Received: February 10, 2014
Accepted: April 10, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014032, DOI 10.4084/MJHID.2014.032
This article is available on PDF format at:

Anna Maria Testi1, Mariella D’Angiò1, Franco Locatelli2, Andrea Pession3 and Francesco Lo Coco4,5
1
Department of Cellular Biotechnologies and Hematology, Sapienza
University of Rome, Italy
2 Department of Pediatric Hemato-Oncology, IRCCS
Ospedale Bambino Gesù, Roma University of Pavia, Italy
3 Department of Pediatric Hemato-Oncology,
University of Bologna, Italy
4 Department of Biomedicine and Prevention,
University Tor Vergata, Rome, Italy
5 Laboratory of Neuro-Oncoematology, Santa
Lucia Foundation, Rome, Italy

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract The
outcome of adults and children with Acute Promyelocytic Leukemia (APL)
has dramatically changed since the introduction of all trans retinoic
acid (ATRA) therapy. Based on the results of several multicenter
trials, the current recommendations for the treatment of patients with
APL include ATRA and anthracycline-based chemotherapy for the remission
induction and consolidation, and ATRA combined with low-dose
chemotherapy for maintenance. This has improved the prognosis of APL by
increasing the complete remission (CR) rate, actually > 90%,
decreasing the induction deaths and by reducing the relapse rate,
leading to cure rates nowadays exceeding 80% considering both adults
and children.[1-9] More recently
the combination of ATRA and arsenic
trioxide (ATO) as induction and consolidation therapy has been shown to
be at least not inferior and possibly superior to ATRA plus
chemotherapy in adult patients with APL conventionally defined as
non-high risk (Sanz score).[10]
Childhood APL has customarily been treated on adult protocols. Data from several trials have shown that the overall outcome in pediatric APL appears similar to that reported for the adult population; however, some clinical and therapeutic aspects differ in the two cohorts which require some important considerations and treatment adjustments. |
Introduction
Epidemiology of Pediatric APL
Compared to that of Adults
In childhood, APL is very rare disease; its incidence seems to be
different according to various geographic areas. In the United States,
as in Central and Northern Europe, the percentage of APL patients is
5-7% of all pediatric acute myeloid leukemia (AML) cases; a higher
frequency (about 20%) is reported in children of Latino/Hispanic
descent.[6-8;11-16]
In the AML Berlin-Frankfurt-Münster (BFM) studies,
8-10 APL children were registered per year, compared to 16-18 pediatric
patients treated, each year, within the Gruppo Italiano Malattie
Ematologiche dell’Adulto (GIMEMA)-Associazione Italiana Ematologia
Oncologia Pediatrica (AIEOP) AIDA trials.[6,14,17]
Both in the
Latino/Hispanic and in Western countries, however, the number of adult
APL is much higher compared to that of children, like it is observed in
the other forms of AML (110-120 new adult APL cases/a year, in
Italy).[5,18]
Consequently, pediatric patients have represented only a
minimum percentage in trials enrolling both children and adults.
Between November 1996 and June 2004, the Programa Espanol de
Tratamientos en Hematologia (PETHEMA) LPA96 and LPA99 studies included
639 consecutive patients with newly diagnosed APL from Spain,
Netherlands, Belgium, Argentina and the Czech Republic; 67 of them
(10.5%) were aged less than 18 years.[2,3]
In the Italian GIMEMA
AIDA-0493 and -2000 protocols, a total of 1095 adults with APL (age
18-61) were included; in this time frame, a total of 247 (22.6%)
children received the same treatments.[5,6,17,18]
Data from single institutions, as well as population-based study,
suggested that the Latino-American population has a higher proportion
of APL among AML diagnoses, which account for as much as 37.5%. In
centers of Brazil, it has been reported that APL represents 28.2% of
all AML cases, a fraction that is very similar to that reported by Melo
et al (28%) in another Brazilian center. These figures have been
confirmed by information from Mexico (20%), Venezuela (27.8%) and Peru
(22%). The increased incidence has also been reported in pediatric
age.[19,22]
In China, the lack of population-based registries makes it difficult to
determine the real incidence of APL, which is estimated on the basis of
its relative frequency among other AML subtypes in large clinical
trials. According to data so far published, it appears that the Chinese
population has a higher prevalence of APL when compared to most
non-Chinese studies.[23,24] The
most striking ethnic difference is
evident in children. In a single-center large series of 629 Chinese
patients with de novo AML, 138 (22%) were diagnosed as having APL,
cytogenetically confirmed; the incidence of APL was higher in the
pediatric age (34%) compared to both adults (19%) and elderly (3%). In
Peking Union Medical College, 51 (31.6%) of the 141 cases of pediatric
AML, registered between 1996 and 2004, were diagnosed as having APL; in
the Children’s hospital of Zhejiang University School of Medicine,
between 1997 and 2005, 49 (26.5%) of the 185 newly diagnosed pediatric
AML, were APL. The percentage of APL could be higher in children than
in adults, in this country, but a population-based cancer registry
would be necessary to confirm this data.[23-25]
Also in low-income
countries such as Iraq, the real incidence of pediatric (< 15
years)
APL is still unknown; it is estimated on the basis of its relative
frequency among other pediatric AML subtypes diagnosed at a single
institution. At the Pediatric Oncology Unit, College of Medicine, in
Baghdad, which represents a referral center for childhood cancer in
Iraq, the overall high incidence of childhood APL is recorded:
approximately 30% of pediatric AML are morphologically diagnosed as
APL.[26] The same high incidence
is not registered among adult and
elderly patients (age > 15 years), but in this country many
adult
patients with leukemia are not referred to a specific oncology center.
