Received: March 19, 2014
Accepted: May 31, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014047, DOI 10.4084/MJHID.2014.047
This article is available on PDF format at:

Grzegorz Helbig1, Malgorzata Krawczyk-Kulis1, Malgorzata Kopera1, Krystyna Jagoda1, Patrycja Rzepka2, Aleksandra Majewska-Tessar2, Marta Hejla2 and Slawomira Kyrcz-Krzemien1
1
Department of Hematology and Bone Marrow Transplantation, Silesian
Medical University, Katowice, Poland.
2 Students Research Group, Department of
Hematology
and Bone Marrow Transplantation, Silesian Medical University, Katowice,
Poland.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Objective.
To evaluate the efficacy and toxicity of autologous hematopoietic stem
cell transplantation (AHSCT) for high-risk acute lymphoblastic leukemia
(ALL).
Material and methods. Overall, 128 high-risk ALL patients at a median age of 26 years (range 18-56 years) at diagnosis received AHSCT between 1991-2008. Induction treatment was anthracycline-based in all patients. Conditioning regimen consisted of CAV (cyclophosphamide, cytarabine, etoposide) in 125 patients whereas 3 subjects received cyclophosphamide and TBI (total body irradiation). Bone marrow was stored for 72 hours in 4oC and re-infused 24 hours after conditioning completion. Bone marrow was a source of stem cells in 119 patients, peripheral blood in 2 and 7 subjects received both bone marrow and peripheral blood. Results. With a median follow-up after AHSCT of 1.6 years (range 0.1-22.3 years), the probability of leukemia-free survival (LFS) for the whole group at 10 years was 27% and 23% at 20 years. Transplant-related mortality at 100 days after AHSCT was 3.2%. There was a strong tendency for better LFS for MRD-negative patients if compared with patients who had positive or unknown MRD status at AHSCT (32% vs 23% and 25%, respectively; p=0.06). There was no difference in LFS between B- and T-lineage ALL as well as between patients transplanted in first complete remission (CR1) and CR2. LFS at 10 years for patients with Philadelphia-positive (Ph+) ALL at transplant was 20% and this was comparable with subjects with negative and missing Ph status (26% and 28%; p=0.97). Conclusions. The results of AHSCT for high-risk ALL remain unsatisfactory with low probability of long-term LFS. |
Introduction
The
clinical outcome of adult acute lymphoblastic leukemia (ALL) remains
far unsatisfactory with a long-term survival of about 50% for patients
<60 years and merely 10% for older subpopulation.[1-3]
An initial
therapy including remission induction followed by an intensified
consolidation results in complete remission (CR) rate of 80%-90% with
early death in less than 10% of treated patients.[2,4,5] The optimal
post-remission therapy for adult ALL represents a major challenge and
further management is usually risk-adapted,[6]
however the role of
hematopoietic stem cell transplantation (HSCT) was found to be highly
controversial as demonstrated by conflicting results of various
studies. Some authors did not demonstrate any differences between
chemotherapy and allogeneic/autologous HSCT[7]
whereas others reported on
a significant superiority of allogeneic HSCT in high-risk ALL
patients.[5] A follow-up of the
French LALA-87 study demonstrated a
trend favoring an autologous HSCT (AHSCT) over chemotherapy in patients
with high-risk features, but there was no difference between
chemotherapy, autologous and allogeneic HSCT in standard-risk ALL
population.[8] The summary of the
three randomized studies from LALA
group comparing chemotherapy and AHSCT in high-risk ALL patients with
no sibling donors found a significantly lower risk of relapse at 10
years in the autologous group with no improvement in overall
survival.[9] Recently published
data indicates that minimal residual
disease status (MRD) before AHSCT is a predictor of better clinical
outcome in high-risk ALL subjects transplanted in first complete
remission (CR1), but further prospective studies are required.[10]
The goal of our retrospective study was to analyze the results of AHSCT
in 128 high-risk ALL patients.
Material and Methods
Study
design.
One hundred and twenty eight patients at a median age of 26 years
(range 18-56 years) received AHSCT in our center in years 1991-2008.
