Received: January 23, 2014
Accepted: March 11, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014029, DOI 10.4084/MJHID.2014.029
This article is available on PDF format at:

Adel A. Hagag1, Nahla A. Nosair2, Fatma M.Ghaith2 and Eman H. Elshenawy2
1
Department of Pediatrics, Faculty of Medicine. Tanta University. Egypt
2 Department of Clinical Pathology, Faculty of
Medicine. Tanta University. Egypt

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Background:
Acute Lymphoblastic leukemia (ALL) is a malignant disorder of lymphoid
progenitor cells that proliferate and replace the normal hematopoietic
cells of the bone marrow. Protease-activated receptors (PARs) comprise
a family of trans-membrane G-protein coupled receptors.
Protease-activated receptor 1 (PAR-1) is a typical member of this
family of receptors that mediate cellular responses to thrombin and
related proteases. PAR1 is expressed by a wide range of tumor cells and
can promote tumor growth, invasion and metastasis. The aim of this work
was to study the role of PAR-1 expression in newly diagnosed ALL
patients.
Patients and methods: This study was conducted on 44 children with newly diagnosed ALL who were admitted to Hematology Unit, Pediatric department, Tanta University Hospital including 24 males and 20 females with their age ranged from 4-17 years and their mean age value of 9.06±3.26. All patients were subjected to complete history taking, thorough clinical examination, bone marrow aspiration and flow cytometric analysis for detection of PAR-1 expression by malignant cells. Results: PAR-1 was positive in 18 cases (41%) and negative in 26 cases (59%) of studied patients. This study showed no significant relation between PAR-1 expression and age, sex and most of the clinical data including hepatomegaly, splenomegaly and purpura while generalized lymphadenopathy was significantly higher in PAR-1 positive group. PAR-1 positive expression was associated with some bad prognostic laboratory parameters including higher hemoglobin, higher white blood cells, higher peripheral blood and bone marrow blast cells, higher serum LDH and lower platelets count. No significant association was detected between PAR-1 expression and immunophenotyping. There were significantly higher remission rates in PAR-1 negative group and significantly higher relapse and death rates in PAR-1 positive group. Conclusion: From this study, it could be concluded that PAR-1 expression on ALL cells represents an important adverse prognostic factor. Recommendations: PAR-1 expression should be routinely investigated for better prognostic assessment of ALL patients at diagnosis and should be taken in consideration in designing future therapeutic strategies based on patients- specific risk factors. |
Introduction
Acute
lymphoblastic leukemia (ALL) is a malignant disorder of lymphoid
progenitor cells that proliferate and replace the normal hematopoietic
cells of the bone marrow resulting in a marked decrease in normal blood
cell production[1] and is the most
common childhood
malignancy, representing nearly one third of all pediatric cancers; the
annual incidence is approximately 9-10 cases per 100.000 populations in
childhood.[2] Typically, ALL
develops quite quickly (acutely) and rapidly becomes worse unless
treated[3] as it spreads into the
blood stream and other vital organs quickly.[4]
Many studies over the past 20 years looked at the role of various
cellular phenotype assessed at initial diagnosis in predicting therapy
response. The associations generally have been strong and are clearly
predictive when coupled with several factors such as age, sex, initial
hemoglobin level, and total leucocytic and platelets counts.[5]
Protease-activated receptors, (PARs) comprise a family of
trans-membrane G- protein coupled receptors that are uniquely activated
by proteolytic cleavage of their extracellular portion. This cleavage
"unmasks" a new N-terminus, which serves as a "tethered" ligand that
binds to the second extracellular domain of the protein, resulting in a
variety of cellular responses.[6]
Protease-activated
receptor 1 (PAR-1) is a typical member of this family of receptors that
mediate cellular responses to thrombin and related proteases.[7]
Physiologically, PAR-1 is expressed by different tissues including
vascular cells, neurons, fibroblasts, epithelial cells and others.[8]
PAR-1 has been shown to be overexpressed in various human cancers
including breast, melanoma, colon, prostate, ovarian, esophagus and
others[9] and has been associated
with several
pro-tumoral responses including primary growth, aggressive behavior,
invasion, metastasis and angiogenesis.[10,11]
PAR-1 is significantly elevated in aggressive leukemias including blast
phase of CML and AML subtypes M4/M5, in contrast to chronic phase in
CML and CLL. Therefore, this protein plays an important biological role
in aggressive leukemias and might offer additional strategies for the
development of new therapies.[12]
Subjects and Methods
This study was done after approval from Ethical Committee of research
Center of Tanta University Hospital and written consent from parents of
included children in this research and was carried out on 44 children
with newly diagnosed ALL who were admitted to Hematology Unit,
Pediatric department, Tanta University Hospital including 24 males and
20 females with their age ranged from 4-17 years and their mean age
value of 9.06±3.26. ALL was diagnosed according to clinical
presentation, morphological, cytochemical smears together with
immunophenotyping and was based on the presence of >= 20% blast
cells in BM according to WHO proposal and MPO negative staining and
immunophenotyping results consistent with ALL.[13]
Patients were followed up for 24 months for clinical outcome and fate
of the disease.
