Received: February 9, 2014
Accepted: March 19, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014030, DOI 10.4084/MJHID.2014.030
This article is available on PDF format at:

Brunella Posteraro1, Riccardo Torelli2, Elena De Carolis2, Patrizia Posteraro3 and Maurizio Sanguinetti2
1
Institute of Public Health (Section of Hygiene), Università Cattolica
del Sacro Cuore, Largo F. Vito, 1-00168, Rome, Italy.
2 Institute of Microbiology, Università
Cattolica del Sacro Cuore, Largo F. Vito, 1-00168, Rome, Italy.
3 Clinical Laboratory, Ospedale San Carlo, Via
Aurelia, 275-00165, Rome, Italy.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Despite
availability of many antifungal agents, antifungal clinical resistance
occurs, perhaps as a consequence of an infecting organism found to be
resistant in vitro to one or more antifungals tested. From what derives
the important current role of the in vitro antifungal susceptibility
testing (AFST), that is to determine which agents are like to be
scarcely effective for a given infection. Thus, AFST results, if timely
generated by the clinical microbiology laboratory and communicated to
clinicians, can aid them in the therapeutic decision making, especially
for difficult-to-treat invasive candidiasis and aspergillosis. Although
recently refined AFST methods are commercially available for allowing a
close antifungal resistance surveillance in many clinical setting,
novel assays such as flow cytometry or MALDI-TOF mass spectrometry are
upcoming tools for AFST. Based on short-time antifungal drug exposure
of fungal isolates, these assays could provide a reliable means for
quicker and sensitive assessment of AFST.
|
Introduction
Although several factors are key
determinants of antifungal clinical resistance,[1]
which is referred to as the persistence or progression of a fungal
infection despite the administration of appropriate antifungal therapy,
there is a general consensus that clinical outcomes are better when
treatments are started early.[2,3]
Almost all the
classes of systemically active antifungal agents available to date,
such as polyenes (i.e., amphotericin B), azoles, flucytosine, and the
newest echinocandins contribute to improve the management of invasive
fungal infections (IFIs).[4-6]
Nevertheless, the rate
of antifungal failures is high, and the emergence of resistant fungal
strains is a growing concern, particularly for strains capable of
exhibiting resistance to commonly prescribed antifungal drugs.[7] Eighteen (11.1%) of 162
fluconazole-resistant bloodstream isolates of Candida glabrata
collected during two large surveillance programs were found to be
cross-resistant to one or more of the echinocandins.[8]
Likewise, patients with chronic pulmonary Aspergillus
infection who receive prolonged (tri)azole therapy are at risk of
resistant aspergillosis,[9] with an
evolving spectrum of resistance owing to the emergence of non-cyp51A-mediated
mechanisms,[10]
as well as are at risk azole-naïve patients due to the presence of
resistant TR/L98H strains (i.e., carrying a substitution at codon 98 in
the cyp51A
gene in combination with a 34 base-pair tandem repeat in the gene
promoter) in the environment.[11,12]
Thus, while two-thirds of surveyed Dutch patients with azole-resistant Aspergillus disease
had not history of previous azole exposure (with all A. fumigatus
isolates from patients with invasive aspergillosis harboring the
TR/L98H mutation),[13]
recent epidemiological data show that this resistance mechanism, first
emerged in the Netherlands, is expanding not only in European countries
but also in China, Iran, and India.[10]
Antifungal Susceptibility Testing to Aid the Management of IFI Patients
The primary utility of antifungal susceptibility testing (AFST) arises
from the concept that susceptibility (or resistance) to an antifungal
agent selected for the therapy would allow some prediction about the
impact that administration of the agent tested in vitro has on the
clinical outcome of infection caused by the treated organism.[14,15]
Therefore, clinical microbiologists are currently engaged to determine
the growth of fungi under different drug concentrations so as to yield
the minimum inhibitory concentration (MIC) for a specific infecting
isolate, that is an in vitro measure of susceptibility (expressed as
growth inhibition) which helps to predict the therapeutic efficacy.[16]
Thus, it is important that MIC results are timely communicated to
physicians to guide them in the therapeutic decision making, in the
same way that antibacterial testing aids in the clinical guidance of
bacterial infections.[17]
As attested by several studies evaluating the role of “real-time” AFST
in managing patients with invasive Candida
infections,[18]
physicians frequently (and appropriately) adjust the therapy on the
basis of MIC results, although a clearly defined association between
the timely receipt of antifungal therapy and poor outcome after Candida bloodstream
infection due to a resistant isolate is lacking to date.[2]
Indeed, Collins et al.[19]
reported that the susceptibility testing (especially when done
in-house) of C. glabrata
isolates may facilitate quicker interventions (i.e., de-escalation of
therapy from an expensive echinocandin to fluconazole) for patients
with documented C.
glabrata fungemia, thereby resulting in lower overall
treatment costs. Likewise, Grim et al.[20]
found that receipt of appropriate early antifungal therapy (i.e.,
administered within 72 h of a positive culture being drawn) was
associated with a significant (P = 0.047) survival benefit for patients
who were effectively treated for ≥24 h, and their results were
supported by the inclusion of routine AFST to optimally assess the
adequateness of therapy.