Thus, the presumed higher prevalence of APL may reflect underestimation
of AML cases. Indeed, it is difficult to identify the reasons behind
the apparent high prevalence of Iraqi childhood APL; in fact,
epidemiological and environmental studies are not carried out, and
pediatric and adult cancer registries are still not available.
Ethnic variability may also account for the different incidence of APL
in the various countries; environmental factors may play a role;
however, the incidence in the different age groups is still not
explained. In addition, the diagnostic poor facilities, have to be
considered as possible bias for the reported higher incidence of
childhood APL in the developing countries.
Are there Clinical and Biological Differences in Pediatric APL Compared to Adults?
Some pediatric trials have shown clinical and biological
differences between adults and pediatric APL. Pediatric APL is
diagnosed at a median age of 9-12 years; diagnosis at age < 1
year
is very rare in all countries. In our GIMEMA-AIEOP AIDA-0493 study, one
of the 124 children was under 2 years, and only one of the successive
123 children treated with GIMEMA AIDA-2000 protocol was aged <
12
months.[6,17]
Among the 66 children included in the PETHEMA LPA96 and
LPA99 studies, only 6 of them were less than 3 years old (9%).[7] The
BFM-AML Study Group reported 81 children and adolescents treated with
three consecutive protocols (AML-BFM-93, -98 and -2004 studies); only
one of them was under 1 year of age and 4% (2 cases) of the 53 children
of the first North American Intergroup trial (INT0129) were aged ≤ 2
years.[11,14]
APL seems to be more frequent in children with an age ≥ 10
years: 61% in the PETHEMA studies, 50% in the Italian GIMEMA-AIEOP AIDA
and 65% in the BFM protocols.[6,7,14]
A higher frequency of APL in older
children has been also described in two small series of Chinese
patients (10/19 and 18/37 children aged over 10 years, in the two
studies, respectively).[23,24]
However it should be noted that, in the
pediatric APL series reported to date in the literature, the median age
ranged widely, from 7.2 years in the German Austrian Swiss study to 15
years in the European APL study.[28,29]
In most countries, the incidence
of the disease increases during the second decade of life reaching a
plateau during early adulthood when the incidence remains constant,
until it decreases after 60 years of age.
The female sex seems to be predominant among children but not in
adults; the predominance of girls (71%) was reported in the French
pediatric study published by de Botton et al., by the Children Cancer
Group (CCG) and Pediatric Oncology Group (POG) (60%) and by the PETHEMA
group (59%) but not in our GIMEMA-AIEOP large group that reported a
female/male ratio of almost 1.[6,8,13]
An equally even distribution of
the two sexes in children and adults had been previously noted by
Guglielmi et al, in a series of 196 Italian APL patients.[30]
High body mass index (BMI) is more frequent in APL than in other AML
subtypes, both in adults and children. Estey reported, in 1999, that an
increasing BMI was strongly associated with a diagnosis of APL among
patients affected by AML; in a cohort of 1245 patients with AML, which
included 120 APL, the mean BMI was 27.6 and 25 in APL and no-APL
patients, respectively.[31] At our
Hematology Department, in the
“Sapienza” University of Rome, 90 patients (62.5%), of a group of 144
consecutive patients, both adults and children, who received the GIMEMA
AIDA protocols, were overweight, and of them 66% were over 40 years.
Increased BMI is present in children with APL, but seems to increase
with age in all APL patients.[32]
Compared to the disease in adults, childhood APL is more frequently
associated with hyperleukocytosis and a higher number of circulating
blasts; in spite of this, the outcome results are comparable. It should
be noted that the three largest pediatric studies reported a relatively
high proportion of children with hyperleukocytosis at presentation,
ranging from 35% to 48%. In both GIMEMA-AIEOP AIDA and European APL
93-2000 trials approximately 35% of pediatric patients were classified
as high risk patients according to WBC count.[6,17] A significantly
higher median value of WBC counts at diagnosis had been previously
reported in children by Guglielmi et al (3.6x109/L
vs 2,6x109/L,
respectively in children and adults).[30]
One large recent European APL
study, reporting the analysis of 84 children treated with 2 consecutive
trials, confirmed this observation and described the difference between
children ≤12 vs ≥ 13 years of age (WBC 10.8 vs 2.6x109/l
respectively). Using the commonly adopted cut-off value of 10x109/L,
the incidence of hyperleukocytosis is clearly higher in children than
in adolescents and in adults, in whom it is usually around 20% to
25%.[33] The 749 adults, who
entered the same European APL protocols, had
a similar median WBC levels (2.3x109/l)
as adolescents; thus the
biggest difference in WBC count at onset is observed between young
children and patients aged more than 13.[28,33] Also in smaller series of
children with APL, a WBC count at presentation over 10x109/l seems to
be more common; in the first French childhood series, 13/31 (42%)
children had WBC greater than 10.0x109/l,
and 7 of them presented WBC
over 25.0x109/l
at diagnosis.[8] In the BFM
pediatric APL series, 30/81
children (30%) presented WBC ≥ 10x109/l,
with 3 of them showing