ALL patients were subjected to the following:
1.Complete history taking
2.Thorough clinical examination: with an especial account on pallor,
purpura, hepatomegaly, splenomegaly and lymphadenopathy.
3.Laboratory investigations.
Specimen
collection and handling:
Four ml of venous blood were collected using sterile needles through
gentle venipuncture after sterilization of the puncture site by
alcohol, and collected samples were divided into; one ml was delivered
on 20 uL EDTA solution for complete blood count including differential
white blood cells count which was done on Leishman stained peripheral
blood smear with evaluation using ERMA PCE-210 N cell –counter[14] and the rest of blood was put in a
plain tube and serum was separated for estimation of LDH.
Bone
marrow aspiration:
Bone marrow aspiration was performed under complete aseptic technique.
Smears of direct bone marrow aspirate were prepared, stained with
Lieshman stain for morphologic study and cytochemical stains with Sudan
black and Myeloperoxidase and Immunophenotyping using the following
panel of fluorescein isothiocyanate / phycoerythrin conjugated
monoclonal antibodies:
Lymphoid cell markers.
T-cell markers (CD2, CD3, CD5, CD7).
B-cell markers (CD10, CD19, CD20, CD22).
Myeloid cell markers (CD13, CD33).[15]
Immunophenotyping
for evaluation of PAR-1:
One ml of bone marrow or peripheral blood samples (with more than 20%
blast cells) were withdrawn on EDTA tubes. Evaluation of PAR-1 was done
using Becton Dickinson FAC Scan flow cytometer (BD FACS).[16]
Monoclonal antibodies PAR-1/APC, anti-human reagent for identification
of cell expression PAR-1 labeled with fluorescein, commercially
available by R&D Systems; FAB3855A. The percentage of blast
cells
positive for PAR-1 was determined as a percentage from the gated blast
cells populations. The negative control was set at 2%. A case was
defined as PAR-1 positive if >=20% of the gated cells expressed
PAR-1.[17]
Follow up of patients was done clinically and by blast cell count in
the bone marrow (BM) on day 21 after induction chemotherapy which
includes: Vincristine 1.5mg/kg/m2/week
IV (days 0, 7, 14, 21, 28, 35), Doxorubicin 25mg/m2/
week IV infusion (days 0, 7, 14, 21, 28, 35), L-Asparginase 6000 u/m2 SC on
alternate days for 10 doses, and Prednisone 40mg/m2/day
for 6 weeks orally. Bone marrow aspiration was done on day 21. In
non-responding cases, we add Etopsoide 100mg/m2/dose
IV (days 22, 25, 29), Cyclophosphamide 750mg/m2/dose
IV infusion (days 22, 25, 29), Aracytin 100/m2/dose
IV (days 22, 25, 29), and methotrexate 5g/m2
over 4 hours on day 28.[18]
Definition
of complete remission and relapse:
Complete remission (CR) is defined as a cellularity of more than 20%
with fewer than 5% blasts in bone marrow after induction chemotherapy.[19]
Relapse is defined by the appearance of one of the following: (1) more
than 50% lymphoblasts in a single BM aspirate; (2) more than 25%
lymphoblasts in BM and 2% or more circulating lymphoblasts; (3)
progressive repopulation of lymphoblasts in excess of 5% culminating in
more than 25% on two or more BM samples separated by 1 week or more;
(4) leukemic cell infiltration in extra medullary organs as gonads; (5)
lymphoblasts in CSF with cell count greater than 5 WBCs/mm3.[20]
Statistical
analysis:
Statistical presentation and analysis of the present study was
conducted, using the mean, the standard error, student t- test and Chi-
square tests by SPSS V17.
