Received: March 28, 2014
Accepted: May 2, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014042, DOI 10.4084/MJHID.2014.042
This article is available on PDF format at:

Elena Maria Elli, Angelo Belotti, Andrea Aroldi, Matteo Parma, Pietro Pioltelli and Enrico Maria Pogliani
Hematology
Division, San Gerardo Hospital, Monza, Italy

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Deferasirox
(DSX) is the principal option currently available for
iron-chelation-therapy (ICT), principally in the management of
myelodysplastic syndromes (MDS), while in primary myelofibrosis (PMF)
the expertise is limited. We analyzed our experience in 10 PMF with
transfusion-dependent anemia, treated with DSX from September 2010 to
December 2013. The median dose tolerated of DSX was 750 mg/day (10
mg/kg/day), with 3 transient interruption of treatment for drug-related
adverse events (AEs) and 3 definitive discontinuation for grade 3/4
AEs. According to IWG 2006 criteria, erythroid responses with DSX were
observed in 4/10 patients (40%), 2 of them (20%) obtaining transfusion
independence. Absolute changes in median serum ferritin levels (Delta
ferritin) were greater in hematologic responder (HR) compared with
non-responder (NR) patients, already at 6 months of ICT respect to
baseline. Our preliminary data open new insights regarding the benefit
of ICT not only in MDS, but also in PMF with the possibility to obtain
an erythroid response, overall in 40 % of patients. HR patients
receiving DSX seem to have a better survival and a lower incidence of
leukemic transformation (PMF-BP). Delta ferritin evaluation at 6 months
could represent a significant predictor for a different survival and
PMF-BP. However, the tolerability of the drug seems to be lower
compared to MDS, both in terms of lower median tolerated dose and for
higher frequency of discontinuation for AEs. The biological mechanism
of action of DSX in chronic myeloproliferative setting through an
independent NF-κB inhibition could be involved, but further
investigations are required.
|
Introduction
Primary
myelofibrosis (PMF) is a clonal chronic myeloproliferative neoplasm
characterized by reactive bone marrow fibrosis, osteosclerosis,
angiogenesis and an abnormal cytokine expression leading to
extramedullary hematopoiesis (EMH) and progressive cytopenia. Clinical
manifestations of PMF include constitutional symptoms, marked
hepatosplenomegaly secondary to EMH, uncontrolled myeloproliferation
manifesting with marked leukocytosis or thrombocytosis or progressive
cytopenias.[1] Current standard
therapies and also experimental
approach with JAK2 inhibitors (as Ruxolitinib) focus on symptom
management, spleen enlargement reduction and optimizing cell counts,
and a mainstay of therapy is transfusion support.[2]
Patients with PMF
frequently may develop anemia from decreased marrow reserve,
ineffective erythropoiesis, splenic sequestration and myelosuppressive
medications.[3] Many patients eventually require red blood cell (RBC)
transfusions, which may lead to iron overload (IOL) from transfused
blood and increased iron absorption;[3]
significant IOL may occur after
as few as 20 RBC units,[4] and
transfusion dependent (TD) patients may
develop cardiac, hepatic and endocrine dysfunction.[5,6]
Organ damage
secondary to TD has been described as detrimental for survival in PMF,
and it presents a prognostic relevance independently from the
International Prognostics Scoring System, IPSS (which considers age
over 65 years, leukocytosis higher than 25 x 109/l,
Hemoglobin level lower than 10 g/dl, peripheral blasts equal to or
higher than 1% and presence of constitutional symptoms), or other
prognostic score.[7] At last, IOL
represents a risk factor for early
hepatotoxicity, and it impacts on survival in patients with PMF
undergoing allogeneic hematopoietic cell transplantation.[8] Iron
related organ toxicity is mediated in part by deposition of iron into
tissues and organs, and in part by the chronic exposure to
non-transferrin bound iron (NTBI), which leads to the formation of
labile plasma iron (LPI) and reactive oxygen species (ROS) capable of
damaging lipids, proteins and nucleic acids, thereby possibly provoking
apoptosis[9,10] and mutagenesis.[11] In particular, LPI represents the
most toxic fraction of NTBI, which is both redox active and chelatable
and, over time, sustained levels of LPI may compromise organ function
and overall survival. LPI is taken up into cells leading to an
increased labile iron pool with rapid generation of ROS. The iron pool
has, therefore, been regarded as one of the main regulators of the
production of ROS in cells. Oxidative stress leads to oxidation of
proteins, lipids and DNA, as well as suppression of the self-renewal of
the hematopoietic stem cells, a decrease in the number of these cells
and increased apoptosis and organ damage.[12]
Transfusion-induced IOL is a frequent problem that clinicians have to
face in the management of patients affected by hematologic
neoplasms.[13-15] For example in
myelodysplastic syndromes (MDS), many
recent studies demonstrated that TD patients in comparison to
transfusion independent (TI) ones have a lower survival which is
proportional to the degree of transfusion dependence.[16-17]
To limit the toxicity of excess iron, patients may receive iron
chelation therapy (ICT). The benefits of ICT in patients with
thalassemia major and IOL are well established,[18]
and ICT has been
shown to reduce LPI levels and oxidative stress[14]
in TD patients.