leucocytes count ≥ 100x109/l.[14] In the Japanese series of 58
children
with APL, 35 (60%) had hyperleukocytosis at disease presentation.[34]
Other characteristics, such as the microgranular M3 variant (M3v
according to French-American-British –FAB- classification) and the
promyelocytic leukemia/retinoic acid receptor-alpha (PML/RARα) isoforms
bcr2 and bcr3 have been reported with increased incidence in children
(25% and 37.5%, respectively) as compared to adults (12% and 25%,
respectively), although isoform bcr1 remains the most common in all age
groups.[8,28]
A higher prevalence of M3v morphology (32% vs 16.5%) and
the bcr3 type of PML/RARα transcript (56.5% vs 35.5%) have been
reported for pediatric APL in some series.[30] However the association
of
M3v and bcr2 and bcr3 isoform with pediatric age is not clearly defined
and not always reported in very recent trials. Bally et al. found
higher incidence of M3v in patients with APL aged < 18 ( 23% ≤12
y,
24% 13 to 18 y; 13 % 19 to 60 y; p=0.03) but no difference regarding
PML/RARα isoforms (bcr1: 50% ≤12 y, 62% 13 to 18 y; 61 % 19 to 60 y;
bcr2: 14% ≤12 y, 9% 13 to 18 y; 9 % 19 to 60 y; bcr3: 36 ≤ 12 y, 29% 13
to 18 y; 30% 19 to 60 y; p= 0.79).[33]
Similarly, M3v and the PML/RARα
isoforms bcr2 and bcr3 were found not to be increased in the
AIEOP-GIMEMA and PETHEMA studies when compared to adults. In fact, the
incidence of 18% and 43% of M3v and of the bcr3 isoform in the PETHEMA
pediatric series do not differ from the 19% and 44% reported for the
whole series of APL patients included in the PETHEMA LPA96 and LPA99
studies.[2,3,5-7]
A low incidence of additional cytogenetic rearrangements has been
reported in pediatric APL by Raimondi et al and in children included
in the European APL93 trial (11% of children vs 27% of adults carrying
chromosomal abnormalities in addition to PML/RARα).[35]
On the contrary,
these findings were not confirmed by Ortega at al in the PETHEMA LPA96
and LPA99 trials in which the proportion of children with additional
chromosomal abnormalities did not differ from that reported in the
PETHEMA study of adult patients.[2,3,7]
FMS-like tyrosine kinase (FLT3) mutations have been examined as a
prognostic indicator in adult and pediatric APL. Mouse models have
demonstrated that FLT3 mutations cooperate with RARα translocations by
conferring a proliferative advantage to cells in maturation arrest. The
differentiation arrest caused by t(15;17) likely cooperates with the
proliferative advantage conferred by FLT3 mutations in APL development
and/or progression. Studies of APL patients (mostly adults) have shown
that 20-30% of patients harbor in their leukemic cell the FLT3/internal
tandem duplication (ITD) and another 10-20% carry the FLT3/tyrosine
kinase domain (TKD) mutation. As to the prognostic significance of FLT3
mutations in APL, there is no consensus at present and divergent
conclusions have been reported in the published studies. The European
cooperative APL Group found that there was a trend toward shorter
overall survival in patients with FLT3/ITD (but not in those with
FLT3/TKD) due to very poor post-relapse survival.[36]
On the contrary, no
correlation between FLT3 mutations and survival have been found by
Stock, et al. in 78 adult patients treated on Cancer and Leukemia Group
B (CALGB) C9710.[37] Nevertheless
in the UK Medical Research Council
(MRC) AML10 and AML12 trials that included 203 adult and children with
APL, patients with FLT3 mutations [both FLT3/ITD and missense mutations
in the activation loop domain of the tyrosine kinase domain (FLT3/ALM)]
had a higher rate of induction death but no difference in relapse risk
or overall survival.[38] The
prevalence and the prognostic significance
of FLT3 mutations have not been well defined in childhood APL. One
earlier study examined FLT3 mutations in a pediatric APL population;
among 29 children, FLT3 mutations were present in 10 (34.5%) of them
and were strongly associated with higher leukocyte count.[39] The largest
study on FLT3 mutations restricted to pediatric patients with APL
examined 104 patients aged < 21 years; 81 treated within
cooperative
group trials CCG-2891 (n=13), CCG-2911 (n=18) and CALGB C9710 (n=50)
and 23 treated according to institutional standard therapy.[40] This
study demonstrated a high prevalence of both FLT3/ITD and FLT3/TKD
mutations in childhood APL (40%). Furthermore, a strong correlation
between FLT3 mutations and WBC count at diagnosis (median diagnostic
WBC count for children with FLT3 mutations 32.95x109/l
compared to
3.6x109/l
in those with wild-type FLT3 -p=0.004) and a significantly
higher proportion of M3v in FLT3 mutated compared to FLT3 wild type
patients (47% versus 15%, p=0.035) were found. In the same study,
analysis of induction death by FLT3 mutational status in the high WBC
count group showed an early death rate (EDR) of 47% and 0% in FLT3
mutant and FLT3 wild type patients, respectively (p=0.052). The
association of FLT3 mutant genotype with induction death in patients
with higher WBC count may indicate direct contribution of the FLT3
activation to coagulation dysregulation. If the link between FLT3
mutated status, coagulopathy and induction death observed in this study
is further substantiated, interruption of the FLT3 signal transduction
pathway by FLT3 inhibitors may represent an attractive therapeutic
strategy to ameliorate the rapidly progressive coagulopathy and
counteract early death risk.