They were recruited from several hematologic institutions. Patients
were eligible for AHSCT if they had no sibling or alternative matched
donor and met the following criteria of high risk (HR) ALL (at least
one criterion must be met): 1) age ≥ 35 years, 2) white blood cell
(WBC) count at diagnosis ≥30x10^9/L for B-cell ALL and ≥100x10^9/L for
T-cell ALL, 3) pro-B, early-T and mature T immunophenotype, 4) second
or subsequent complete remission (CR) and 5) the presence of adverse
cytogenetics: (Philadelphia-positive ALL, i.e. t(9;22) and/or BCR-ABL
transcripts; ALL with 11q23 abnormality and/or MLL-AF4 transcripts; ALL
with t(1;19) and/or E2A-PBX1 transcripts; ALL with complex karyotype
and hypodiploidity). Due to the fact that some patients were referred
for AHSCT from other centers, not all data were available for all
patients.
Induction
treatment.
Induction treatment was anthracycline-based in all study patients, but
chemotherapy regimens varied depending on study protocol used. Most
adults ALL patients received therapy according to the Polish Adult
Leukemia Group (PALG): 4-91 (n=20); 4-96 (n=46); 4-2002 (n=28)[11-13]
or German Multicenter Study Group for Adult ALL (GMALL; n=34).[14]
Central nervous system (CNS) prophylaxis consisted of intrathecal
administration of methotrexate, cytarabine and steroids and/or cranial
irradiation (depending on study protocol and year of diagnosis). One
hundred and seven patients achieved CR1 (84%) whereas 21 subjects had
CR2 at transplant (16%).
Investigation
of minimal residual disease.
Minimal residual disease (MRD) was evaluated in bone marrow samples on
the day of aspiration by multiparametric flow cytometry: EPICS-XL
(Beckman Coulter Inc. Marseille, France) and FACSCanto II (Becton
Dickinson, Biosciences, San Jose, CA, USA). The
“Stain&Lyse&Wash” method for staining of the surface
markers
and Fix&Perm Cell Permeabilization Kit (Invitrogen) for
simultaneous staining of surface and intracellular markers were applied
according to the producer’s instructions. At least 2-3 different
aberrant phenotypes were examined to avoid false negative results. The
isotypic negative controls and normal bone marrow samples were used to
avoid false positive results. At diagnosis bone marrow samples were
stained with a panel of 3-18 triple combinations of antigens. Only
phenotypes that have been found to be informative for MRD examinations
(>50% expression on leukemic cells before treatment and
<0.1% in
normal bone marrow) were used for disease monitoring. The 3 following
aberrant phenotypes were analyzed: 1) ectopic phenotypes 2)
asynchronous antigen expression/overexpression within the same line and
3) co-expression of antigens from different cell lines. The “quadrant”
and “empty spaces” techniques were applied for searching of these
abnormal phenotypes. MRD status was assessed before conditioning
regimen using a panel of combinations of antibodies and level of 0.1%
was used as a cut-off point.[14]
In sum, data on MRD were available for
57 patients; 40 were MRD negative and 17 MRD positive. The results of
MRD were missing for 71 subjects.
All BCR-ABL positive patients had p190 transcripts and all they were
found to have Philadelphia chromosome on cytogenetic study. BCR-ABL
status was monitored using real-time polymerase chain reactions (PCR)
assays.[15] Data on Philadelphia
status at transplant was known for 53
patients; and 5 out of them were Ph+ at transplant (9%). No patient
received tyrosine kinase inhibitors, both before and after
transplantation. No other cytogenetic high-risk patients were
identified in our study group.
Transplant
details. The conditioning regimen consisted of CAV
(cyclophosphamide 60mg/kg on days -3,-2; cytarabine 2000mg/m2 on days
-3,-2,-1 and etoposide 800mg/m2
on days -3,-2) in 125 patients whereas 3 subjects received
cyclophosphamide 120mg/kg on days -6,-5 and total body irradiation
(TBI) with 12 Grey on days -3 to -1. Bone marrow was collected in
general anesthesia, stored for 72 hours in 4oC
without any processing and re-infused 24 hours after conditioning
ending. Bone marrow was a source of stem cells in 119 patients,
peripheral blood in 2 and 7 subjects received both bone marrow and
peripheral blood. A median number of transplanted nuclear cells and
CD34+ cells was 2.11x10^8/kg body weight (range 0.86-7.04) and
1.7x10^6/kg body weight (range 0.16-36.2), respectively. A median time
to achieve an absolute neutrophil count >1.0x10^9/L and platelet
count >50x10^9/L was 16 days. No granulocyte colony stimulating
factor (G-CSF) was used. Patients demographic and transplant data were
shown in table 1.
