Received: March 5, 2014
Accepted: June 20, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014044, DOI 10.4084/MJHID.2014.044
This article is available on PDF format at:

Adel A Hagag1,
Ghada Elmashad3 and Aml Ezzat Abd El-Lateef 2
1
Pediatrics Department, Faculty
of Medicine, Tanta University, Egypt
2 Clinical Pathology Department,
Faculty of Medicine, Tanta University, Egypt.
3 Pediatric Department, Faculty of Medicine,
Elmenofia University, Egypt.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Background:
Sickle cell disease has a worldwide distribution. Vaso-occlusive crisis
(VOC) is one of the most important clinical features of the disease.
Thrombospondin (TSP1) and Placenta growth factor (PlGF) have been
reported to be involved in sickle cell diseases (SCD).
Objective: The aim of this study was to assess the clinical significance of Thrombospondin and Placenta growth factor profiles in patients with sickle cell disease. Patients and methods: This study was carried out in sixty patients with sickle cell anemia who were attendants to Hematology units, Pediatric Departments, Tanta and Elmenofia University Hospitals in the period between December 2011 and May 2014 including thirty patients during vaso-occlusive crisis and thirty patients out of crisis. Also this study included twenty healthy children of matched age and sex as a control group. Serum TSP1 and PlGF levels were analyzed by ELISA. Results: In SCA patients with crisis the mean serum Thrombospondin level was 902.5±280.89 ng/mL; in SCA patients out of crisis the mean serum Thrombospondin level was 462.5±190.2 ng/mL and in controls the mean value was 236.66±58.29 ng/mL. In SCA patients with crisis the mean serum Placenta growth factor level was 19.97±1.28 pg/ml; in SCA patients out of crisis the mean serum Placenta growth factor level was 13.12±1.82 pg/ml and in controls the mean value was 9.89±1.20 pg/ml. All paired comparisons for Thrombospondin and Placenta growth factor reached statistical significance (P< 0.001). There was significant positive correlation between serum Thrombospondin and Placenta growth factor levels in sickle cell anemia patients during crisis (r=0.848, p=<0.001). Conclusions: This is the first study to show TSP1and PlGF concentration changes in patients with SCD in a large cohort study from Middle East, and to show correlation between both markers; therefore TSP1and PlGF may be useful VOC markers in SCD patients. Recommendation: To further assess TSP1 and PlGF as a marker of VOC in patients with SCD, further studies should be conducted to determine the exact point before VOC, when serum TSP1 and PIGF levels begin to increase. This requires monitoring of the TSP1 and PIGF levels in sickle cell patients out of crisis, showing how rapidly these levels increase just before VOC development. |
Introduction
Sickle
cell disease (SCD) is hereditary hemoglobinopathy characterized by
abnormal hemoglobin production, hemolytic anemia, and intermittent
occlusion of small vessels, leading to acute and chronic tissue
ischemia, chronic organ damage, and organ dysfunction.[1]
Sickle hemoglobin (Hb S) is common and clinically significant
hemoglobin structural variant.[2]
Hb S is caused by β-globin gene mutation in which the 17th nucleotide
is changed from thymine to adenine and the 6th
amino acid in the β-globin chain becomes valine instead of glutamic
acid; this mutation produces a hydrophobic motif in the deoxygenated Hb
S tetramer that results in binding between β1 and β2 chains of two
hemoglobin molecules. This crystallization produces a polymer nucleus,
which grows and fills the erythrocyte, disrupting its architecture and
flexibility and promoting cellular dehydration.[3]
Damage to the erythrocyte cell membrane occurs as it passes through the
microcirculation, shortening its life span and causing chronic
hemolytic anemia.[1] Also Hb S
polymerizes when sickle
RBCs are exposed to hypoxic conditions in the microcirculation, leading
to increased cellular adhesiveness, nitric oxide depletion and
vaso-occlusion.[4] Most patients
will have severe pain due to occlusion of blood flow to bones, muscles,
arms, legs, back, abdomen, and chest.[5]
Cytokines and adhesion molecules play an important role in the
pathophysiology of vaso-occlusion in SCD.[6]
Placenta growth factor (PlGF) is released by immature erythrocytes and
is elevated in SCD and may play a role in the pathophysiology of sickle
cell disease-associated pulmonary hypertension by inducing the release
of vasoconstrictor substance called endothelin-1.[7]
Platelets are activated in SCD particularly during vaso-occlusive
episodes (VOE).[8] Increased
platelet activation likely plays a catalytic role in vaso-occlusion and
vasculopathy in SCD[9,10] by
increasing the adhesion of sickle RBCs to the endothelium[11] via secretion of fibrinogen, von
Willebrand Factor[12] and
Thrombospondin-1 (TSP1)[13] and
promoting further intimal damage. [14]
TSP1 is multifunctional glycoprotein containing domains for adhesive
proteins, enzymes, cell receptors that is abundantly present in
platelet α-granules, and is a key player in vascular biology.[15] TSP1 is the major secretory product
of activated platelets, which is increased in VOE.[8]
TSP1, via its cognate receptor CD47, modulates vascular responses to
hypoxia, regulates vaso-constriction, inhibits angiogenesis, and
promotes adhesion of sickle RBCs to the endothelium.[16]
Moreover, TSP1 inhibits NO signaling pathway through binding to the
receptors CD36 and CD47 expressed on endothelial cells and platelets[17,18] thus; TSP1 represents a plasma
biomarker of disease severity in SCD.[8]
Aim of this Study
The aim of this study was to assess the clinical significance of
Thrombospondin and Placenta growth factor profiles in patients with
sickle cell disease during crisis and in steady state.