Other reported characteristics of childhood APL, compared to adults,
include more frequent organomegaly and a higher incidence of the
expression of the T-antigen CD2 and of the stem cell marker CD34, which
are generally also correlated with bcr3 isoform and M3v.[30]
The true incidence of central nervous system involvement (CNS) at
diagnosis is unknown both in children and adults. One small pediatric
study described initial CNS leukemia in 3/40 (7,5%) patients while a
large more recent pediatric trial reported an incidence of less than
2-5%.[6-8;14,41] Patients with APL commonly present
with coagulopathy.
Approximately 80% of them have a prolonged INR, elevated fibrinogen
degradation products, low fibrinogen, and thrombocytopenia, all
potential causes of the severe hemorrhages. Therefore, it is
recommended in modern guidelines that lumbar puncture at diagnosis is
not performed in light of the high risk of bleeding.
Current Treatment Approach in Childhood APL
Specific therapeutic
strategies for pediatric APL have been derived from adult trials that
included children. Most of these approaches include the simultaneous
combination of ATRA and anthracycline-containing chemotherapy. In the
firstly published study of a German-Austrian-Swiss group, 95% of the 22
children treated with ATRA followed by chemotherapy achieved CR and the
5-year Overall Survival (OS) and Event-Free-Survival (EFS) were 87% and
76% respectively.[29] The European
APL93 study included 31 children
receiving ATRA followed by or combined to daunorubicin and cytarabine;
the CR rate was 97%, and the 5-year OS and EFS were 90% and 71%,
respectively.[8] The Italian
GIMEMA-AIEOP AIDA-0493 trial (ATRA and
idarubicin as induction followed by 3 polychemotherapy consolidation
courses), the largest pediatric APL series during the ATRA era,
reported a CR rate of 96% and a 10-year OS and EFS of 89% and 76%
respectively. Similar results were obtained in children treated with
PETHEMA LPA96 study that included the same idarubicin and ATRA
combination followed by three anthracycline-based consolidation courses
(CR 92%; OS 71%).[7] The main
characteristics and therapeutic results of
these studies are summarized in Table
1. All of these studies confirm
the virtual absence of leukemia resistance using state-of-the-art
treatment. Sample size, eligibility criteria, and some differences in
patients characteristics with potential impact on responses to therapy
can explain the apparently different results, which are not
statistically significant.
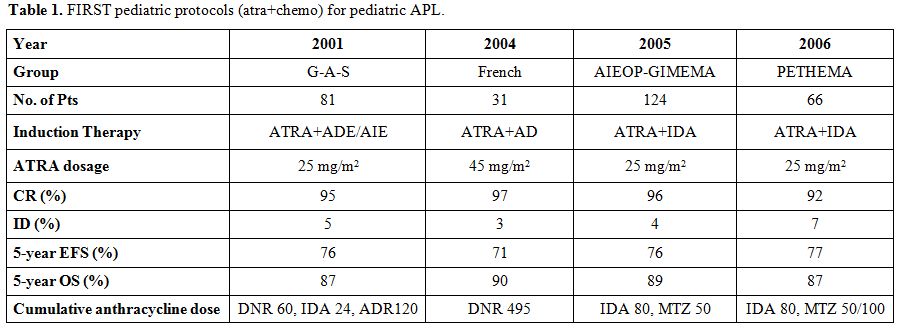 | Table 1. FIRST pediatric protocols (atra+chemo) for pediatric APL. |
Since the
introduction of ATRA, the EDR, mainly because of bleeding, is
dramatically reduced in the pediatric APL studies (3-7%); similar
results have been reported in most of adult cooperative group trials
(5-10%).[5,9]
However significant higher EDR have been recently observed
in unselected population-based studies by Park et al[42] and Lehamann et
al[43] (17.3% and 29%,
respectively, with a higher incidence for older
patients), in which all adult patients with newly diagnosed APL are
reported. The apparently lower EDR in adults and especially in children
enrolled in the clinical trials may partially reflect earlier referral
to specialized centers, without delay in ATRA administration. In
children, the fewer age-related comorbidities could explain the lower
EDR. However, to better establish the size of the problem, all authors
of clinical trials studies should be requested to report all
information for patients who were excluded from the study because of
eligibility criteria.