More recent studies suggest that administration of ICT may improve
survival in patients with MDS and IOL.[19,20]
Deferasirox (DSX) is the principal option currently available for oral
ICT.[21] DSX has been demonstrated
to decrease NTBI, to maintain or
reduce body iron (as assessed by serum ferritin) and to have a good
tolerability profile with no severe adverse effects in pre-treated or
therapy-naïve MDS patients.[21,22]
This oral ICT also seems to induce a
hematologic improvement that leads to a significant reduction or
complete interruption of blood transfusions in MDS patients, in
addition to improving the survival.[23]
Hematologic responses also in
term of increase in platelet and neutrophil count have been observed in
MDS setting.[24] The exact
mechanism of the hematologic response to ICT
is unknown, and the relationship between DSX and erythroid improvement
has yet to be elucidated. It has been hypothesised that DSX acts not
only reducing the high levels of LPI into the plasma but also in the
bone marrow through a direct and protracted effect on the
microenvironment and against the neoplastic clone. As compared to other
iron chelators, DSX is a potent NF-kB inhibitor and is able to increase
glutathione (GSH) in red blood cells, thus protecting them from
oxidative insults.[25] Despite
these observations, the role of ICT in
PMF remains largely undefined, and a few reports are present in the
literature regarding this specific setting of Philadelphia-negative
chronic myeloproliferative neoplasm (MPN Ph-),[26-29]
reflecting the
limited expertise in this field. Few data have shown a survival benefit
associated with the use of ICT in patients with TD anemia and PMF,[29]
similar to MDS, but more importantly there are no sufficient studies
that have examined the effectiveness in terms of hematological
response, particularly erythroid response, together with the safety
profile in patients with PMF. Here we reported our experience in TD-PMF
patients treated with DSX from September 2010 to December 2013, in
order to evaluate the efficacy and safety profile of this approach in
MPN Ph- setting.
Material and Methods
We identified in our MPN Ph- database, 154 patients affected by
myelofibrosis, referred to our division from 1990 to 2012. We
identified 47 patients with PMF (30,5%) presenting TD anemia at onset
or during follow-up of disease; of whom, we analyzed 10 TD-PMF patients
treated with oral DSX, from September 2010 to December 2013, starting
from a dose of 10 mg/kg/day, up to the maximum tolerated dose. In this
way, all the patients were evaluable for toxicity and hematologic
response (≥ 6 months of treatment). Criteria for initiating ICT were an
estimated life expectancy of at least 1 year and at least one of
elevated ferritin level (over 1000 μ/l), transfusion of at least 20 RBC
units, or organ dysfunction from IOL, refractoriness and/or absence of
concomitant therapy with stimulant erythropoietic agents (recombinant
erythropoietin, steroids, immunosuppressive therapy). Clinical evidence
of IOL was determined retrospectively as organ dysfunction in the
absence of other etiology. In particular cardiac dysfunction was
defined as left ventricular enlargement or decreased ejection fraction,
clinical signs of systolic or diastolic dysfunction or arrhythmia.
