Received: April 11, 2014
Accepted: April 16, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014043, DOI 10.4084/MJHID.2014.043
This article is available on PDF format at:

Lorenzo Zammarchi1, Filippo Bartalesi2 and Alessandro Bartoloni1,2
1
Infectious Diseases Unit, Department of Experimental & Clinical
Medicine, University of Florence School of Medicine, Florence, Italy.
2 SOD Malattie Infettive e Tropicali, AOU
Careggi, Firenze, Italy.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract About
95% of cases and 98% of deaths due to tuberculosis (TB) occur in
tropical countries while, in temperate low incidence countries, a
disproportionate portion of TB cases is diagnosed in immigrants.
Urbanization, poverty, poor housing conditions and ventilation, poor nutritional status, low education level, the HIV co-epidemic, the growing impact of chronic conditions such as diabetes are the main determinants of the current TB epidemiology in tropical areas. TB care in these contests is complicated by several barriers such as geographical accessibility, educational, cultural, sociopsychological and gender issues. High quality microbiological and radiological facilities are not widely available, and erratic supply of anti-TB drugs may affect tropical areas from time to time. Nevertheless in recent years, TB control programs reached major achievements in tropical countries as demonstrated by several indicators. Migrants have a high risk of acquire TB before migration. Moreover, after migration, they are exposed to additional risk factors for acquiring or reactivating TB infection, such as poverty, stressful living conditions, social inequalities, overcrowded housing, malnutrition, substance abuse, and limited access to health care. TB mass screening programs for migrants have been implemented in low endemic countries but present several limitations. Screening programs should not represent a stand-alone intervention, but a component of a wider approach integrated with other healthcare activities to ensure the health of migrants. |
Introduction
Despite
encouraging progress, the burden of tuberculosis (TB) remains enormous
with about one third of the World population latently infected with the
etiologic agent Mycobacterium
tuberculosis,[1] 8.7
million new cases of active disease and 1.4 million people died in
2011.[2] Some authors state that
95% of all cases and 98% of deaths due to TB, occurs in tropical
countries.[3]
In the matter of facts among the 22 high burden countries that account
for more than 80% of the worldwide incident cases of the disease, 19
have territories geographically located, at least in part, within the
tropics (Table 1),
indicating
tropical areas as the most affected by TB in the World. In high income
industrialized countries, the majority of which are located outside the
tropics, the overall TB incidence is low, as expected given the inverse
correlation between economic development of the country and its TB
diffusion.[4] A disproportionate
and growing portion
of subjects affected by TB in industrialized countries are migrants
from tropical countries,[5,6]
configuring this group of subjects as a TB vulnerable population in low
endemic areas.
The aim of this review is to give an overview on the historical,
epidemiological, clinical and microbiological characteristics and
recent control strategies of TB in tropical countries and migrant
populations.
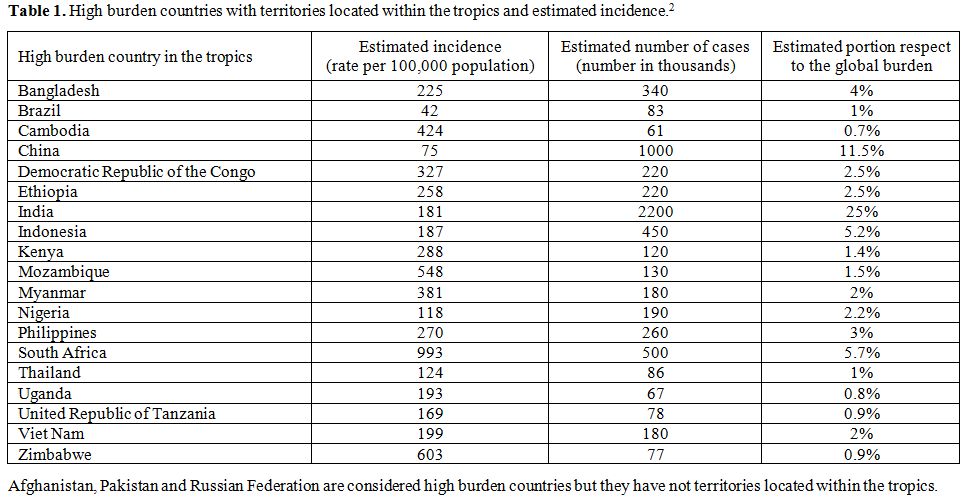 | Table 1. High burden countries with territories located within the tropics and estimated incidence.[2] |
History of TB in Tropical Areas and Migration
Current evidence supports the so called "Out-of-and-back-to-Africa"
scenario in explaining the origin and global spread of human TB.[7] Human M. tuberculosis complex
probably originated in Africa and accompanied the Out-of-Africa
migrations of modern humans approximately 70,000 years ago.[7] The three phylogenetically ‘modern’
lineages of M.
