Received: March 2, 2014
Accepted: April 27, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014035, DOI 10.4084/MJHID.2014.035
This article is available on PDF format at:

Umberto Ricardi, Andrea Riccardo Filippi*, Cristina Piva and Pierfrancesco Franco
Department
of Oncology, Radiation Oncology, University of Torino, Torino, Italy.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Radiation
therapy has a key role in the combined modality treatment of
early-stage Hodgkin’s Lymphoma (HL). Nevertheless, late toxicity still
remains an issue. A modern approach in HL radiotherapy includes lower
doses and smaller fields, together with the implementation of
sophisticated and dedicated delivery techniques. Aim of the present
review is to discuss the current role of radiotherapy and its potential
future developments, with a focus on major clinical trials,
technological advances and their repercussion in the clinical
management of HL patients.
|
Introduction
In
the era of modern chemotherapy and new highly effective targeted
agents, many clinicians may perceive external beam radiotherapy (RT) as
an old-fashioned treatment for Hodgkin’s Lymphoma (HL). In fact, the
initial demonstration of X-ray effectiveness in HL was made a century
ago,[1] while the first clinical
results on disease control and survival have been published in 1935 and
1950.[2,3]
However, we are still using this powerful single agent, albeit in a
very different way than in early years. For decades Extended Fields
Radiotherapy (EF-RT) has been considered the standard treatment for
early stage HL, on the basis of the ground-breaking work published by
Kaplan in 1968.[4] It has later
become evident that
EFRT was associated with a high risk of treatment-related
complications, mainly represented by heart diseases, secondary cancers
and endocrine dysfunctions.[5,6,7]
Concomitantly, chemotherapy has been shown to improve results when
combined with radiation in early stages.[8]
A large number of subsequent randomized controlled trials, designed and
conducted over the last 20 years, lead to re-think the role of RT,
modifying its indications and use and questioning its incorporation in
such combinations because of concerns about late toxicity. The
technological “revolution,” occurred over the last 15 years in
Radiation Oncology, made also possible a different technical approach
to HL, by applying the new concepts of high-precision image-guided and
intensity-modulated RT, even when doses in the range of 20-30
Gy
were delivering.
Aim of this review is
a) to summarize and discuss the main changes and the current role of RT
in the treatment for HL, and
b) to delineate the present and future research paths in RT, focused on
maintaining efficacy while minimizing late effects on long-term
survivors.
Overview of Clinical Trials
The initial use of RT was based upon extensive treatment volumes
covering both involved and uninvolved lymphatic sites. For the most
common presentations in early stages, for example, neck and
mediastinum, this approach consisted of sub-total nodal irradiation
(STNI), to the dose of 40-44 Gy. The results obtained in the time lapse
1962-1984 by the Stanford group in early stages with EFRT show complete
remission rates of 100% and recurrence-free survival (RFS) rates of 80%
in stages IA, IIA and IIB without large mediastinal tumors.[9]
In the eighties (1988–1994), the German Hodgkin Study Group (GHSG)
designed the HD4 trial, one of first studies to address a specific
RT-related question. The major aim of HD4 was to show whether the
radiation dose to the non-involved lymphatic regions could be reduced
while maintaining an effective tumor control. Patients with early stage
HL without risk factors (large mediastinal mass, extra-nodal extension,
massive spleen involvement, > 3 lymph node areas, high
Erythrocyte
Sedimentation Rate) were randomized between 40 Gy EF-RT (arm A) and 30
Gy EF-RT plus additional 10 Gy to the Involved Field (IF) region (arm
B). Results showed no statistically significant differences in RFS and
overall survival (OS) between the 2 treatment arms, but the overall
recurrence rate approached 20%. As relapsing patients underwent an
effective salvage therapy, RFS after 7 years came up to 80%, with an
overall survival rate of 93%.[10]
For this study,
GHSG promoted the creation of a task force for quality assurance (QA).