Unlike Candida
infection, there is only a limited number of reported Aspergillus infection
cases that could elucidate the clinical impact of azole resistance on
the patient’s outcome,[21] and
this situation has hindered the wide application of in vitro AFST of Aspergillus
species. However, in an attempt to establish clinically derived
breakpoints for Aspergilli
that
would help physicians to interpret the MIC values as produced from the
clinical microbiology laboratory, a pragmatic (and not formal) approach
was followed by Verweij et al.[21]
Thus, taking MIC
distribution, pharmacokinetic/pharmacodynamic parameters of antifungal
azoles, in vivo experimental correlation between cyp51A
point mutations and failure, and clinical experience into account,
interpretive breakpoints were proposed, that is MICs >2 μg/ml
for
itraconazole and voriconazole and >0.5 μg/ml for posaconazole.[21]
These breakpoints were able to discriminate between wild-type (that
refers to isolates without mutational or acquired mechanisms of
resistance) and non-wild-type (that refers to isolates with mutational
or acquired mechanisms of resistance) MIC distributions for
itraconazole and voriconazole among 325 consecutive clinical A. fumigatus
isolates from the Nijmegen fungus culture collection.[21]
Based on these findings, a 4-well azole-agar dilution (4D) plate (i.e.,
3 wells were each containing one of azoles: itraconazole 4 μg/ml,
voriconazole 1 μg/ml, or posaconazole 0.5 μg/ml; and the fourth
azole-free well served as control growth) was developed as a screening
test for identifying potentially resistant A. fumigatus
isolates.[22] In parallel, Pfaller
et al.[23] used a collection of
637 geographically diverse, clinical isolates of A. fumigatus
tested against itraconazole, posaconazole, and voriconazole, to assess
the wild-type MIC distribution and epidemiological cutoff values
(ECVs), that is MIC threshold values for differentiating wild-type
isolates from non-wild-type isolates, for A. fumigatus and
the mold-active triazoles.
By contrast, due to scarce (and less frequent than for azoles) tendency
to carrying out AFST for Aspergillus
isolates,[24] perhaps as a result
of technical difficulties and suboptimal reproducibility of the methods
employed,[25] echinocandin
resistance in Aspergillus species is much less known.[22]
Although the caspofungin is recommended as a second line treatment
choice for invasive aspergillosis,[26]
and often administered in combination with amphotericin B,[21] however, breakthrough infections
(though sporadic) have been reported in patients under caspofungin
therapy,[27-29] and they involved A. fumigatus
isolates, with elevated minimum effective concentrations (MECs) to
caspofungin. The MEC endpoint, defined as the lowest drug concentration
that leads to the growth of small, rounded, compact hyphal forms as
compared to the hyphal growth seen in the growth control well, was
suggested for testing antifungal susceptibility of Aspergilli to
echinocandins, rather than the MIC;[25]
nonetheless, MEC remains technically difficult to determine.
Antifungal Susceptibility Testing in the Daily Laboratory Practice
Several recommendations for routine use of AFST of Candida species in
the clinical microbiology laboratory have been developed.[18] They include testing of fluconazole
and an echinocandin against C.
glabrata isolated from deep sites and, possibly, against
other species of Candida,
unless their antifungal susceptibility pattern is predictable (i.e.,
for Candida krusei);
use of clinical breakpoints (CBPs) or ECVs to interpret MIC values as
appropriate; considering cross-resistance between fluconazole and all
other triazoles (itraconazole, posaconazole, and voriconazole) to be
complete for C. glabrata;
and careful choice of susceptibility testing methods.[18]
In essence, a selective application of AFST, together with a precise
identification of Candida
to the species level,[30] should
be useful in selecting agents for primary therapy as well as in a
de-escalating strategy,[18]
especially in difficult-to-manage cases of invasive candidiasis.[31]
With regards to Aspergillus
species, it is currently recommended to perform AFST of clinically
relevant Aspergilli
(with isolates at least identified to the species level)[32]
as an adjunct to the treatment for IFI patients when therapeutic
failure of initial therapy or breakthrough infection occur, and for
patients with disease and long-term triazole treatment and/or recurrent
isolation of an Aspergillus
species.[25] Also, whereas
isolates of Aspergillus species known to be intrinsically
drug-resistant (e.g., A.