tuberculosis
complex (namely the East Asian, the Central Asian/Delhi and the
Euro-American lineage) seeded in China, India and Europe, respectively
where human population strongly grew during the last few centuries.[7]
As overcrowding conditions and the urbanization increased, TB expanded
in these areas and concomitantly spread globally through waves of human
migrations.[7] Through European
colonization, the Euro-American lineage of M. tuberculosis
complex reached other regions such as the Americas in the mid
nineteenth century and sub-Saharan Africa at the beginning of the
twentieth century.[7,8] Historians
and
paleopathologists, supported by the detection of mycobacterial DNA in
pre-Columbian human remains, suggests that TB was already present in
pre-Columbian America. Today most TB in the Americas is caused by the
Euro-American lineage, but in the pre-Columbian period, the etiologic
agent may have been Asian forms, as would be expected given the
original human colonization of the Americas via the Bering Strait.
Alternatively, pre-Columbian TB might have been caused by mycobacterial
lineages which are now extinct perhaps because they were outcompeted by
the Euro-American lineage following the massive influx of Europeans
into the Americas between the early eighteenth and early twentieth
century.[7]
Epidemiology and Determinants of Tuberculosis in Tropical Areas
The majority of the known risk factors for acquiring TB infection and
for progress to TB disease after the infection are widespread and
responsible for the high burden of TB in tropical areas.
About 59% of new estimated TB cases occur in South East Asia and the
Western Pacific Regions,[2]
where some of the most populated countries (India, China, Indonesia)
and some of the most crowded cities of the World are located.[9]
Urbanization and the consequent overcrowded living conditions, through
the increase of shared airspace between individuals, are among the
well-known risk factors to acquire TB.[10]
Urbanization is a relatively new, but growing phenomenon in Africa,
which is substantially less populated than Asian regions.[9]
However, countries of the African Region account for 26% of the World's
TB cases and they have the highest incidence rate of cases and death per capita.[2]
In Africa, the TB epidemic is overlapped and strongly driven by HIV
infection which is the most powerful risk factor for developing active
TB disease in subjects infected with M. tuberculosis.[11]
In this region, 46% of subjects who develop active TB are estimated to
be co-infected with HIV (ranging from 8% in Ethiopia to 77% in
Swaziland).[2] In extreme settings,
such as
gold-mining workforce in South Africa, the annual incidence reaches
value of 2,000-3,000 per 100,000 population due to the high rate of HIV
co-infection (up to 80% among subjects with active TB) and silicosis.[12]
Concerning countries of the region of Americas, only Brazil, is
considered a high burden country given its relevant contribution to the
absolute number of TB cases, despite a relatively low overall incidence
rate (less than 50 per 100,000 population). However, the burden of TB
in Brazil is not uniformly distributed in the national territories with
more 70% of cases concentrated in 315 over 5,564 municípios
corresponding to those hosting the large metropolitan cities where
overcrowding and extreme poverty is more frequent.[13]
In some districts of São Paolo (Brazil) where the Human Development
Index is particularly low, TB incidence is 167 per 100,000 population.[14]
Some tropical countries such as Haiti, Peru, Bolivia and Suriname have
the highest incidence of TB in the Americas (between 100 and 200 per
100,000 population).[15] HIV
co-epidemic is probably
one of the most important determinant of the high incidence found in
Sub-Saharan Africa, as well in Brazil and Haiti, where about 20% of
incident TB cases is HIV co-infected.[15]
Re-infection of subjects with previous latent tuberculosis infection
(LTBI), which account for up to 40% of the general population in
countries like India,[16] may play
an important role.