Hepatic dysfunction included clinical signs of liver disease or alanine
aminotransferase (ALT) or aspartate aminotransferase (AST) greater than
1.5 times the upper limit of normal. Endocrine dysfunction included
glucose intolerance or diabetes, and thyroid stimulating hormone level
above the upper limit of normal. 4/10 patients at baseline were
assessed by noninvasive liver iron concentration (LIC) measurement
using R2-magnetic resonance imaging for evaluation of hepatic damage;
only one patient with severe serological hepatic dysfunction underwent
to hepatic biopsy with histological confirmation of iron damage.
Unfortunately, none of these patients was monitored over time using
R2-magnetic resonance imaging, in order to re-evaluate the hepatic iron
deposits after ICT.
Assessment
and statistical methods:
We evaluated the efficacy of ICT in PMF patients in term of reduction
in serum ferritin levels and hematologic responses. Efficacy of ICT was
assessed evaluating the changes from baseline in serum ferritin levels
after 6, 12 and 18 months of treatment with DSX and at the end of
treatment. Details of ongoing RBC transfusion were recorded throughout
the study. Transfusional iron intake, expressed in mg of iron, was
calculated as the total amount of pure RBC transfused X 1.08.[30]
The IWG 2006 criteria[31] were
used to evaluate erythroid, platelet and
neutrophil response during DSX treatment. Time to hematologic response
was assessed as the number of days from the first dose of DSX to the
onset of hematologic response. According to erythroid response, defined
as complete response (CR: transfusion independent patients), partial
response (PR: reduction in transfusion requirement or increases in Hb
levels) or absence of response, the patients were divided into 2
subgroups: hematologic responder (HR: CR + PR) and non-responder (NR)
patients.
Non parametric analysis, Fisher's exact test and Mann-Whitney test,
were performed to evaluate, respectively, the qualitative and
quantitative variables in HR and NR patients of collection. In order to
evaluate the efficacy of ICT, the non-parametric Wilcoxon signed-rank
test was used to calculate P-values for changes in serum ferritin
levels, in the course of ICT compared to baseline, in each HR e NR
group; the Mann-Whitney test was used to compare the difference in the
changes of serum ferritin levels of the two groups between them.
We considered the safety and tolerability of treatment, in term of
transient or definitive discontinuation of DSX for drug-related adverse
events (AEs) and median tolerated dose of DSX. Safety and tolerability
were evaluated throughout the study by monitoring the incidence and
type of adverse events (AEs) and by assessing routine laboratory
parameters. AEs were assessed according to CTCAE Version 4 (2009)
definition. Overall survival (OS) was defined as the time from the date
of PMF diagnosis to the date of death from any cause. AML
transformation (PMF-BP) was defined as the appearance of > 10%
circulating blasts in the peripheral blood and/or at least 20% blasts
in the bone marrow.[26] Patients
still alive were censored at the last
known date of follow up.
This study was notified and performed with the requirements of the San
Gerardo Hospital Institutional Research Ethics Board. All procedures
were followed according to the Helsinki Declaration.
Results
We treated from September 2010 to December 2013, 10 TD-PMF patients,
with a median age of 70.5 (range 55-81) years at onset of ICT.
Principal clinical and laboratory features are summarized in Table 1. Median
hematologic at diagnosis were: Hemoglobin 8.95 (7.3-9.6) g/dl, platelet
count 228 (12-1050) x 109/l
and WBC count 8.96 (2.71-23.8) x 109/l.
The median baseline serum ferritin level was 1702 (range 1173-3198) μ/l.
As showed in Table 2,
starting
dose of DSX was 10 mg/kg/day, increasing up to the maximum tolerated
dose, for a median time of exposure to ICT of 11 (range 1–33) months.