Results
Table 1
shows no
significant differences between PAR-1 positive and PAR-1 negative
patients regarding age, sex, pallor, purpura, hepatomegaly and
splenomegaly while there was statistically significant difference
between PAR-1 positive and PAR-1 negative patients regarding
lymphadenopathy with a higher incidence of lymphadenopathy in PAR-1
positive patients.
Table 2
shows
statistically significant differences between PAR-1 positive and PAR-1
negative patients regarding hemoglobin and LDH levels, total white
blood cells and platelets counts, peripheral blood and BM blast cells
percentage with higher hemoglobin and LDH levels, total white blood
cells count, peripheral blood and BM blast cells percentage in PAR-1
positive patients while there were significantly lower platelets counts
in PAR-1 positive than PAR-1 negative patients and no significant
difference between PAR-1 positive and PAR-1 negative patients regarding
immunophenotyping.
Table 3
shows statistically significant difference between PAR-1 positive and
PAR-1 negative expression regarding relapse, death and remission rates
with higher relapse and death and lower remission rates in PAR-1
positive group (Fig. 1
and 2).
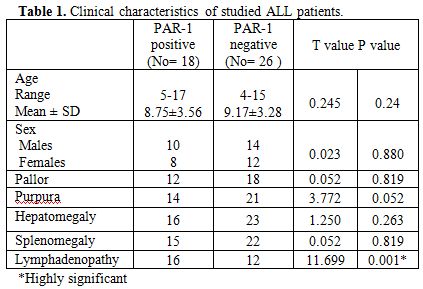 | Table 1. Clinical characteristics of studied ALL patients. |
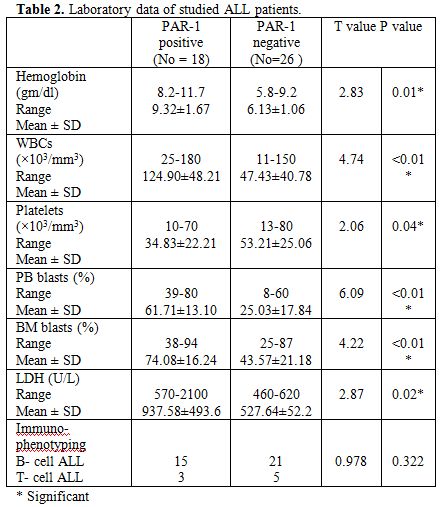 | Table 2. Laboratory data of studied ALL patients. |
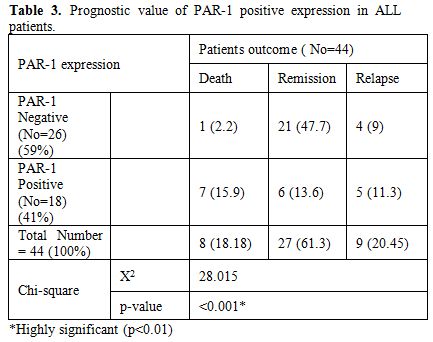 | Table 3. Prognostic value of PAR-1 positive expression in ALL patients. |
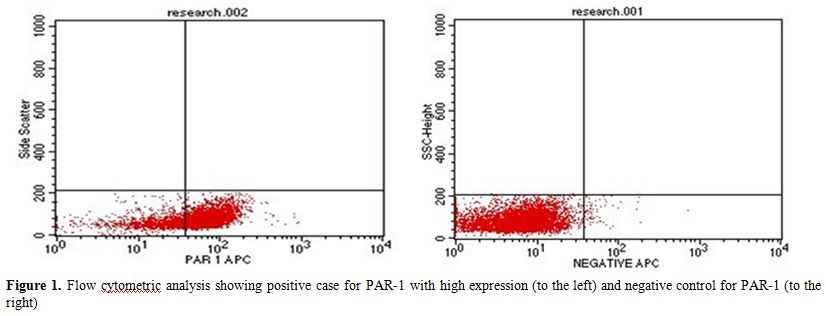 | Figure 1. Flow cytometric analysis showing positive case for PAR-1 with high expression (to the left) and negative control for PAR-1 (to the right) |
Discussion
ALL is the most common childhood malignancy, representing nearly one
third of all pediatric cancers. It has become a curable disease in over
than 80% of patients with current treatments. However, the treatment of
ALL results in significant morbidity and mortality. The use of
risk-adapted treatment protocols has improved cure rates while limiting
the toxicity of therapy.[21] PAR-1
plays an important
biological role in aggressive leukemias and might offer additional
strategies for the development of new therapies.[12]
The present research was done to evaluate the prognostic value of PAR-1
expression in 44 children with newly diagnosed ALL who were admitted to
Hematology Unit, Pediatric department, Tanta University Hospital
including 24 males and 20 females with their age ranged from 4-17 years
and their mean age value of 9.06±3.26 and they included 18 PAR-1
positive patients and 26 PAR-1 negative patients.