Even if people with LTBI, have a markedly lower risk of developing TB
disease after a re-infection if compared with previously uninfected
subjects,[17] in endemic areas the
contribution of re-infection may account up to 70% of TB relapse cases.[18]
A very important, even if distal, determinant of TB in tropical areas
is poverty that affects housing conditions, ventilation, nutritional
status, education and the access to health care system.[19]
In some areas of India, for example, the amount of monthly earning as
well as the schooling degree have been correlated to TB prevalence.[19]
About two third of cases are diagnosed between 15-44 years of age in
countries such as South Africa and India. The impact on the health
status of young adults in their most economically active years makes
that not only does poverty predispose one to TB, but also TB can
increase poverty.[19] In India
three to four months
of work time, the equivalent to 20–30 per cent of annual household
income, are typically lost because of TB.[19]
A growing role of emergent risk factors for progression from latent to
active TB, such as certain chronic conditions, have been observed more
recently in tropical areas. Smoking doubles the risk of TB and might
account for up to half of all deaths in men with TB in India.[20]
Diabetes is associated with an about three-times increase in TB risk
accounting for about 20% of smear-positive tuberculosis cases in India
in 2000.[20] Helminthic
infestations that are endemic
in tropical countries are strongly suspected to negatively impact on TB
diseases inducing immunological alterations including alternatively
activation of macrophages and Th1-lymphocytes response impairment.[21] In a cohort of HIV-infected Ugandan
adults, Schistosoma
mansoni infestation was associated with an increased risk
of TB progression.[22] Finally,
according to a recent review of the literature on racial difference in
susceptibility to infection by M.
tuberculosis,
black skin people may have consistently higher susceptibility to TB if
compared to whites skin peoples due to environmental, immunologic, and
genetic factors.[23]
Epidemiology and Determinants of Tuberculosis in Immigrants
TB is a well-known phenomenon linked to migration. By the time of the
Italian migration to America between the XX and the XXI century,
Italian migrants, resettled in New York city, worked in the factories
of the metropolis in very poor housing and living conditions. In this
setting, Italian migrants experienced a very high number of TB cases
with tens of cases per household and the block where they lived was
named “lung’s block”.[24]
Today, migration is a global social phenomenon that may be defined as a
movement of people within and among countries as a consequence of
wealth disparity, poverty, wars, natural disasters and political
persecution.[19,25]
To date there are an estimated 740 million internal migrants and 200
million of international migrants (Figure
1),[26] without
considering irregular migrants of which it is difficult to make an
affordable estimate.
Many migrants originate from countries where TB have a high incidence,
such as tropical countries, and resettle in higher income countries,
such as Unites States, Canada, Australia, New Zealand and western
Europe, where TB incidence is now very low (less than 10 per 100,000
population) (Table 2).[25]
In the United States (US), TB cases in foreign-born persons accounted
for 62% of total TB cases in 2011 with Asians accounting for 29% and
Hispanics/Latinos for 21% of all cases.[6]
Considering
the countries belonging to the European Economic Area (EEA), foreign
origin persons represented 26% of cases diagnosed in 2011.[5]
However, this percentage rises to more than 40% in the western European
countries holding the highest proportions of migrants in Europe (Table 2).[5]
In the EEA the majority of foreign origin subjects diagnosed with TB in
2009 originated from Asia (34.2%), Africa (28.6%) and other European
countries (19.9%).[27]
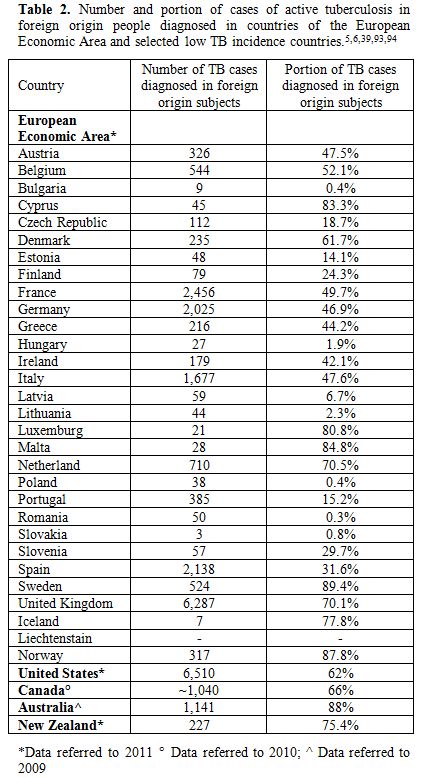 | Table 2. Number and portion of cases of active tuberculosis in foreign origin people diagnosed in countries of the European Economic Area and selected low TB incidence countries.[5,6,39,93,94] |
Immigration
is playing an important role in the epidemiology of TB in certain high
burden countries with emerging economies. In some districts of São
Paolo (Brazil), the portion of TB cases diagnosed in Bolivian migrants
grew up to 53% of total cases in the period 1998-2008,[14]
while migrant workers from rural areas of China resettled in the
district of Shanghai accounted for 67.4% of cases diagnosed in
2006-2008.[28]
It is clear that migrants currently play an important role in
determining the current epidemiology of TB in countries where they
settled. However reports from different high income countries with
well-performing screening and treatment systems have shown that
foreign-born TB patients do not contribute importantly to TB
transmission in the native population.[25,29,30]
Based on genotyping analysis, a variable portion of TB cases in native
populations (ranging from 2% to 17%) has been attributed to
transmission from foreign-born subjects.[31,32]
In
more recent study, performed in Denmark, transmission from Danes to
migrants occurred 2.5 times more frequently than vice-versa.[30]
Migrants are exposed in their country of origin to several risk factors
for TB infection and progression as already explained in the above
paragraph.