For all patients enrolled in the study, a treatment plan was given by
the radiotherapy reference Centre based on the documentation of the
disease extension on case report forms. After completion of EF-RT, an
expert panel analyzed simulation and verification films of every
individual patient, as well as treatment data. This retrospective
quality control study showed that deviations of radiation treatment
portals and radiation doses from prospective treatment prescriptions
were unfavorable prognostic factors.[11]
Second
generation of trials compared, both in favorable and unfavorable
presentations, EFRT vs. IFRT in combination with chemotherapy. Very
valuable data came from these studies, which completely changed the
previous treatment paradigm, by showing that the combination of
systemic agents and RT was superior to EFRT alone, both in terms of
disease control and inferior toxicity. Moreover, these trials
demonstrated that, when combined with chemotherapy, RT could be safely
reduced to the IF region.[12,13,14]
This evolution
also led to an initial important reduction of late toxicity, as
described by the 2005 Cochrane review focused on the therapy of early
stage HL and second cancer risks.[15]
At the end of
the nineties, a decisive step towards a further reduction of the
therapeutic burden was made by GHSG in 2 key studies, the HD10 ad HD11
(1998–2002). In these trials, irradiation was performed as IF-RT only
in all treatment arms, with reduced total doses in combination with
different chemotherapy schedules. The whole treatment strategy was
based upon a proper selection of patients by known prognostic
factors. In HD10, stage I-II patients without risk factors
(no
bulky disease, less than 3 involved sites, low ESR values) were
randomized in a four-arm study between an IF-RT dose of 30 Gy vs. 20 Gy
and 2 vs. 4 cycles of ABVD. Meanwhile, an extensive quality assurance
program has been made in order to ensure that IF-RT was performed
exactly according to the RT-prescriptions of the protocol.
Results of HD10 were published in 2010:[16]
the 2
chemotherapy regimens did not differ significantly with respect to
freedom from treatment failure (FFTF) (p=0.39) or OS (P=0.61). At 5
years, the rates of FFTF were 93.0% (95% confidence interval [CI], 90.5
to 94.8) with the four-cycle ABVD regimen and 91.1% (95% CI, 88.3 to
93.2) with the two-cycle regimen. When the effects of 20-Gy and 30-Gy
doses of radiation therapy were compared, there were also no
significant differences in FFTF or OS (p=0.61). HD10 showed that the
treatment with two cycles of ABVD followed by 20 Gy of IF-RT was
equally effective, and less toxic (acute toxicity), compared to
treatment with 4 cycles of ABVD followed by 30 Gy IF-RT. Therefore, 2
ABVD cycles plus IFRT 20 Gy emerged as the standard treatment worldwide
for low risk patients. The GHSG HD11 trial,[17]
in
patients with unfavorable early stage disease presentation (bulky
disease, multiple involved sites, high ESR values), showed that, after
4 cycles of BEACOPP, IF-RT 20 Gy was not inferior to 30 Gy, whereas
inferiority of 20 Gy cannot be excluded after 4 cycles of ABVD.
At the same time, other research groups tested a chemotherapy alone
strategy in early stage HL, based on similar criteria for patients’
selection (low risk of treatment failure). Some of these studies were
conducted on children and/or young adults. The CCG 5942 trial showed
inferior 10-year event-free survival for the no RT versus the RT arm
(82.9% vs. 91.2%, p=0.004). After stratification for risk factors, a
significant difference was evident for the low risk patients (89.1% vs.