The APL trials conducted in the 1990-2000 decade provided an important
source for the investigation of prognostic factors to be used for
treatment stratification. In particular the so called Sanz’s score for
the relapse risk (low: initial WBC < 10x109/l
and platelet count
> 40x109/l;
intermediate: WBC < 10x109/l
and platelet count ≤
40x109/l;
high-risk WBC ≥10x109/l)
was developed to dissect relapse
risk categories for patients receiving AIDA-like regimens adopted by
the GIMEMA and PETHEMA groups.[1,9] This in turn allowed the design of
distinct strategies which were aimed at sparing unnecessary toxicity
for patients with low-risk (WBC < 10x109/l),
whereas more intensive
post induction chemotherapy including cytarabine were adopted for high
risk (WBC > 10x109/l)
patients. The results of both GIMEMA and
PETHEMA trials using a risk adapted approach for adult APL, showed a
significant improvement in patient outcome.[18,44] Other large trials
conducted by the French European APL group, the British MRC, the
Japanese Adult Leukemia Study Group (JALSG) and the German AML
Cooperative Group (AMLCG) confirmed the advantage of risk adapted
strategies using mainly WBC count as a prognostic factor. Overall,
studies reported in recent years with ATRA-based and risk adapted
chemotherapy, resulted in CR rates of up to 95% and OS rates >
85%
for adult APL.[45] In the
pediatric setting, initial WBC count is the
most important prognostic factor influencing the outcome and children
with WBC count higher than 10x109/l
(who usually have a younger age as
compared to children with low WBC counts) have a higher risk of
relapse. In the pediatric series of GIMEMA-AIEOP AIDA 0493 trial, a
leukocyte count at diagnosis ≥ 10x109/l
had a negative impact on EFS
(59% vs 83% at ten years); the 5-year cumulative incidence of relapse
(CIR), among the 61 children treated with PETHEMA trials, was higher
for those with presenting WBC ≥ 10x109/l,
compared to those with lower
WBC count (31% vs 3.5%).[6,7] The
PETHEMA LPA 99 and GIMEMA AIDA-2000,
risk-adapted trials, were adopted also in the pediatric population and
confirmed the improvement in results as reported in adults. For
children in the previous PETHEMA LAP96 study, the 5-year disease-free
survival (DFS) was 75%, whereas, in the LPA99 study, it was 89%.[7] The
6-year OS and DFS rates for the 123 children treated with AIDA 2000
risk-adapted regimen resulted superior compared to those achieved in
children who received the AIDA 0493 protocol (96% vs 89.7% and 82.5% vs
73.1%, respectively). For the low-risk children, the less intensive
anthracycline-based plus ATRA consolidation was equally effective as
the previous cytarabine-containing regimen (6-year OS and DFS 94.2% and
95.6% vs 76.7% and 82.7%, respectively). The role of ATRA combined with
cytarabine and anthracyclines during consolidation resulted in a
significant improvement in OS and DFS in the high-risk group (96.8% and
82.3% vs 81.6 and 65.2, respectively for AIDA-2000 vs-0493).[17]
As mentioned above, the incidence of CNS involvement in APL, both at
diagnosis and relapse, remains to be established, as does the need for
prophylactic intrathecal chemotherapy in children and adolescents as an
integral part of first-line therapy. Approximately 10% of relapses have
a CNS component.[1] The risk of CNS
involvement seems to be extremely low
in patients without hyperleukocytosis at diagnosis and, in any case, it
is more frequent for those with initial WBC > 10x109/l. Other
risk
factors for CNS recurrence remain a controversial matter. Some authors
have previously suggested that FLT3-ITD mutation, which correlate with
hyperleucocytosis and an increased expression of adhesion molecules,
such as CD56, can promote leukemic infiltration in the CNS. In the
PETHEMA LAP96 and LAP99 studies, CNS relapse was associated with CNS
hemorrhage before or during induction treatment, which emerged as a
novel and independent prognostic factor.[46]
This has not been reported
before and could have potential therapeutic implications. Therefore,
CNS prophylaxis could be considered at least for high-risk patients. In
addition, high-dose cytarabine, that ready penetrates the blood-brain
barrier could represent a valuable tool to prevent CNS involvement in
APL.
Has Cytarabine, at High Doses, a Role in the Consolidation Treatment of Children with APL?
The PETHEMA-LPA2005 study suggested that the addition of high-dose cytarabine (1 g/m2/day x 4 days) to consolidation therapy could reduce the incidence of relapse in patients defined at high risk. Other European adult trials have confirmed this finding. [44,45,47] Two questions remain unanswered: 1) can cytarabine replace anthracycline in the consolidation treatment of APL? 2) is there any benefit of adding cytarabine to the consolidation schedules? Both questions are a matter of investigation in children, mainly in those at high-risk. The 5-year DFS of PETHEMA LPA96 (anthracycline monotherapy in consolidation) and GIMEMA-AIEOP AIDA 0394 (polychemotherapy combination with high-dose cytarabine in consolidation) showed no clear difference in the outcome of pediatric APL (5-year EFS 76% vs 77%).[6,7] Luo et al. suggested that the children included in the PETHEMA trials (without cytarabine) had a significant higher EFS (3.5-year EFS 79.6% vs 37.5%; p 0.012), lower frequency of sepsis during treatment (7.7% vs 78.8%; p 0.0015) and lower hospitalization cost than those treated with protocols containing high-dose cytarabine (USA $ 4,700 vs 20,000; p < 0.0001).[48] On the contrary the BFM protocol, in line with other reports, combined cytarabine at intermediate-high dose to anthracyclines for consolidation therapy of pediatric APL and reported 5-year EFS and OS rates of 73% and 89%, respectively.[14] These results support the efficacy of high-dose cytarabine in combination therapy for pediatric APL. In the Japanese childhood acute myeloid leukemia cooperative study (AML99-M3), cytarabine was combined with ATRA and anthracycline both in induction and consolidation; the 7-year EFS and OS of the 58 enrolled children were 91% and 93%, respectively, and the CIR plateaued at 3.6% after 2 years. In this last trial, the addition of ATRA in the consolidation phase also contributed to improve the results (Table 2).[34] Furthermore, the European Leukemia Network recommended including at least one consolidation cycle of high-dose cytarabine for young high-risk patients.[1]
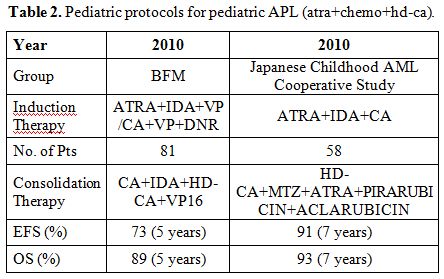 | Table 2. Pediatric protocols for pediatric APL (atra+chemo+hd-ca). |
Are there Particular Treatment Issues for Children with APL?