The incidence in the countries of origin is the strongest predictor of
TB incidence in migrants according to some authors.[33]
However in other studies the TB incidence in selected migrant
communities was found to be lower or higher if compared with the
incidence in the country of origin according to the degree of
socio-economical integration of the community.[34,35]
After migration, foreign born people are exposed to a series of
additional factors that have been associated with an increasing risk of
acquiring or reactivating TB infection such as poverty, stressful
living condition, material deprivation, social inequalities,
unemployment, fewer educational opportunities, overcrowded housing,
malnutrition, substance abuse, and limited access to health care.[36]
TB in migrants may occur as a consequence of a reactivation of a LTBI
acquired in the country of origin, but also because of a new infection
acquired in the host country after resettlement or during travel in the
country of origin. Molecular epidemiology studies have helped to
understand the relevance of LTBI reactivation in the pathogenesis of TB
in migrants. In these studies, clustered cases (defined as two or more
cases with clonally related TB strains) are assumed to belong to a
chain of recent transmission, while cases whose M. tuberculosis
isolates display unique patterns are regarded as sporadic and assumed
to be caused by reactivation.[35]
According to the different studies, 10%-45% of TB cases diagnosed in
foreign-born patients are clustered,[35,37,38]
this means that a relevant proportion of active TB cases is probably
caused in immigrants by new infection acquired after migration, even if
the majority of cases are due to LTBI reactivation acquired before
migration.
As well known a considerable portion (23-53%) of TB cases in migrants
is diagnosed in the first years (2-4) after resettlement in the host
country.[6,39-41]
However, the
reasons for this phenomenon are not completely clear. Some authors
suggest that the stressful and socioeconomically disadvantaged living
conditions in the first years after migration could contribute to the
reactivation of TB early after arrival.36 However, the risk of TB in
migrants was found to persist for their life time.[42]
For example, in one study, one third of TB cases in Australia migrants
were diagnosed 10 years after arrival, and this interval was larger
when considering European migrants only.[43]
An increased risk of TB is still present in second generation migrants
in which a link to endemic countries persists after migration through
social networks or travel in the country of origin of their ethnic
minority group.[44,45] In United
Kingdom (UK), for example, the highest incidence rates in UK born
subjects are in ethnic minority groups.[46]
The role of travel to visit friends and relatives on the risk of TB
infection during an international travel is not exactly known. However
the risk for an international traveler approximates the risk of
transmission in the local population of the country of destination,[47] and it is associated with duration
of travel.[48]
Among travelers, immigrant visiting friends and relatives, especially
children, are likely to represent a group at higher risk, perhaps due
to their closer contact with the local population as shown by several
studies that report an association between TST positivity and return to
the country of origin.[49]
TB Diagnosis and Management in Tropical Areas
The most common symptom of pulmonary TB is a productive cough for more
than 2 weeks, which may be accompanied by other respiratory symptoms
(shortness of breath, chest pains, hemoptysis) and/or constitutional
symptoms (loss of appetite, weight loss, fever, night sweats, and
fatigue).[50] The presence of
those symptoms are
enough to met the definition of suspected TB case according to the
World Health Organization (WHO).[50]
For a patient living in a remote tropical village that has cough for
more than 2 weeks, the way to achieve the correct diagnosis of TB, to