terreus against amphotericin B) need to be not usually
tested,[25] MIC determination
could be useful to monitor the emergence of polyene resistance in Aspergillus species
such as A. flavus.[33]
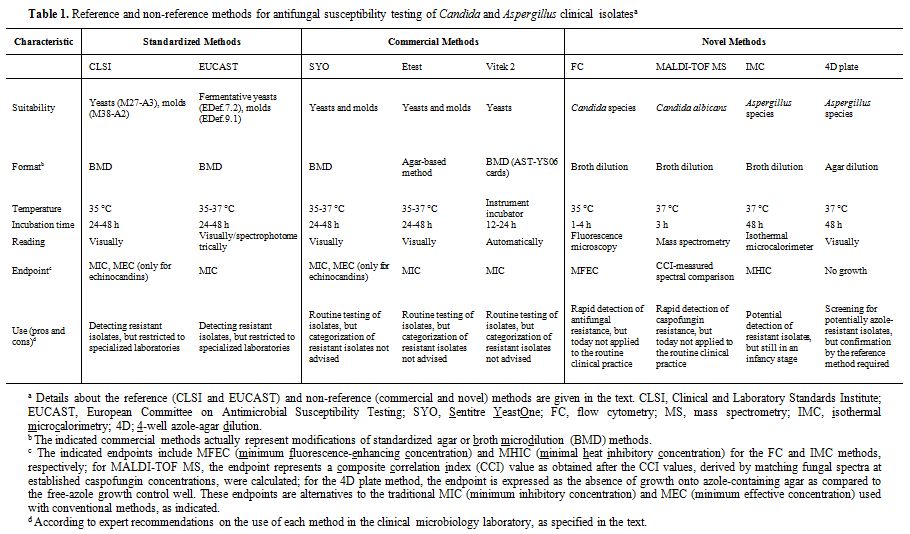
Conventional and Novel Laboratory Assays for Testing Antifungal Susceptibility
Standardized microdilution-based procedures by the Clinical and
Laboratory Standards Institute (CLSI) and the European Committee on
Antibiotic Susceptibility Testing (EUCAST),[34-38]
are universally accepted for performing AFST (Table 1), but these
procedures are complex, time-consuming, and not intended for routine
use.[39]
As a result of a multistep process based on the analysis of MIC
distribution curves for wild-type populations and the clinical
relationship between MIC values and efficacy,[40,41]
CLSI/EUCAST MIC breakpoints (i.e., obtained with CLSI/EUCAST reference
methods in specialized mycology laboratories) are to date available to
interpret the AFST results of amphotericin B, azoles, and echinocandins
for Candida, and amphotericin B and azoles for Aspergillus.[42]
Besides to be an important step in establishing fungal CBPs, the MIC
distributions of wild-type fungal populations provide a measure of the
ECVs, which, in the absence of specific CBPs, may be very useful in
antifungal resistance surveillance to monitor the emergence of
resistant isolates (i.e., those with gene mutations associated with
reduced therapeutic responses).[7]
Also, AFST using
the CLSI/EUCAST reference methods is a precious tool for studying the
in vitro activity of new and experimental compounds, as well as the
epidemiology of antifungal-resistant fungi. Finally, through recently
refined AFST methods,[24] coupled
with detection of molecular fungal alterations conferring reduced
antifungal drug susceptibility,[43]
often directly from clinical specimens,[44,45]
it is now possible to ensure a close antifungal resistance surveillance
in many clinical settings. The detection of cyp51A gene
mutations in primary clinical specimens is still the sole strategy for
detecting Aspergillus
resistance to triazoles in the absence of culture confirmation, which
occurs in most cases of invasive and chronic pulmonary aspergillosis,
making an MIC determination impossible.[21,22]
However, these nucleic acid-based assays, though permitting quicker
detection of azole-resistance in culture positive samples, are to date
not standardized or practical for most clinical laboratories,[42] in addition to be unable to reveal
the influence from other resistance mechanisms.[21,22]
Given these concerns and the aforementioned increasing number of
resistance cases, performing susceptibility testing of Aspergillus
isolates before and during antifungal treatment can be clinically
relevant.[22] Yet, since obtaining
repeated Aspergillus
positive cultures from patients receiving antifungal therapy (that
would allow to prove that a treatment failure is actually due to an
antifungal-resistant organism) is an uncommon clinical scenario,
monitoring of the galactomannan (GM) biomarker through serial GM index
measurements following antifungal treatment[46,47]
could be effective for detecting resistance to antifungal therapy.