The
optimal pediatric dose of ATRA has not yet been established; the
idiopathic intracranial hypertension, commonly called pseudotumor
cerebri (PTC), can complicate the treatment of APL with ATRA. The
diagnosis of PTC is based on increased intracranial pressure with
normal cerebrospinal fluid composition and negative cerebral imaging
studies (computed tomography or magnetic resonance imaging scan). This
side effect is more common in children and adolescents, but the
incidence decreases with the use of a lower dose of ATRA, without
apparently compromising the outcome results. In the European APL 93
trial with the dose of 45 mg/m2
severe headache episodes were more
frequent in the pediatric population than in adults (16% vs 1-2%).[8,28]
Several studies have also reported increased neurotoxicity of ATRA in
children, particularly in younger age (<10 years). In an attempt
to
reduce ATRA related toxicity the daily dose administered in children
treated according to GIMEMA-AIEOP AIDA and PETHEMA protocols, was
reduced to 25 mg/m2.[6,7] This dose proved to be equally
effective with a
lower incidence of side effects in a previous adult APL dose reduction
trial. Available data also suggest that a half dose of ATRA can be as
effective as the standard dose of 45 mg/m2
per day.[49] The apparently
lower incidence of PTC and headache, together with the excellent
therapeutic results obtained with ATRA at 25 mg/m2
suggest that such
dose could be the recommended standard for children.[50]
Another issue of particular importance in children with APL is the
difficulty in swallowing the 10-mg gel caps of ATRA, since the
medication is not available in liquid form. Some studies suggest that
the contents of an ATRA capsule can be mixed with milk and administered
in a nasogastric tube, for comatose patients, reaching a high serum
level.[50,51] Similarly, in very
young children it is possible to soften
capsules in warm milk and so chew them alone or mixed in a spoonful of
soft food. An intravenous liposomal formulation of ATRA has been tested
in newly diagnosed patients with APL unable to swallow or absorb
medications and has been shown to be effective in inducing CR. Unlike
oral ATRA, liposomal ATRA was able to produce molecular CR without
addition of chemotherapy. In patients with newly diagnosed APL, using
polymerase chain reaction (PCR) assay with a sensitivity level of 10-4,
Estey et al reported that liposomal ATRA monotherapy induced molecular
CR in a significant fraction of patients within 3 months. In more than
30% of patients, PCR negativity persisted for years thereby indicating
that liposomal ATRA as a single agent may be curative in APL. However,
this ATRA formulation is no longer available.[52]
A relevant problem, linked to the use of chemotherapy in the pediatric
population, is a risk of cardiomyopathy, a real threat for children
with APL treated with regimens that include high doses of
anthracyclines. As suggested by Van Dalen et al, the risk of developing
clinical heart failure is dose-dependent, increasing from 0% for 150
mg/m2
of cumulative anthracycline dose, up to 14.3% for doses of 600
mg/m2.[53] Relatively high-dose anthracyclines
(450–750 mg/m2)
used in
modern chemotherapy plus ATRA regimens have proven successful to
achieve high cure rates in adults and children with APL, although the
high cumulative anthracycline dose was potentially associated with high
risk of late cardiotoxicity.[4,6,7,44]
Although no severe acute
cardiotoxicity was observed in our first GIMEMA-AIEOP study, longer
follow-up is needed to define the late cardiotoxicity of anthracycline
regimens. Late subclinical cardiotoxicity was observed in 52% of the
adult survivors of APL treated on the GIMEMA AIDA-0493 and-2000
protocols.[54] To reduce the risk
of developing clinically significant
cardiotoxicity and heart failure, which is approximately 5% at 15 years
after anthracycline therapy for childhood cancer, the AML BFM study
group limited the cumulative anthracycline dose to 350 mg/m2 in most
APL patients obtaining results comparable to those reported for studies
with higher doses.[14] In the
PETHEMA 2005 study, a lower anthracycline
dose provided equivalent efficacy with less myelosuppression in
patients at low and intermediate risk.[44]
Based on these findings, the
International Consortium for Childhood Acute Promyelocytic leukemia
(ICC APL) was established and a new study (ICC APL 01) was launched and
is still actually ongoing with the goal to investigate the safety and
efficacy of a regimen with a reduced cumulative anthracycline dose of
355-405 mg/m2
in combination with ATRA and a high dose of cytarabine in
one consolidation course for low risk and two courses for high risk
patients. Due to the obvious concerns of irreversible heart failure in
children who receive high cumulative doses of daunorubicin, also the
CALGB C9710 study utilized a cumulative dose of daunorubicin of 500
mg/m2
for those >15 years of age and 400 mg/m2
in children 3–14
years of age.