100%, P=0.001), but not for the intermediate and high-risk groups
(78.0% vs. 84% and 79.9% vs. 88.5%, respectively).[18]
Conversely, the GPOH-HD95 trial showed that the omission of RT was safe
only for low-risk patients with complete response after chemotherapy
(PFS of 96.8% versus 93.6%, p=0.42), whereas this strategy was not
proven to be safe for the intermediate and the high risk groups (PFS
69.1% vs. 92.4%, p<0.001 and 82.3% vs. 90.7%, p=0.08,
respectively).[19]
In adults, the largest study to compare chemotherapy alone with
combined modality therapy was the intergroup HD.6 study (NCIC),
designed with the aim of comparing chemotherapy alone (4-6 ABVD cycles)
to RT only or with 2 ABVD cycles (according to risk groups), with
subtotal nodal irradiation 35 Gy.[20]
An obvious
critical point is that STNI is no more part of current treatments
protocols, and thus a direct comparison on late toxicity versus
chemotherapy alone is unbalanced. In 2010, Herbst et al published a
systematic review with meta-analysis of randomized controlled trials
comparing chemotherapy alone with CMT in patients with early stage
Hodgkin’s lymphoma with respect to response rate, tumor control and
overall survival. Five randomized controlled trials involving 1,245
patients were included. The hazard ratio was 0.41 for tumor control and
0.40 for OS for patients receiving CMT compared to chemotherapy alone.[21]
The results of these studies raised an important debate in the
scientific community, still ongoing at present. An individual patient
meta-analysis was recently undertaken to compare HD10 and HD11 results
with HD.6 study. On 406 patients who fulfilled the eligibility
criteria, combined modality therapy was shown to give better time to
progression (HR=0.44); PFS was superior but without reaching
statistical significance, and overall survival superimposable.
Remarkably, the difference between the two treatments was particularly
evident among patients in partial remission after chemotherapy.[22]
The following logical step was to try to better select patients at
lower/higher risk of relapse, and consequently to better adapt the use
of consolidation RT. FDG-PET emerged as a powerful tool to predict
early chemo-sensitivity in advanced stages,[23]
and
was consequently introduced in early stages to stratify patients with
different response to chemotherapy. In these studies, functional
imaging was used to modulate therapy, comparing chemotherapy alone
strategy to combined modality treatment, consisting of a brief
chemotherapy followed by low-dose IF-RT, in patients achieving complete
remission at FDG-PET. Three major trials were designed over the last
years according to this principle, the H10 trial (EORTC/GELA/FIL), the
GHSG HD16 trial and the UK NCRI RAPID trial. In all studies, a panel of
expert Nuclear Medicine physicians reviewed FDG-PET imaging results.
H10 compared ABVD + RT vs. an experimental arm where the treatment was
driven by interim (after 2 ABVD cycles) FDG-PET results. Notably, H10
represented a very innovative step for radiotherapy, introducing the
new concept of “Involved Node Radiotherapy” (IN-RT), a further
reduction of radiation volumes on the basis of pre- and
post-chemotherapy imaging.[24]
Patients with
favorable presentations according to EORTC criteria were randomized to
ABVD x 3 + IN-RT 30 Gy vs. ABVD x 2 and, if PET negative, 2 more ABVD
cycles (chemotherapy alone). This trial is now closed, and the final
results will be available within next 2 years. An independent data
monitoring committee advised to stop the chemotherapy alone arm due to
an excess number of relapses (in both favorable and unfavorable arms).[25]
This decision was deeply discussed, as probably a difference in
failure-free survival between the 2 arms (the primary endpoint for
non-inferiority), was to be accounted in the statistical design at the
beginning, even in patients in metabolic complete response. Overall
Survival is expected to be the same for both arms after adequate
salvage therapy. The ongoing GHSG HD16 trial has more “contemporary”
design with regards to RT doses and compares, in favorable patients
(according to GHSG criteria), a standard arm consisting of 2 ABVD
cycles followed by 20 Gy IF-RT to a PET-guided experimental arm
consisting of 2 ABVD and observation (if negative) or IF-RT 20 Gy (if
positive). The purely RT-related question on the potential equivalence
of IF-RT and IN-RT is being investigated in a parallel trial, the GHSG
HD17.[26]
In UK NCRI RAPID trial, low-risk patients with a PET negative finding
after 3 ABVD cycles were randomized either to 30 Gy IF-RT or to
observation only. Patients with a positive PET were treated with one
more ABVD cycle plus 30 Gy IF-RT. Preliminary findings were disclosed
firstly at the 2012 ASH meeting[27]
and then, in updated version, at the ISHL 2013 meeting in Cologne.[28]
The number of events needed to complete the statistical analysis is not
reached yet, but results suggest, as expected, slightly inferior RFS
for chemotherapy alone in comparison with chemo-radiotherapy in PET
negative patients, representing 75% of patients using a prudential
cut-off for positivity at Deauville’s score 3 (3-year PFS: 90.8% vs.