start anti-tubercular treatment and to complete it successfully may be
very long and full of hurdles. According to a systematic review, in
resource limited countries the average patient delay (time from the
onset of symptoms until the patient see the first health care
practices) and average health system delay (time from the first health
care seeking for diagnosis until the diagnosis made) are 31.7 days and
28.5 days, respectively.[51]
Low educational level, low awareness and knowledge about TB and
sociopsychological barriers, gender inequalities, are the first
bottlenecks for the initial health access.[52]
Believing TB incurable or caused by evil spirit, possible social
exclusion following the diagnosis of TB (stigma), fear of revealing HIV
status to neighbors, since TB is closely related to HIV in tropical
areas, are some the factors conditioning health seeking behaviors and
the diagnostic delay in tropical countries.[52]
Rural residence and other geographical barriers are further limiting
factors in the diagnostic path. Ideally a health facility able to start
the clinical management of a suspected TB case should be within 1-day
walking distance as many patients have limited access to motorized
vehicles.[52] However, in 2011 the
WHO estimates that
only 15 of the 22 high TB burden countries met the target of having 1
microscopy centre per 100,000 population and 17 of the 36 countries
with a high burden of TB and multidrug-resistant (MDR) TB have the
recommended capacity of 1 laboratory to perform culture and drug
susceptibility test (DST) per 5 million population.[2]
Initial visit to a governmental low-level healthcare facility, initial
visit to traditional or unqualified practitioner or even a visit to a
private practitioner are factors associated with further diagnostic
delay.[52] The delay in diagnosis
from this point
forward reflects a lack of effective diagnostic tools and follow-up
routines since a correct diagnosis requires both good training and
available diagnostic facilities.[52]
Table 3
reports the list of the most important factors associated with
diagnostic delay according to a systematic review.[52]
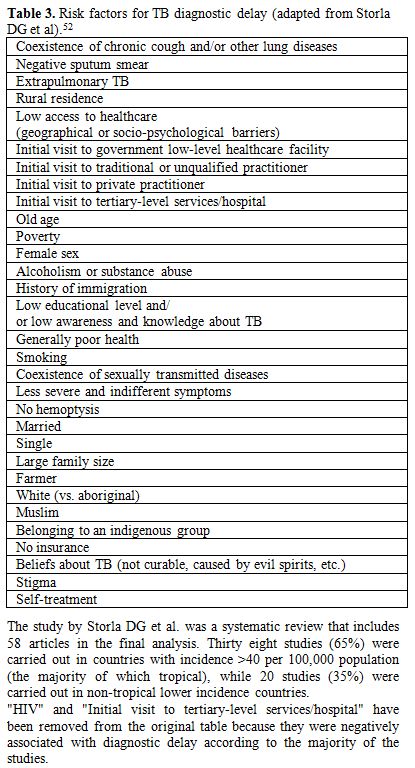 | Table 3. Risk factors for TB diagnostic delay (adapted from Storla DG et al).[52] |
Diagnosis
of TB is a challenge not only in tropical countries, but anywhere
resources are limited. Conventional microbiological methodology such as
direct microscopy and culture, when available, have intrinsic
limitation that have constrained TB care and control up to now.[2] Smear microscopy has a low
sensitivity (about 64%),[53] which
is even lower in HIV positive patients[54]
and in children.[55]
Culture is considered the gold standard but requires some weeks to give
a positive result and even new liquid culture techniques, which are
more sensitive and allow a faster grown of mycobacteria, are seldom
available in resource-constrained settings largely because of cost.[56]
Radiology has an important role in the diagnosis of TB but the
equipment is expensive to obtain, maintain, operate and experienced
radiologist are required in order to interpret the often non-specific
radiological signs of TB.[3] Few
years ago, the
situation of radiological manpower and facilities in sub-Saharan Africa
was reported to show a desperate shortage of radiologists,
radiographers and equipment, with most of services located in the
capital with few at rural hospital and CT scanners or high resolution
ultrasound machines available only in 40% of these countries.[57]
In view of the paucity of diagnostic tools available, the challenge of
TB diagnosis in the tropics may be related to problems of differential
diagnosis. In the tropics, pulmonary TB must be distinguished from
other rare endemic and ubiquitous conditions such bacterial pneumonia,
histoplasmosis, paracoccidioidomycosis, coccidioidomycosis,
melioidosis, actinomycosis, paragonimiasis, echinococcosis, nocardial
and aspergillus mycetoma, dirofilariosis, neoplasm, sarcoidosis,[58,59] which could be a hard task,
given the limited diagnostic resources available.