Role of Arsenic Trioxide (ATO) in Pediatric APL
Treatment strategies
for childhood APL aim to decrease the incidence of relapse and
chemotherapeutic toxicity. The introduction of ATRA has been crucial
for both antileukemic efficacy in APL and for reducing EDR.
Furthermore, in more recent studies, Arsenic Trioxide (ATO), first
introduced for the treatment of relapsed patients has resulted highly
effective in achieving high cure rates in association with reduced
toxicity in adults with APL.[55-59]
Arsenic compounds had been used as therapeutic agents for more than
2000 years in Western and Eastern medicine, particularly in China.
Despite the advent of new cytotoxic drugs the empirical use of arsenic
as an antileukemic agent continued in China throughout the past century
leading ATO to be introduced into the treatment of APL in the 1970s.
The specific mechanism of ATO in APL treatment is still under
investigation. A dual mechanism of action has been described; as
demonstrated by Chen et al[55] and
Shao et al,[56] at low
concentration ATO
exerts a partial differentiating effect by inducing the stimulation of
both PML-RARα and PML leading to the degradation through the proteasome
pathway. On the contrary at high concentrations, ATO induces apoptosis
through caspase activation, reactive oxygen species (ROS) production
and induction of mitochondria mediated intrinsic apoptotic pathway.
Several studies have shown the benefits of ATO in the treatment of
relapsed APL, with a remission rate of 80-90% and long term DFS of
60-80%, and also as induction and consolidation therapy for adults with
newly diagnosed APL, demonstrating that ATO is the most active single
agent in this disease.[55-59]
However, experience with ATO for treating pediatric APL is limited
compared to that achieved with adults APL studies. Two small but
significant series[27,60] reported the use of ATO a single
agent for both
remission induction and post-remission therapy in children with newly
diagnosed APL. In both studies, the morphological CR rate was
approximately 90% (91% and 89.5% respectively in George et al[60] and
Zhou et al[27]), and no resistant
cases were recorded. The use of ATO as
a single agent in post-remission therapy led to an estimated 5-year OS
and EFS of 91% and 81% and 84% and 73%, respectively in George’s[60] and
Zhou’s[27] series, respectively.
These results were comparable with those
achieved with ATRA plus chemotherapy. ATO-related toxicity was minimal
and transient during induction, and neutropenia was the most common
side-effect during the 3-year post-remission ATO therapy.[27,60]
A striking convergence regarding the antileukemic effects of ATRA and
ATO is the degradation of PML/RARα through distinct pathways, with ATRA
targeting the RARα and ATO targeting the PML moieties of the fusion
protein. The combined use of ATRA and ATO synergistically induces
differentiation, apoptosis and accelerates tumor regression in vivo.
Shen et al[61] suggested that
ATRA-ATO combination for treating adult APL
significantly shortened the time to achieve CR, reduce the disease
burden and improved DFS compared with approaches based on the use of
either ATRA or ATO alone.[61]
These benefits of combining ATRA and ATO
have been confirmed by several groups in adults and, more recently, in
pediatric APL.[27,62]
The retrospective analysis at Pediatric Department,
Pecking University, Medical School, showed that the application of ATRA
and ATO as induction and consolidation therapy for newly diagnosed
children with APL resulted in excellent outcomes and improved the
long-term prognosis. Children treated with ATRA-ATO combination had
significant better CR and EFS rates, compared to those who received
ATRA-based regimes (CR 95.3% and 80%; EFS 92.5% and 70.4%,
respectively). Arsenic was well tolerated in children and was devoid of
major acute side-effects.[63] Table 3 summarizes
the results in pediatric
APL with ATO as first-line therapy.
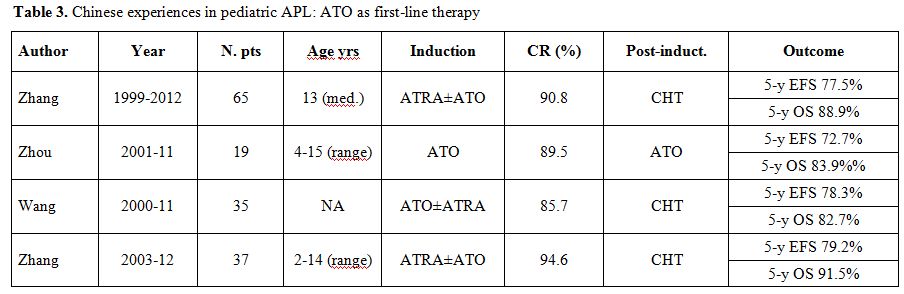 | Table 3. Chinese experiences in pediatric APL: ATO as first-line therapy. |
The long-term safety
of ATO in children is unclear. Reported late
effects include hyperpigmentation, neutropenia, muscular atrophy and
peripheral neuropathy. One study reported a significant association
between the children’s neurocognitive function and their chronic
environmental ATO exposure.[50]
What is the Role of Stem Cell Transplant in Pediatric APL?
Several
large multicenter studies have shown that regimes combining upfront
ATRA and chemotherapy and/or ATO lead to a high curability rate in APL,
suggesting that hematopoietic stem cell transplantation (HSCT) is not
recommended as consolidation therapy for patients in first complete
remission (CR1). However, despite optimal therapeutic results, relapses
still occur in 15% to 25% of the cases.