The median dose tolerated of the DSX was 750 (range 500-1500) mg/day,
i.e. 10 mg/kg/day. Treatment with DSX started after a median interval
from diagnosis of 43,5 (range 7-207) months.
 | Table 1. Demographics and principal characteristics of all patient’s collection, hematologic responder (HR) and non-responder (NR) patients. |
 | Table 2. DSX dosing and exposure in all patient’s collection, hematologic responder (HR) and non-responder (NR) patients. |
Before starting ICT,
the median number of RBC transfusions received by
patients was 28 (range 10-150) RBC units/patient. The corresponding
median transfusional iron intake was 0.27 (range 0.07-1.11) mg/kg/day.
No patients presented cardiac or endocrine dysfunction at baseline.
Three patients (30%) showed hepatic dysfunction at serological test or
iron hepatic damage at R2-magnetic resonance imaging or hepatic biopsy
at onset of ICT.
We reported only 3 transient interruption of treatment for grade 2
extra-hematological toxicity: in particular 1 cutaneous rash, 1
diarrhea and 1 transaminitis. 5/10 patients (50%) experienced a
definitive discontinuation of the drug for grade 3/4 AEs (1 hepatitis,
1 cutaneous rash with mucous membrane ulceration, 1 intestinal
malabsorption and 2 renal failure). Drug-related AEs were reported
after a median time of 135 (range 15-612) days from start of ICT. Two
patients interrupted DSX for PMF-BP, 1 patient developed PMF-BP after
discontinuation of DSX. Overall, only 3/10 patients (30%) continued
permanently oral ICT.
Effect
of DSX on hematologic parameters:
HR and NR patients: According to IWG 2006 criteria, erythroid responses
with DSX were observed in 4/10 patients (40%), after a median of 150
(range 94-352) days; in particular we reported 2 PR: 1 patient with
reduction in transfusion requirements and 1 patient with hemoglobin
improvement. Two patients (20%) obtained transfusion independence, i.e.
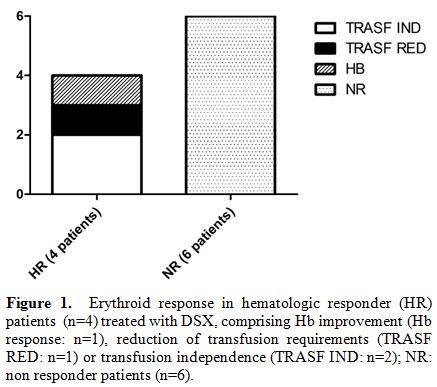
CR, as showed in Figure 1.
Six
patients did not achieve any hematologic response. One patient achieved
an improvement of liver function. At last, we did not record platelet
and neutrophil response during DSX treatment. The demographics and
principal characteristics in HR and NR patients were summarized in Table 1.
The HR patients were
younger at onset of ICT (61.5 vs 74.5 years, p =
0.007), the median time from diagnosis to start of ICT was longer in HR
group (69.5 vs 16.5 months, p = 0.05) even if the distribution of IPSS
score was similar in two groups; transfusion history prior to ICT, both
in terms of absolute number of RBC units/patient and transfusional iron
intake was not significantly different in HR respect to NR patients
(40.5 vs 28 RBC units/patient and 0.21 vs 0.29 mg/kg/day,
respectively). The median daily dose of DSX received by patients was
similar in each group (HR: 12 mg/kg/day vs NR: 10 mg/kg/day, p = NS),
even if we noted a trend for a shorter median exposure time at DSX in
NR patients compared to HR patients (6.5 vs 14.5 months). Of note,
there was no difference in drug exposure between the two groups in the
first 6 months of ICT: only 1 patient in HR group (for cutaneous rash)
and 2 NR patients discontinued DSX within 6 months of treatment (1
patient for hepatitis and 1 patient for PMF-BP). Furthermore,
transfusional iron intake during ICT was also similar in two groups
(0.65 vs 0.47 mg/kg/day, respectively in HR and NR group).