Hopefully, the recent availability of new rapid tests could
revolutionize TB care in endemic and tropical countries. The new test
Xpert MTB/RIF, which has been endorsed by WHO in December 2010, is a
cartridge-based automated diagnostic test that has three main
advantages if compared with older tests: 1) it enables simultaneous
detection of M.
tuberculosis
complex and rifampicin-resistant associated genotype; 2) provides
accurate results in less than two hours so that patients can be offered
proper treatment on the same day; 3) has minimal bio-safety
requirements, training, and can be housed in non-conventional
laboratories.[60] According to a
meta-analysis, the
pooled sensitivity was 98.7% for pulmonary sputum positive TB and 75%
for sputum negative TB with an overall specificity of 98.4%, while the
sensitivity on non-respiratory clinical samples resulted to be 80.4%.[61,62]
Xpert MTB/RIF showed dramatic cut of the time needed to start
treatment, especially in smear negative cases, and to obtain rifampicin
susceptibility result.[63] With
the introduction of
Xpert MTB/RIF, there has been also an increase of the number of
microbiologically confirmed TB in children,[62]
and an increase of the number of pulmonary TB cases detected in HIV
positive patients when compared with microscopy.[62]
Between its endorsement by WHO and the end of June 2012, 1.1 million
test cartridges were procured in 67 (46%) of the 145 countries eligible
to purchase them at initial concessional prices (9.98 $ per test from
August 2012).[62] Currently, WHO
strongly recommends
the use of Xpert MTB/RIF for use, as the primary diagnostic test, in
individuals suspected of having MDR or HIV-associated TB and in testing
cerebrospinal fluid specimens from patients presumed to have TB
meningitis; furthermore, WHO provides “conditional
recommendations” for its use in other settings.[64]
However, several weakness of this new tool have already been
highlighted, including elevated cost of the platform (17,000$), the
sophisticated hardware needing calibration and maintenance, need of
continuous electrical power supply and air conditioning, short shelf
life of cartridges needing good procurement system, need for cartridges
storage at 2-28°C and system for disposal after use.[62]
Concerning other relatively recent diagnostic tools such as interferon
gamma release assays (IGRA) and serological test for TB, WHO
recommended against their use in middle and low income countries for
the diagnosis of both active and LTBI.[2]
Directly Observed Treatment (DOT) of TB reduces TB related death,
disability and transmission, and it is highly cost-effective
intervention even in the lowest income countries.[2]
Treatment of a drug-sensitive TB, case, takes 6 months, while treatment
for MDR TB case takes 18-20 months according to the WHO
recommendations.[2] The target of
85% of treatment
success for new TB cases has been achieved at global level, but it is
still under the goal threshold in African (73%), Americas (74%) and
European Regions (74%), with the lowest rate (53%, possibly
underestimated) reached by South Africa.[2]
Concerning patients with MDR-TB, that represent a growing portion of
cases, only 44% to 58% completed treatment successfully according to
different Regions.[2] In Africa,
19% of patients with MDR-TB is not able to complete the treatment
because of death.[2]
TB and HIV are strictly related, and the management of the two
conditions must go hand in hand. To date only 40% of patients with TB
are tested for HIV, with the African Region performing better than all
other regions (69%).[2] However
only 56% of people eligible for antiretroviral therapy is receiving it
in Africa.[65]
The assessment of the HIV status in a patient with TB is essential
since the timely start of antiretroviral therapy has been demonstrated
to reduce significantly the mortality of the patient.[66-68]
Treatment success of TB is hampered by several problems that may be
amplified especially in tropical areas, such as problematic access to
health care facilities, poor adherence to treatment, availability of
quality drugs, high rate of MDR cases, and HIV co-infection. Treatment
default implies persistence of infection source, increased mortality,
increased relapse rates and increased risk of the development of
resistant strains.