 | Table 1. Reference and non-reference methods for antifungal susceptibility testing of Candida and Aspergillus clinical isolatesa |
Commercially
available tests, such as Sensititre YeastOne, Etest, and the fully
automated Vitek 2 yeast susceptibility system (Table 1),
all easy-to-use modifications from the CLSI/EUCAST reference methods
are widely used for testing antifungal susceptibility of relevant Candida and Aspergillus
species.[7]
While the commercial tests show a good essential agreement (defined as
MICs within 2 dilutions) with the reference methods, the categorical
agreement (i.e., agreement in the categorization of an isolate as
susceptible, intermediate, or resistant) may be lower, especially for
the echinocandin class of antifungal agents.[48-50]
Thus, it was noted that clinical fungal isolates should not be
classified as resistant in vitro by commercial methods, unless
standardization processes and setting of their own breakpoints have
been undertaken.[39] As MIC
determination by reference methods is highly recommended for patient
management,[51]
periodical epidemiological surveys of deep, blood, and mucosal
infections should be done to monitor antifungal susceptibilities of Candida and Aspergillus.
So, local surveillance MIC data, derived from a routine microbiology
laboratory workflow, can be used to develop treatment strategies,
particularly by clinicians who prescribe preemptively or empirically
antifungals in hematology, transplantation, or intensive care units. In
parallel, antifungal resistance surveillance studies should also
investigate air samples for the presence of A. fumigatus
resistant to medical triazoles in the hospital environment to ascertain
the local resistance risk among filamentous fungi. Therefore, both
clinical and environmental samples can be screened using the
aforementioned 4D plates[52] to
evaluate to what extent exposure to azoles in patients[9]
or in the environment[11]
contributes to antifungal resistance in the hospital setting.
New diagnostic approaches, based on emerging technologies such as flow
cytometry (FC), MALDI-TOF mass spectrometry (MALDI-TOF MS), and
isothermal microcalorimetry (IMC) (Table
1),
have been developed to expand, and potentially improve, the capability
of the clinical microbiology laboratory to yield AFST results. By flow
cytometry (FC), the effects of a given antifungal drug can be
appreciated by observing alterations in the fungal cell viability
(rather than the growth inhibition as in conventional methods) that
will be identified via changes in the measured cell fluorescence;[53]
this led to assess the minimum fluorescence-enhancing concentration
(MFEC), that is the lowest concentration of antifungal agent to which
the percentage of cells showing altered fluorescence is superior to a
predetermined cutoff value (set at 50% for C. glabrata and C. krusei, and at
40% for Candida
parapsilosis).[54]
Using MALDI-TOF MS, a simple and rapid AFST assay (named ms-AFST) was
established to discriminate susceptible and resistant isolates of Candida albicans
after a 3-h incubation in the presence of “breakpoint” concentrations
of caspofungin; after the fungal spectra at concentration 0, 0.03, or
32 μg/ml of caspofungin were compared to create individual composite
correlation index (CCI) matrices, the tested isolates were classified
as susceptible or resistant to caspofungin if the CCI values of the
spectra at 0.03 and 32 μg/ml were, respectively, higher or lower than
the CCI values of the spectra at 0.03 and 0 μg/ml.[55]
Finally, IMC was evaluated for “real-time” susceptibility testing of Aspergillus
species, by measuring the thermal variations induced by the action of
antifungals; this led to define the minimal heat inhibitory
concentration (MHIC), that is the lowest antifungal concentration which
inhibits 50% of the total heat produced by the growth control at 48 h
or, only for anidulafungin and caspofungin, the lowest antifungal
concentration which reduces the heat-flow peak by 50%.[56]
It should be noted that while the time-to-result of an IMC assay is
surely not different from that of conventional MIC methods (Table 1),
the susceptibility endpoints for the echinocandins are hard to
determine due to significant trailing growth, and the MEC reading is
actually subjective and poorly reproducible.[22]
As
an alternative to the classical MIC, the new endpoints could then
provide a simple, reliable, and accurate means of identifying
antifungal-resistant isolates, thus potentiating the practicability and
the clinical utility of AFST. However, further studies need to be
undertaken to improve reproducibility and standardization of the recent
developments in AFST, in order to transform them in clinical useful
assays in the next future.
Conclusions
Although AFST is considered currently a valid method, it remains a very dynamic field of clinical microbiology, as further research is needed before MICs are independently used to guide treatment decisions[15] and before the standardization process is completed to include all known antifungal compounds and fungal species.[42] While a crucial issue is whether current AFST methods and antifungal breakpoints are capable of identifying resistant fungal isolates, associated with treatment failures, new alternate AFST methods should be introduced to improve the detection of antifungal resistance, which is perhaps the most challenging goal in clinical microbiology.
References






















































[TOP]