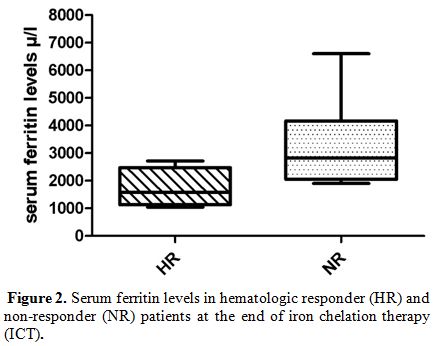
The median serum ferritin levels at baseline were comparable in both HR
and NR patients (1988 vs 1702 μ/l, respectively). Analyzing serum
ferritin levels at 6, 12 and 18 months from start of ICT, median serum
ferritin levels at 6 months, respect to baseline, were already reduced
in HR patients (ferritin level at baseline 1988 μ/l, and ferritin level
at 6 months 1756 μ/l) without reaching statistical significance. In NR
patients, it was not observed any reduction in the serum ferritin
levels at baseline respect of any time of ICT. In fact, this group
presented a significant increase of serum ferritin levels at any time
until the end of treatment (p = 0.03), comparing with HR patients, as
reported in Figure 2.
In other words, the reductions in median serum ferritin levels were
obtained at any time during ICT only in HR patients, as showed in Figure 3.
Furthermore, comparing two groups, the difference in the median changes
of serum ferritin levels (Delta ferritin evaluation) was statistically
significant already at 6 months of ICT, as showed in Figure 4: HR
patients experienced at 6 months of ICT a significant reduction in
serum ferritin levels of – 17.5 μ/l (range: - 1102 to 370 μ/l) compared
with an increase of + 464 μ/l (range: 108 to 723 μ/l) in NR group (p =
0.028).
 | Figure 2. Serum ferritin levels in hematologic responder (HR) and non-responder (NR) patients at the end of iron chelation therapy (ICT). |
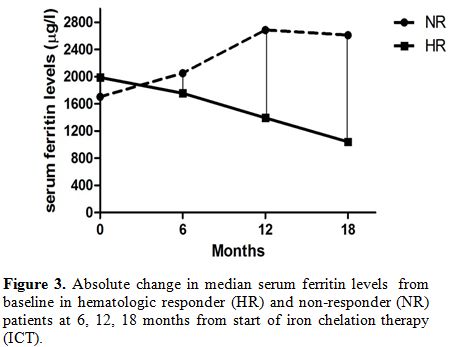 | Figure 3. Absolute change in median serum ferritin levels from baseline in hematologic responder (HR) and non-responder (NR) patients at 6, 12, 18 months from start of iron chelation therapy (ICT). |
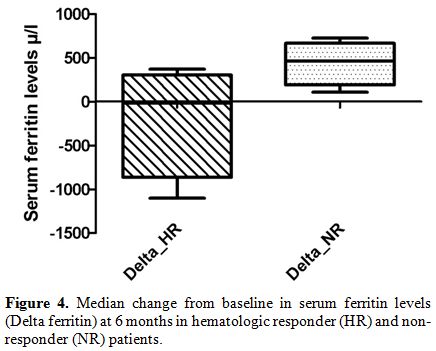 | Figure 4. Median change from baseline in serum ferritin levels (Delta ferritin) at 6 months in hematologic responder (HR) and non-responder (NR) patients. |
In patients who met
the criteria for hematologic erythroid response,
the frequency of drug-related AEs was similar to NR patients, but the
incidence of definitive discontinuation of ICT for AEs was higher in NR
group (75% vs 25%). However, the median time of onset of AEs was later
in NR patients compared to HR group (248 vs 119 days).
PMF-BP evolution was reported only in 3 NR patients. Finally, HR
patients seem to present a better OS than NR patients (median OS: 93 vs
35.5 months, respectively). OS at 5 years was greater in HR respect to
NR patients (75% vs 33%, respectively). There were 7 deaths, 5 of them
in NR patients. Causes of the 5 deaths in NR were: 3 PMF-BP, 1 sepsis
and 1 bleeding. The 2 deaths in HR patients were 1 bleeding and 1
sepsis occurred 45 days after bone marrow transplantation.