While, for patients relapsing after ATRA plus chemotherapy, ATO as
salvage for re-induction is an established recommendation, the
post-consolidation approach to relapsed or refractory APL is less
clearly defined. Although most relapsed patients (> 80%) can
achieve
a second CR (CR2) with ATRA, ATO, chemotherapy (CT), or a combination
of these agents, only occasional cases have demonstrated second
remission for as long as 8-10 years following treatment with
chemotherapy and/or ATRA.
For this reason most relapsed APL patients would receive autologous
(auto), allogeneic (allo) or haploidentical cell transplantation
(HSCT). Auto-HSCT is one of the therapeutic options for patients who
achieve a CR2 and are in molecular remission (MR). Allo-HSCT may be
useful to consolidate patients in CR2 and to treat patients with
persistent minimal residual disease due to a potent graft versus
leukemia effect, the greatest asset of an allo-HSCT in this setting.[64]
In the largest study by Sanz et al,[65]
the investigators retrospectively
analyzed outcomes in 625 patients with APL who had been treated as part
of the European Cooperative Group for Blood and Marrow Transplantation
(EBMT) and who underwent either an auto- or allo-HSCT. In this registry
survey, similar outcomes were shown for both auto and allo-HSCT,
although the allo-HSCT showed a higher incidence of non-relapse
mortality (transplant related mortality TRM), whereas the auto-HSCT
group had a higher risk of relapse. This study including patients in
CR1 or CR2 suggested that, even in the ATRA era, HSCT had to be
considered as consolidation therapy, especially for patients in CR2.[65]
The majority of reports on the use of HSTC for treatment of relapsed or
refractory APL deal primarily with adult patients making the benefit of
these therapies for children still unclear. The Dana Farber Cancer
Institute reported a favorable outcome among children undergoing
allo-HSCT for relapsed and refractory APL with a probability of
survival at 5 years of 73%. This group also reported a TRM of 33% and a
low relapse rate (15%).[66] The
largest study by Dvorak et al.[67]
reported
32 pediatric patients underwent auto or allo-HSCT for treatment of
primary-refractory (3 patients) or relapsed (29 patients) APL. The
incidence of TRM and relapse for auto-transplant in children were 0%
and 27%, and the 5 year EFS and OS of 73% and 82%, respectively; for
the allo-HSCT the incidence of TRM and relapse were 19% and 10%; the 5
year EFS and OS were 71% and 76%, respectively. This study demonstrates
that auto or allo-HSCT are both effective therapies for the treatment
of children with relapsed or refractory APL without significant
difference in EFS and OS but, as previously described in adults, with a
low TRM for auto-HSCT and a low incidence of relapse for allo-HSCT.[67]
Notwithstanding the successful results, the limits of the above
mentioned studies are the retrospective nature, the relatively small
number of patients included, mainly as concerns pediatric age, and the
different therapeutic strategies, according to the single institute
policy.
The optimal stem cell transplantation strategy for advanced APL still
remains controversial, as several factors influence the choice. The
type of salvage regimens, donor selection, conditioning regimens and
graft versus host disease (GVHD) prophylaxis should be included in the
context of prospective clinical trials. However, the superior
protection from relapse afforded by allo-HSCT and the favorable
survival described in the few pediatric experiences reported, suggest
performing allo-HSCT for children with relapsed or refractory APL for
whom a suitable HLA-donor is available. On the other hand, children,
without a suitable donor, achieving molecular remissions after salvage
therapy can be considered candidates for high dose chemotherapy and
auto-HSCT as a valid post-consolidation strategy.
Future Directions
For patients with newly diagnosed, non-high risk
APL, the front-line use of ATRA-ATO combination is extremely
encouraging and will probably become the standard regimen also in the
pediatric age. Future controlled prospective trials should address the
role of ATO in induction and consolidation chemotherapy and establish
whether ATO could reduce cytotoxic chemotherapy intensity in children
with APL. Oral arsenic is also very effective, and its combination with
oral ATRA warrants further investigation also in the pediatric
setting.[68,69] Next ATO-based
pediatric APL trials should include
longitudinal assessment of neurological and neurocognitive outcomes,
evaluation of the growth assessment in treated children and
pharmacokinetic studies will be necessary to investigate how the faster
metabolism in children can promote drug excretion and influence the
right dose to administer, in particular in younger patients.
New drugs, such as FLT3 inhibitors, anti-CD33 monoclonal antibodies and
tamibarotene (a synthetic retinoid more potent and less toxic than
ATRA) may offer additional options for patients with high-risk or
relapsed/refractory APL.
Future efforts should focus on decreasing the delay in referral and
diagnosis; unfortunately intracranial bleeding is still the major cause
of early death and a small percentage of patients, adults and children,
in all countries, are still diagnosed after the occurrence of
life-threatening bleeding. More importantly, as reported by Park et
al,[42] there is a clear need to
provide the knowledge necessary to
recognize APL as a medical emergency, which requires specific and
simultaneous actions, including a prompt initiation of ATRA, aggressive
supportive care to counteract the coagulopathy, and patient referral to
experienced medical centers when the disease is first suspected.
Finally, more studies are warranted to clarify the reasons for the
different epidemiology of pediatric APL in several countries.
References




































































[TOP]