There were no significant differences between PAR-1 positive and PAR-1
negative patients regarding age, sex, pallor, purpura, hepatomegaly and
splenomegaly while there was statistically significant difference
between PAR-1 positive and PAR-1 negative patients regarding
lymphadenopathy with a higher incidence of lymphadenopathy in PAR-1
positive patients. These findings were consistent with Mook et al.,
2004[22] who found the same
results.
In the present series, there were normocytic normochromic anemia,
leukocytosis and thrombocytopenia in studied leukemic patients. This
was in agreement with Biswas et al., 2009[23]
who
found the same results and explained this by direct result of the
diffuse and heavy BM and peripheral blood infiltration due to
uncontrolled proliferation of lymphoblasts.
In our study PAR-1 positive expression at diagnosis was significantly
associated with bad clinical and laboratory prognostic factors
including lymphadenopathy, higher hemoglobin levels, higher white blood
cells, higher peripheral blood and bone marrow blast cells and higher
serum LDH and lower platelets count. These findings were consistent
with Boire et al., 2005[24] and
Salah et al., 2007[11]
who demonstrated that positive PAR-1 expression was associated
significantly with various clinicopathologic features and several
pro-tumoral responses including primary growth, invasion, lymph node
metastasis and depth of tumor invasion and Veiga et al., 2011[12]
who found significantly higher circulating peripheral blood and BM
blasts in PAR-1 positive ALL compared to PAR-1 negative cases.
In this study, 81% of patients were B-ALL, and 19% were T-ALL. This was
in agreement with Ahmed and Hassab 2008[25]
who found that 83.3% of patients were B-ALL and 14.6% were T-ALL. There
were no significant statistical association could be observed between
PAR-1 expression and immunophenotyping of ALL. This finding was in
agreement with Veiga et al., 2011.[12]
In our study, there were statistically significant differences between
PAR-1 positive and PAR-1 negative expression regarding relapse, death
and remission rates with higher relapse and death and lower remission
rates in PAR-1 positive group. This was in agreement with Veiga et al.,
2011[12] who stated that positive
PAR-1 expression
was significantly elevated in aggressive leukemias, including blast
phase of CML, AML subtypes M4/M5 and B cell ALL in contrast with CML,
in chronic phase, and CLL and was associated with poor treatment
outcome, Depasquale and Thompson 2008[26]
who
demonstrated that PAR-1 expression is a negative prognostic factor in
melanomas and strongly correlates with tumor stage and Meis et al. 2010[27]
who found decreased long-term survival in PAR-1 expressing patients
with lung adenocarcinoma compared with PAR-1 negative patients.
It was found that PAR-1 can promote tumor growth, invasion and
metastasis.[24] In addition PAR-1
activation stimulates proliferation and decreases idarubicin induced
cell death in vitro.[28]
The zinc-dependent matrix metalloprotease 1 (MMP-1), also known as
interstitial collagenase, has been reported to promote tumor growth and
invasion through activation of PAR-1, providing an important link
between tumor-generated metalloproteases and PAR-1 expression (Boire et
al., 2005).[24]
PAR-1 plays a primary role in the process of metastasis by stimulating
the secretion of matrix metalloproteinase by virtue of their ability to
degrade the extracellular matrix (ECM) barrier. However, MMPs are also
capable of cleaving non-ECM molecules. The protease-activated receptors
(PARs) are the latest MMP targets. The thrombin receptor PAR-1 has now
been shown to be cleaved and activated on the tumor cell surface by
stromal-derived MMP1. The resulting PAR1 activates intracellular G
proteins to turn on the migratory and invasive program in tumor cells.
This MMP-PAR axis may represent a novel signaling pathway communicating
between tumor and stromal cells during tumor progression.[29]
Conclusion
PAR-1 expression on ALL cells represents an important adverse prognostic factor and therefore its expression should be routinely investigated for better prognostic assessment of ALL patients at diagnosis and should be taken in consideration in designing future therapeutic strategies based on patient- specific risk factors.
References























[TOP]