Discussion
PMF is a myeloproliferative neoplasm frequently complicated by TD
anemia. Given the detrimental effects of anemia and of IOL due to a
prolonged transfusional support, any treatment able to improve anemia
and transfusion dependence could have a significant impact on patient’s
quality of life and life expectancy.[26]
Our preliminary data open new
insights regarding the benefit of ICT not only in MDS, but also in PMF
patients with TD anemia, with the possibility to obtain a partial or
complete erythroid response, overall in 40% of them.
Several emerging lines of evidence actually indicate that ICT can
improve hematopoiesis and leads to a reduction or abolition of
transfusion dependence in PMF.[26-29,32] Therefore these data are very
sparse and mainly deriving from single case descriptions, but they are
suggestive of a real biological phenomenon. A similar positive impact
on transfusion dependence has been also described in patients with MDS
thus suggesting the absence of a specific correlation between
hematopoietic improvement due to ICT and the type of disease.[19,23,24]
Our study represents the first attempt to assess the efficacy, safety
and potential benefit of ICT in this specific setting of patients,
although with the limitation of a small series. However, we stress as
in the inclusion criteria, we deliberately excluded the patients who
were concomitantly in treatment with stimulant erithropoietic agents,
in order to remove any confounding factor on the hematologic response,
with consequent reduction of analyzed cases.
Our results in PMF patients, in term of improvement of hematopoiesis,
reproduce those obtained in MDS setting on a larger series of patients.
Gatterman et al.[24] reported a
post-hoc analysis of haematological
response to DSX in a cohort of 247 iron-overloaded patients with MDS
enrolled in the EPIC trial. Erythroid, platelet and neutrophil
responses were observed in 21.5% (53/247), 13.0% (13/100) and 22.0%
(11/50) of the patients after a median of 109, 169 and 226 days,
respectively. Of the patients with an erythroid response, 28 (11.3%)
had only a transfusion response and 22 (8.9%) had only a haemoglobin
response. Three patients (1.2%) had both transfusion and haemoglobin
responses.
In our study, erythroid responses were observed in a high proportion of
patients (40%), after a median time of 150 days after starting DSX. Of
the patients with an erythroid response, 2 patients obtained a
transfusion independence (20%), 1 patient (10%) had a partial
transfusion response and 1 patient (10%) had only a improvement in
haemoglobin levels. Conversely, we did not record platelet and
neutrophil response during DSX treatment.
ICT therefore may have a role in the management of anemia in PMF, in
all stages of the disease, in patients treated with conventional
cytoreductive therapy but especially in consideration of new drugs that
are now used in this setting, ie JAK2 inhibitors. One of the main side
effects of these drugs, linked to the intrinsic mechanism of action in
JAK2-STAT pathway, seems to be the induction or the worsening of the
degree of anemia in PMF patients, especially in the first 6 months of
therapy; consequently the use of RBC transfusion is critical in order
to avoid the tapering of drug. ICT in these patients can reduce the
amount of IOL and prevent organ damage, keeping the effective dose of
the drug stable and potentially contributing to the potential
hematologic improvement with JAK2 inhibitors.
The tolerability of the drug seems to be lower in PMF compared to MDS
patients, both in terms of lower median tolerated dose (10 mg/kg/day)
and of a higher frequency of discontinuance for drug related AEs (50%).
Nevertheless, among the patients treated with DSX, we identified a
subgroup that responds to ICT, achieving a hematologic improvement or
even a transfusion independence. Furthermore, HR patients had a
significant and progressive reduction in serum ferritin levels and we
demonstrated an improvement in survival and a lower incidence of PMF-BP
in this group, suggesting a potential advantage also in long term
survival of treatment with DSX. In fact, based on the evaluation of
serum ferritin levels in our patients in the course of ICT, hematologic
responses seem to be observed in patients with greater reductions in
serum ferritin levels, suggesting that hematologic response might be
dependent, at least partially, on reductions in levels of body IOL.