Patients and Methods
This study was done after approval from ethical committee of research
center in Tanta and Elmenofia University Hospitals and informed written
parental consent from every case that participates in this research and
was carried out on 60 cases with sickle cell disease (HbSS) who were
admitted or under follow up at Hematology unit, Pediatric department,
Tanta and Elmenofia University Hospitals in the period between December
2011 and May 2014, including thirty patients with sickle cell anemia
during vaso-occlusive crisis (18 males and 12 females) and thirty
patients in steady state out of crisis (15 males and 15 females). Also
this study included twenty healthy children of matched age and sex as a
control group. To ensure that the patients is not in crisis samples
were obtained from patients who had no acute sickle events, fever, or
infections 3 weeks before or 3 weeks after the blood sample and were
not transfused within the last 90 days.[19]
Vaso-occlusive crisis is acute painful condition at any site of the
patient's body due to occlusion of blood flow to bones, bone marrow,
muscles, organs, arms, legs, back, abdomen, or chest.[5]
For
all patients the following were done:
• Complete history taking
• Thorough clinical examination with especial account on pallor,
jaundice, leg ulcers, hepatomegaly and splenomegaly.
• Laboratory investigations including:
a) Complete
blood count.
One ml of venous blood were collected using sterile needles through
gentle venipuncture after sterilization of site of puncture by alcohol,
and collected samples were delivered on 20 uL EDTA solution for
complete blood count including reticulocytic count and differential
count which was done on leishman stained peripheral blood smear with
evaluation using ERMA PCE-210 N cell –counter.[20]
b) Serum
thrombospondin levels.
Two ml of venous blood samples from patients and controls were
collected in citrated tubes and immediately transferred to laboratory
at 4OC.
The tubes were inverted 8–10 times and then subjected to double
centrifugation at 1500g at 4OC
to obtain platelet poor plasma (PPP).
The supernatant was aliquoted into cryotubes and stored at −80OC
until
the day of testing by ELISA. PPP were thawed and assessed for levels of
TSP1 by ELISA in duplicate (R&D Systems, Minneapolis, MN).[8]
c) Serum
placenta growth factor levels.
Two ml of Heparinized venous blood samples was obtained from patients
with SCD and healthy controls. The blood samples were centrifuged at
0OC
- 4OC
and 1000g for 15 minutes and plasma was separated within 2
hours of sample collection and stored at –80OC
until it was assayed.
PlGF concentration was determined on cell-free heparinized plasma using
ELISA.[19]
Statistical
analysis.
Data were collected and analyzed using SPSS for windows (version 12).
All Data were expressed as in terms of mean values ±
SD. Comparisons of
parameters among groups were made using the paired t test. Two-group
comparisons were performed non-parametrically using the Mann-Whitney U
test. All statistical tests were two tailed, and P < 0.05 was
considered statistically significant.
Results
There were no statistically significant differences between sickle cell
anemia patients with and without vaso-occlusive crisis as regards age,
sex, pallor, jaundice, leg ulcers, hepatomegaly and splenomegaly (Table 1).
There were statistically significant differences between patients with
or without VOC and control group as regards platelets; RBCs and WBCs
but there were no statistically significant differences between
patients with and without VOC (Table
2).
Mean serum Thrombospondin levels were significantly higher in sickle
cell anemia patients with crisis than those out of crisis and were
significantly higher in sickle cell anemia patients with or without
crisis than control group (Table
3).