94.5%, per protocol). PET positive patients had 86.2% PFS rate. OS was
equivalent, with most relapsing patients receiving efficient salvage
therapies (not always including ASCT). Table 1 summarizes
the results of major clinical trials with radiotherapy-related
endpoints in early stage HL.
 | Table 1. Summary of clinical trials investigating for radiotherapy-related endpoints. |
The impact of such studies on the current role of RT outside clinical trials is difficult to evaluate; however, data suggest that the omission of RT, even in selected patients, may lead to inferior relapse-free survival rates. On the other side, the entity of the difference is small and overall survival rates are probably similar. Nevertheless, the use of early PET findings to guide therapy outside clinical trials is generally considered not appropriate, for two main reasons: an unclear role as a prognostic marker in early stage in comparison with advanced stages, with controversial retrospective findings,[29,30] and the need to have a strict quality control on images interpretation in daily clinical routine (in all trials, PET images were centrally reviewed by a panel of nuclear medicine experts).
Innovations in Radiotherapy and Strategies to Minimize Radiation-Induced Late Toxicity
During the time interval when most of the aforementioned clinical
studies were designed and conducted, the world of radiation oncology
deeply changed. The transition from EF-RT to IF-RT was relatively easy
since IF were “sub-volumes” of EF, and the fields delineation was based
on the anatomical boundaries typical of 2D RT, as exemplified by J.
Yahalom and P. Mauch in their 2002 classic article.[31]
When CT simulation and 3D reconstruction software became available,
radiation oncologists began to delineate smaller involved fields
volumes, corresponding to a new way of considering IF-RT in comparison
with the 2D era. At the same time, pre-chemotherapy imaging (CT and
CT-PET) became the basis for radiotherapy volumes delineation, actually
corresponding to involved sites at diagnosis. This concept has been
recently defined as “involved-site radiotherapy” (ISRT), according to
the HL radiotherapy guidelines, published by the International Lymphoma
Radiation Oncology Group (ILROG),[32]
and was developed on the basis of the INRT concept defined by EORTC in
H10 trial.[24]
In both INRT and ISRT, the pre-chemotherapy involvement determines the
clinical target volume, and the resulting irradiated volume is
significantly smaller than with IFRT. When pre-chemotherapy imaging is
available, the contouring process could be divided into 4 steps: 1.
delineation of the initially involved lymphoma volume on
pre-chemotherapy CT (GTV-CT) as determined by morphology; 2.
delineation of the
initially involved lymphoma volume on pre-chemotherapy PET/CT (GTV-PET)
as determined by FDG uptake; 3. pre-chemotherapy PET/CT images
co-registration with post-chemotherapy planning CT scan (the GTV-CT and
GTV-PET are imported from the pre-chemotherapy CT to the
post-chemotherapy CT); 4. delineation of the post-chemotherapy volume
using the information from both pre-chemotherapy PET and
pre-chemotherapy CT, taking into account tumor shrinkage and other
anatomic changes. In this way, a CTV is obtained encompassing all the
initial lymphoma volume while sparing normal tissues that were never
involved such as lungs, chest wall, muscles and mediastinal structures.
