. Of the 124 women enrolled in the
study, the umbilical cord blood of only 17 (13.7%) did not contain
adequate volume of blood despite the fact that untrained technicians
were present at the delivery to collect the UCB. An additional 25.8% of
the UCB units had to be excluded either because some data were missing,
or because the UCB did not reach the lab in the required time for
processing. Among the 75 units that met the predefined eligibility
criteria, the volume, nucleated cell dose and CD34 count was similar to
what has been previously published.[5,7,8,10,14]
Previous studies concluded that UCB yield of TNC, CD34+
cells, and CFU-GM is influenced not only by neonatal and maternal
factors but also by ethnicity of the parents.[7,15]
In this study, a total of 75 cord blood samples from Jordanian neonates
were analyzed in order to investigate any neonatal and maternal factors
that might influence UCB unit in terms of TNC and CD34+
cell content, and CFU-GM yields. In the current study, the average age
of the donor mothers included was 28 years and both the TNC and CD34+5,8-10] We found no significant
correlation between birth order and UCB volume, TNC, and CD34+
levels. Our findings are different from prior studies which showed that
women with few previous live births produced UCB units with higher TNC
yields.[9,10] Cell yield was not influenced by maternal age. While the majority of
published data showed similar observation, the study by Nakagawa et al.
which analyzed 572 UCB units, showed that increasing mother’s age was
associated with lower TNC cell yield.
The volume of UCB collected was not influenced by any of the maternal
or neonatal factors except the umbilical cord length, as longer cords
were associated with higher volume collected. This correlation has only
been observed in one prior study from Japan.[8]
We also found an inverse correlation between the CFU-GM concentration
and gestational age, which indicates that there is a loss of
hematopoietic potential with a longer gestational age. In a study by
Ballen et al. of 1269 UCB units, there was an 11% decrease in CFU-GM
with each additional week of gestation.[10]
Since TNC and CD34+
cell counts are the most important predictors of the outcome following
cord blood transplant, collecting units with a larger volume is
desired. In our current study, the only positive predictor of improved
cell count is the volume of UCB collected.
The chance of finding a matched related donor in Jordan is
significantly higher than what has been observed in other countries
(65% versus 25%) due to more homogeneous ethnic group in the region.[16]
Approximately 10-16% of UBC units collected in the international cord
blood banks have TNC of more than 1.2 x 109.
In Our study, 10.6% of UCB units collected have TNC of more than 1 x 109 and 4% of
more than 1.2 x 109.
Although our cell dose was slightly lower than what has been reported
by established cord blood banks, this is dependent on the experience of
the collection staff which always improves with time.
We believe that establishing a cord blood bank in Jordan will further
increase the possibility of identifying donors for patients who lack
related donor options. Taking into consideration the geographical and
cultural similarities between Jordan and its neighboring Arabic
countries, a cord blood bank in Jordan will help patients throughout
the region. Additional training and better logistical support are
needed to collect UCB units in order to decrease the percentage of
unacceptable units collected. We need also more efforts towards
education of the parents about the benefits and safety of UCB
collection, as only 70% of the approached mothers agreed to participate
in this study. A proper cost-effective analysis should be carried out
before establishing national cord blood banks in countries with limited
resources.
In conclusion, we have found that collection of cord blood units in
Jordan is feasible and can result in similar cell content compared to
other developed countries. Efforts toward establishing public cord
blood banks in our area are warranted.
Acknowledgments
We are grateful for the effort of the attending physicians, residents
and nursing staff of Obstetrics and Gynecology department at Al-Isra'a
Hospital. We gratefully acknowledge the Deanship of Research and
Graduate Studies, The Hashemite University, Jordan for financial
support.
Author contribution: Study design (AAH, RMB, LHT, AZE), study analysis
and interpretation of the data (AAH, RMB, LHT, HF, AZE). All authors
contributed to the writing of the manuscript and approval of the final
version.
References
















[TOP]