Mean serum Placenta growth factor levels were significantly higher in
sickle cell anemia patients with crisis than sickle cell anemia
patients out of crisis and were significantly higher in SCA patients
with or without crisis than controls (Table 3).
Significant positive correlation was found between serum Thrombospondin
and Placenta growth factor levels in sickle cell anemia patients during
crisis (Figure 1).
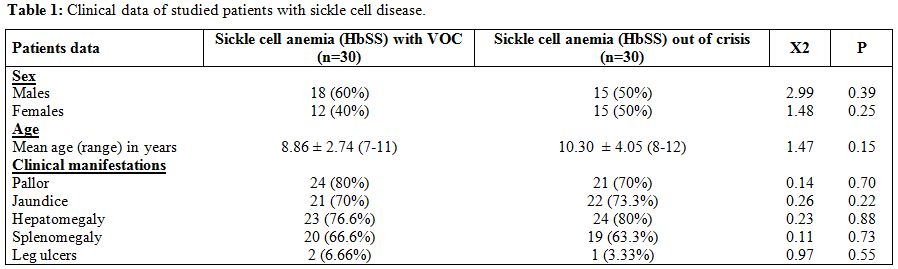 | Table 1. Clinical data of studied patients with sickle cell disease. |
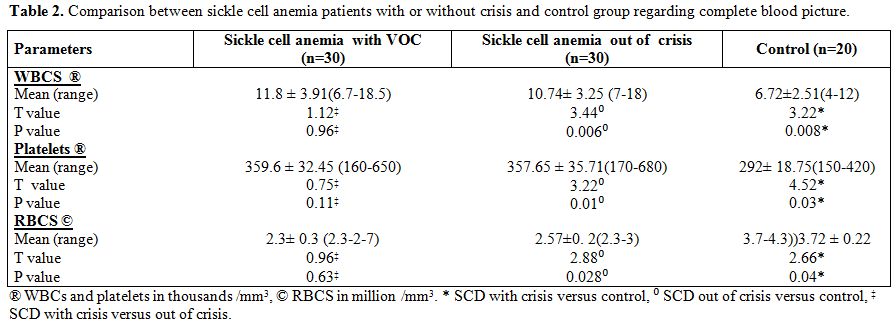 | Table 2. Comparison between sickle cell anemia patients with or without crisis and control group regarding complete blood picture. |
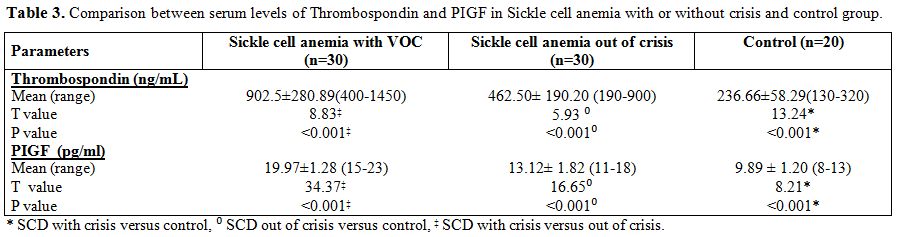 | Table 3. Comparison between serum levels of Thrombospondin and PIGF in Sickle cell anemia with or without crisis and control group. |
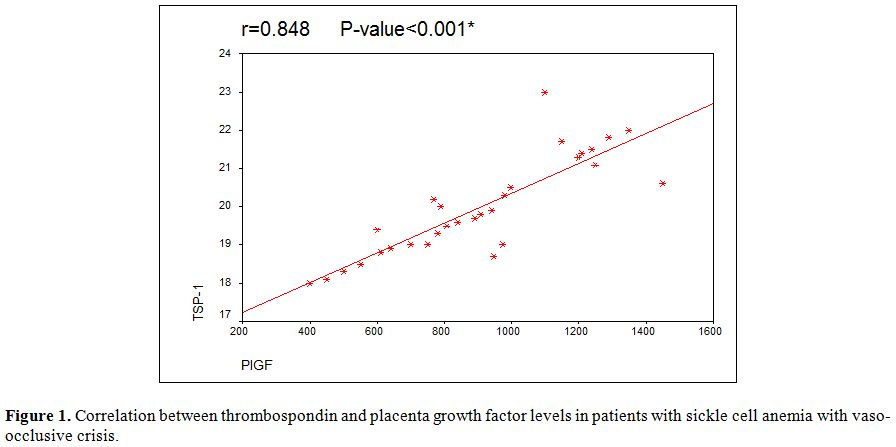 | Figure 1. Correlation between thrombospondin and placenta growth factor levels in patients with sickle cell anemia with vaso-occlusive crisis. |
Discussion
Sickle cell disease is one of the most important single gene disorders
of human beings[21] that affects
1/400 individuals of African descent, as well as people of Arab, Indian
and Hispanic descents.[22]
VOC has a complex nature, involving interactions between sickle red
blood cells, endothelium, and leucocytes. Endothelial damage due to
recurrent adhesion of sickle RBCs may disrupt endothelial function,
leading to altered cytokine release. Altered balance of proinflammatory
and anti-inflammatory cytokines plays an important role in a painful
crisis in SCD patients.[23]
Placenta growth factor is angiogenic growth factor released by immature
erythrocytes and is elevated in SCD.[24,25]
Thrombospondin-1 is the major secretory product of activated platelets
and is a key player in vascular biology that is increased in VOE.[8,15]
In this study Thrombospondin and Placenta growth factor were measured
by a commercially available ELISA kits in 60 sickle cell disease
patients including 30 cases in steady state and 30 cases in a painful
crisis compared with 20 normal controls.