INRT actually represents a special form of ISRT, in which
pre-chemotherapy imaging is ideal for post-chemotherapy treatment
planning. Outside clinical trials specifically investigating new
radiation volumes (i.e. H10 or HD17), radiation fields currently used
in clinical routine (henceforth to be called IS-RT) are significantly
different from the traditional approach of IF-RT. High-quality
retrospective clinical data show that INRT is safe and effective in
terms of disease control.[33-35]
Beyond the IS-RT/IN-RT concept, the technological break-troughs in
radiation oncology also led to the introduction in clinical practice of
highly conformal techniques such as Intensity Modulated Radiotherapy
(IMRT). Standard radiation technique consisted in the past of simple
parallel-opposed anterior-posterior fields (AP-PA); also in the era of
3D-conformal radiation therapy, the AP-PA approach still represented
the most classical solution. Reduced and better defined radiation
volumes, together with the advances in treatment planning tools, now
allow for the utilization of more conformal radiation therapy, based on
more consistent imaging and advanced radiation delivery techniques. As
underlined in the ILROG guidelines,[32]
although the
advantages of IMRT include the tightly conformal doses and steep
gradient next to normal tissues, target definition and treatment
delivery verification need even more attention than with conventional
RT to avoid the risk of geographic miss and subsequent decrease in
tumor control. Image guidance may be required to ensure full coverage
during the whole treatment; preliminary retrospective clinical data on
the combination of image guidance and IMRT with reduced volumes (ISRT)
support the safety of this approach.[36]
Comparative
planning studies showed both that INRT may offer a substantial
dosimetric benefit in comparison with IFRT and that IMRT may result in
a better dose distribution around the target volumes, especially in
unfavourable mediastinal presentations (bulky disease, involvement of
the anterior mediastinum).[37-42]
IMRT can also reduce the mean dose received by critical thoracic
structures such as heart and coronary arteries. Figure 1
illustrates an example of the dose distribution achievable with IMRT,
in comparison with 3D-CRT, in a mediastinal presentation. The
dosimetric gain on healthy tissues achievable with IMRT is usually
associated with a larger amount of normal tissues (for example breasts
or lungs) receiving very low doses (1-2 Gy out of 30 Gy), with a
potential negative impact on radiation-induced secondary malignancies
risk. Historically, the shrinkage of radiation fields from EF-RT to
IF-RT has been shown to decrease the risk of second cancers, as
reported by De Bruin et al.[43]
This effect might be
significant also when shifting from IF-RT to ISRT/IN-RT, especially in
specific disease presentations (according to the disease extent and the
involved lymph nodes anatomical location). Few interesting modeling
studies were conducted with the aim of evaluating both the impact of
reduced volumes and IMRT on secondary cancers risk in early stage HL.[44-47]
Results showed that INRT, at least theoretically, reduces the risk of
secondary cancers in comparison with IFRT; the findings on IMRT vs.
3D-CRT were rather unclear, depending on both the IMRT technique and
the radiobiological models used for risk estimation. Valuable clinical
data on the incidence of secondary tumors after combined modality
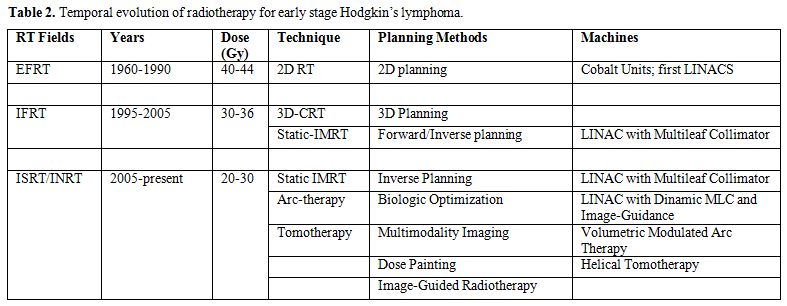
therapy with INRT-IMRT will only become available over the next years. Table 2 illustrates
the time trend in radiotherapy volumes/dose/technology evolution since
1960 to present.
 | Table 2. Temporal evolution of radiotherapy for early stage Hodgkin’s lymphoma. |
Conclusions
Early stage HL patients should be possibly included in clinical trials investigating for treatment optimization. In clinical routine, combined modality therapy still represents the standard, with radiation oncologists now having the opportunity to minimize the risks of late toxicity by using a large armada of technological improvements. Long-term follow-up is needed to clarify the clinical impact of these technical advancements on late morbidity.
References












































[TOP]