In different case control studies, frequently identified risk factors
associated with a default of the patients under TB treatment in
tropical areas were inadequate knowledge on TB,[69,70]
illiteracy or low education level,[70,71]
herbal medication use,[69] low
income,[69] alcohol abuse,[69-71] HIV co-infection,[69,71]
male gender[69] poor
patient-provider interaction,[70]
side effects to anti TB drugs.[70]
The erratic supply of drugs that may affect some areas is another
relevant problem. A survey carried out in Ethiopia in 2008 showed that
the first line drugs for TB treatment were not available in about 20%
of 48 health facilities that were supposed to have.[72]
Doctors without Borders recently reported a drug supply crisis in
Mthatha (South Africa) started in 2013.[73]
During a survey done in May 2013 in the area, still 40% of facilities
suffered stock-outs of antiretroviral drugs and/or TB drugs with a
median duration for reported stock-outs of 45 days.[73]
TB Diagnosis and Management in Immigrants
The access to health system, including TB diagnostic and treatment
services is lower in migrant populations compared to native subjects.
Migrants have a longer patient diagnostic delay for TB (defined as the
time elapsed from the onset of symptoms and the first medical
consultation), while natives have a longer health care diagnostic delay
(defined as the time elapsed between the first medical consultation and
the initiation of treatment).[74,75]
The increased
patient delay is possibly due to a combination of reasons that hinder
migrants of using the available TB services. Among those factors, there
are language barriers, possible lack of medical insurance, fear of
deportation (for illegal migrants) or discontinuation of their
employment[74,75] and competing
socio-economic
priorities may prevail over health issues. Even if in most of countries
TB diagnosis and treatment are provided for free at government health
facilities to all migrants, including illegal migrants, additional
costs of transport and the time needed to perform medical consultations
may represent significant obstacles for access to health system for
migrants on low wages.[76] The
longer health care
diagnostic delay in native subjects can be explained by the lower TB
incidence among native subjects in low endemic countries, that leads
physicians to reduce their index of suspicion regarding the possibility
of TB diagnosis and ordering other tests rather than TB-diagnostic
tests.[74,75]
TB treatment in migrant populations can be challenging due to lower
adherence to treatment.[77-79]
According to a recent study, loss to follow-up in TB cases in UK
appears to occur primarily in young male adults and in subjects born
outside the UK, particularly those who migrated within the 2 years
prior to diagnosis.[77] Moreover,
this study showed that lost to follow-up patients were more frequently
infected with a resistant M.
tuberculosis
strain compared to patients who completed or were still on treatment
(11% vs. 7.4%), highlighting the vicious circle among poor compliance
to treatment and resistance to antitubercular drugs.[77]
Higher therapeutic abandonment has been recently found also in foreign
born patients if compared to natives in Granada (Spain)[79]
and in Chinese internal migrants if compared to permanent residents.[78]
Another challenging issue in the management of TB in migrants, in low
endemic countries, is the high frequency of MDR-TB in this population
if compared to natives (Table
4).
The majority of European and other low prevalence countries, excluding
some of the high priority countries in the WHO European Region (such as
Latvia, Lithuania, Bulgaria and Estonia), report higher prevalence of
MDR-TB cases in migrants if compared to the native population.[80]
This is probably due to the high prevalence of MDR-TB in migrants
countries of origin and possibly to the low compliance to treatment
that characterizes migrants and leads to acquire drug resistance.
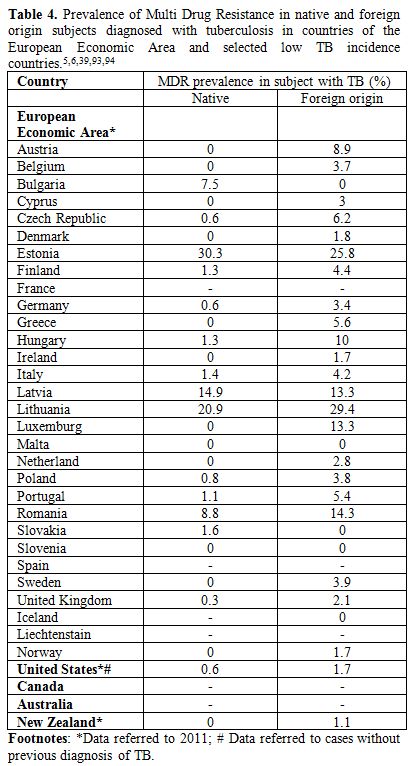 | Table 4. Prevalence of Multi Drug Resistance in native and foreign origin subjects diagnosed with tuberculosis in countries of the European Economic Area and selected low TB incidence countries.[5,6,39,93,94] |
Given
the epidemiological importance of migrant subjects in determining the
epidemiology of TB in industrialized countries, many of those countries
implemented different control measures for TB, including mass screening
programs. The rationale of these programs is the early detection and