In the present study mean, serum Thrombospondin levels were
significantly higher in SCA patients with crisis than patients out of
the crisis and were significantly higher in SCA patients with or
without crisis than controls. This datum was in agreement with Novelli
et al 2013[26] who found the same
results and Novelli et al 2012[8]
who tested 27 patients in steady state and 14 patients with VOE, as
well as 17 healthy controls and found the same results with a positive
correlation between TSP-1 levels and vaso-occlusive complications and
history of acute chest syndrome8 and explained this by increased
platelet activation and degranulation, that can lead to increased
plasma levels of TSP1 in patients with sickle cell anemia with or
without crisis, in accordance with a prior study that showed increased
platelet activation in VOE.[10]
In the current study placenta, growth factor levels were significantly
higher in SCA patients with crisis than patients out of crisis and were
significantly higher in SCA patients with or without crisis than
controls. This datum was in agreement with Bottomley et al 2000,[27] Natalya et al 2003,[19]
Nitin et al 2009[28] and Nitin et
al 2010[29]
who found significant positive correlation between PlGF concentrations
and incidence of VOC and they concluded that PlGF could contribute to
vascular occlusion and might modulate clinical severity, since PlGF
causes a significant increase in proinflammatory cytochemokines mRNA in
monocytes.[19] These
proinflammatory molecules
contributed to the activation of leukocytes and endothelial cells, a
phenomenon observed in SCD at steady state,[30]
and
may be responsible for the increased incidence of vascular occlusions
in SCD subjects. The leukocytes adhesion to endothelium is a primary
event in initiating vascular occlusion and secondarily causes RBCs to
adhere to leukocytes or to endothelium.[31]
Brittain et al 2010[32]
found significantly elevated PlGF in SCD compared with healthy controls
but did not observe any association of PlGF with the frequency of acute
pain episodes or history of acute chest syndrome.
In this work, there were significant positive correlations between
serum TSP-1 and PlGF levels in patients with sickle cell anemia during
vaso-occlusive crisis. This study is, to our knowledge, the first to
correlate these two parameters. The significant positive correlation
between serum TSP-1 and PlGF levels in this study could be explained by
hypoxia, which was shown to be a strong stimulus for angiogenesis in
numerous disorders including sickle cell anemia.[33]
Hypoxia inducible transcription factors induce the expression of
several angiogenic factors including VEGF, nitric oxide synthase, PlGF
and TSP-1.[33] Both of TSP-1 and
PlGF increase together during hypoxia in sickle cell anemia especially
in vaso-occlusive crisis.[33]
Also, Placenta growth factor is released by immature erythrocytes and
is elevated in SCD due to hyperactive bone marrow. Concomitantly
Thrombospondin is released from activated platelets in case of SCD
particularly during vaso-occlusive episodes.[7,8]
On the basis of our results, we concluded that the increased TSP1 and
PIGF levels could be considered as a marker of VOC in SCD. Further
studies should be performed to determine the exact timing of TSP1 and
PIGF levels increase, in relation to the episodes of VOC. The
monitoring of TSP1 and PIGF levels in patients with sickle cell out of
the crisis appears necessary to this scope.
Acknowledgments
We are thankful for the patients of Sickle Cell anemia in Tanta and
Elmenofia Hematology Units for participation in this study.
References
































[TOP]