Delta ferritin evaluation at 6 months could represent a significant
predictor for a different survival and PMF-BP: absolute changes in
median ferritin levels were statistically greater in HR respect to NR
patients and they correspond to a lower incidence of PMF-BP. PMF-BL
occurred indeed only in NR patients. This phenomenon could be secondary
to potentially mutagenic effect of ROS, as has been suggested in
MDS.[26,32]
HR patients seem to have also a better survival respect to
NR group (median OS 93 versus 35.5 months, respectively). This survival
improvement seen in PMF patients receiving ICT is encouraging. However,
because the study is retrospective and the patients’ collection is
small, it is subject to the potential biases of any analysis that is
non-randomized and non-controlled. To minimize the possibility of
selection or referral bias favoring ICT patients, multiple baseline
characteristics could be compared, showing no significant differences
between groups in most factors. In our study, with the limitations
mentioned above, we can say that the distribution of patients by IPSS
prognostic scoring system risk and incidence of JAK2V617F mutation as
well as the median serum ferritin levels at baseline, the transfusion
history, the median daily iron intake before starting ICT and the
median dosage of DSX were not different between two groups. We find
some differences statistically significant comparing two groups in term
of age: HR patients are younger at diagnosis and at the time of ICT
respect to NR patients. In addition, HR patients seem to have a longer
median time from diagnosis to onset of ICT (69.5 vs 16.5 months),
without evidence of a more intensive transfusion requirement or daily
iron intake pre-treatment. This group receives a similar daily dosage
of DSX, with a trend for a longer median exposure time at the drug
(14.5 versus 6,5 months) but transfusional iron intake during ICT was
similar in HR and NR patients.
HR patients seem to present a lower incidence of definitive
discontinuation of ICT for drug-related AEs respect to NR patients (25%
versus 75%). However, the median time of onset of AEs is later NR
compared to HR patients (248 vs 119 days). Therefore, the impact of
drug-related AEs does not appear to affect the achievement of erythroid
response, which is relatively early, as previously discussed.
All these consideration, in our opinion, could instead explain the
improvement in OS in HR patients. Several possible mechanisms by which
ICT can improve erythropoiesis and survival have been proposed: a
direct cytoreductive effect of ICT on the neoplastic clone, a reduction
of oxidative species, which are believed to correlate with inefficient
erythropoiesis, or an inhibition of NF-κB leading to a reduced
transcription of anti-apoptotic factors.[25,33-35] In this specific
myeloproliferative setting, the biological mechanism of action of DSX
may depend mainly from the peculiar mechanism of NF-κB inhibition,
independent from reactive oxygen species scavenging properties of the
drug, that are common features also of other ICT, such as Deferiprone
or Deferoxamine. In fact, previous studies have suggested the
involvement of NF-κB pathway in the pathogenesis of the disease. In
particular, Komura et al.[33] have
speculated a role of NF-κB pathway
in transforming growth factor-beta1 production in PMF; Wagner-Ballon et
al.[34] have reported as
Bortezomib, a proteasome inhibitor, impairs
both myelofibrosis and osteosclerosis induced by high thrombopoietin
levels in mice. These encouraging results in vitro were not confirmed
by phase II clinical studies in vivo,[35]
probably because NF-κB
pathway is not the one primarily involved in the pathogenesis of PMF.
Therefore an involvement of this pathway in the mechanism of action of
DSX could explain a direct action on the malignant clone during in vivo
therapy, even if partial, inducing a hematopoietic improvement, but
further investigations are required.
Conclusions
ICT with DSX, although not routinely recommended by current guidelines
of PMF management, should be proposed in clinical practice of TD
patients, taking into account the possible anti-leukemic effect and the
improvement of survival, besides a potential direct action of ICT in
enhancing erythropoiesis of PMF patients. Further prospective and
larger studies are required in order to confirm the exact role of DSX
in the improvement of erythropoiesis and survival of patients with PMF
and to clarify the mechanism(s) underlining this phenomenon.
Acknowledgments
This work was supported By “Luce e Vita” Association.
References



































[TOP]