treatment of active and then contagious TB cases, in order to prevent M. tuberculosis
transmission within the host country.[81]
Indeed screening for active TB may decrease the period of
infectiousness by as much as 33%.[82]
Secondary benefits of immigration screening are reduced transmission of
TB in the country of origin and during travel.[81]
A recent survey showed that high-income industrialized countries have
widely different approaches to the screening of migrants arriving in
their territories.[83] In the
majority (23 of 25,
92%) of cases, screening is performed after the arrival, while only 36%
(9 of 25) and 20% (5 of 25) of countries perform also pre-arrival and
at-arrival screening respectively, according to the different type of
immigrants.[83] The majority of
countries (25, 86.2%) screens for active TB and most commonly (76% of
cases) the screening is compulsory.[83]
The most commonly used tool for screening for active TB in adult
migrants is a chest radiograph, which is used by 22 of 25 (88%)
industrialized countries alone or in combination with clinical
examination and less commonly, with tuberculin skin test (TST).[83]
However, screening protocols based on chest x-ray only are unable to
detect cases of extrapulmonary TB, which represent a not negligible
portion of TB cases in migrant patients (24% of cases diagnosed in non
US born patient in US).[84]
Moreover, concerning
pulmonary TB, chest x-ray shows a sensitivity of 86-97% and a
specificity of 75-89% according to the different criteria used for
imaging interpretation.[85] The
median yield of
screening for TB disease (portion of patients with active TB among
those screened) has been found quite low (0.18%) in the EEA.[86]
Only 16 of 29 (55.2%) \countries, inquired in the above survey, screens
for LTBI by using TST in 68.8% of cases or TST plus a confirmatory IGRA
in 25% or an IGRA alone in 18.8%.[83]
Some authors
strongly support the implementation of screening for LTBI based on the
evidence that the majority of active TB cases, diagnosed in migrants,
are due to a LTBI reactivation acquired in the country of origin[38] and on the findings of
cost-effectiveness analysis.[87,88]
According to a cost-effectiveness study, the most suitable strategy
would be to screen with an IGRA (in particular QuantiFERON-TB Gold
In-Tube. Carnegie, Cellestis, Australia) test all migrants coming in UK
from countries with an incidence of more than 250 cases per 100,000
(incremental cost-effectiveness ratio [ICER] of £ 21,565 per prevented
case of TB) without port of arrival chest x-ray.[88]
However these results are opposite to those of a previous study done in
Canada that found chest radiograph the most and Quantiferon the least
cost-effective strategy to screen migrants.[89]
The
discordance of the findings are probably explained by the different
assumptions done in the models such as high rates of acceptance and
completion of chemoprophylaxis assumed in the English study and low
prevalence of latent infection in new immigrants assumed in the
Canadian study.
While the attention of the different governmental programs and several
scientists seems to focus on mass screening programs for active TB
and/or LTBI, this kind of interventions should not represent a
stand-alone intervention, but a component of a wider approach.[86] The six points proposed by the STOP
TB strategy (Table 5),[80]
which address the activities to deal with TB at global level, could be
a useful paradigm for drawing a more comprehensive approach for TB
control in migrant populations.
Implementing DOT in low endemic areas or newer socially and culturally
acceptable programs to sustain treatment adherence, address MDR-TB,
contributing to health systems strengthening with the presence of peer
educators and culturally-oriented health staff, engaging all care
providers including members of Non Governmental Organizations and
voluntary associations, empowering migrants communities and promoting
research to find new possible operational solutions and tools for TB
care and prevention could likely be of benefit for future TB control
programs in migrants.[90]
In conclusion TB care should be offered and integrated with other
healthcare activities within the context of a holistic approach to
ensure the health and wellbeing of new entrant migrants.[76,91,92]
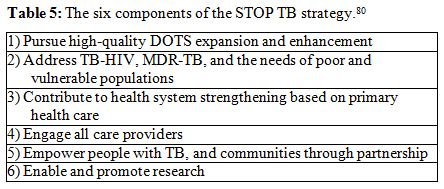 | Table 5. The six components of the STOP TB strategy.[80] |
References































































 .
. 
[TOP]