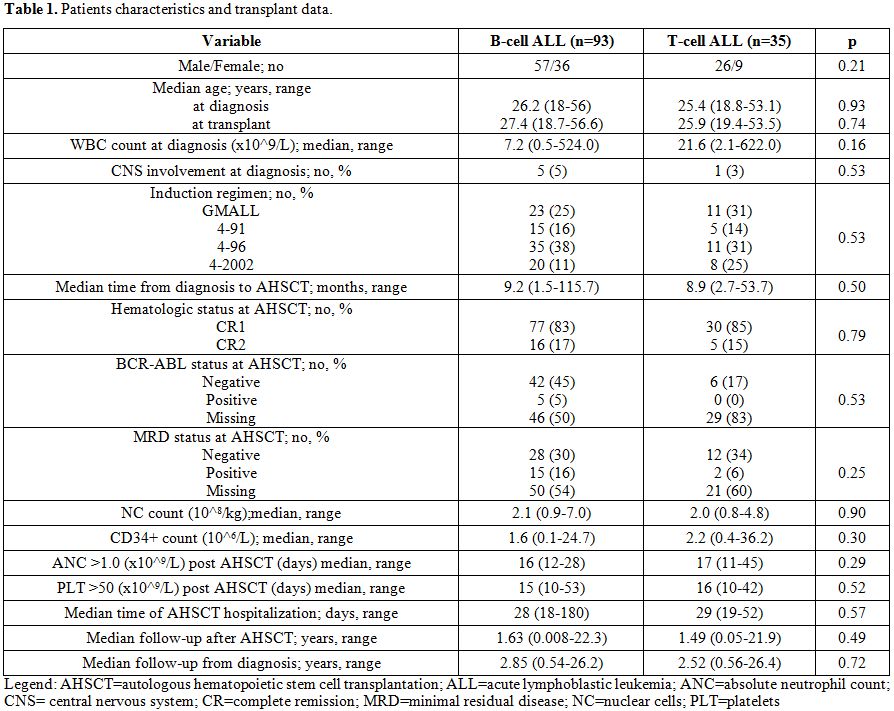 | Table 1. Patients characteristics and transplant data. |
Statistical analysis. The probability of leukemia-free survival (LFS) was defined as the time interval form transplant to the relapse or death in CR and it was calculated according to Kaplan-Meier method. All calculations were made from the date of transplantation. Comparisons between the variables were carried out by log-rank test. Statistical significance was defined at a P value <0.05. A Cox model was used to identify prognostic variables. The following variables were included in Cox regression model: gender, age, the type of induction protocol, WBC count at diagnosis, disease immunophenotype, CNS involvement, disease status at transplant, MRD status at transplant, year of transplant (before and after 2000), time from diagnosis to transplant, the number of transplanted NC and CD34+ cells. Transplant-related mortality (TRM) was defined as death within 100 days post autologous HSCT not related to the disease, relapse and progression.
Results
One hundred and twenty eight patients at median age of 27 years at transplant (range 18.7 – 56.6 years) were analyzed in this retrospective study. A median time from diagnosis to transplant was 9.2 months (range 1.5-115.7 months). With a median follow-up after AHSCT of 1.6 years (range 0.1-22.3 years), the probability of LFS for the whole group at 10 years was 27% and 23% at 20 years (Figure 1). Four patients died due to infectious complications early after transplant and one died due to intracerebral hemorrhage (TRM=3.2%). The reason of death in the remaining subjects was disease relapse with subsequent resistance to re-induction treatment or therapy-related complications. One relapse occurred >10 years after transplant. Details of transplant-related complications were shown in table 2.
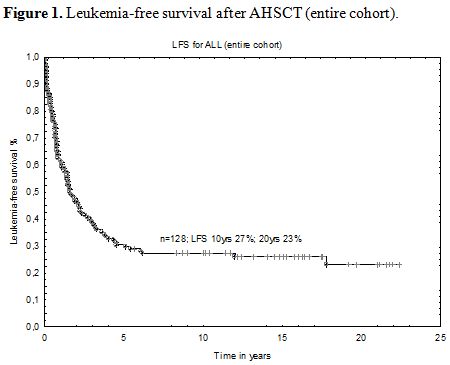 | Figure 1. Leukemia-free survival after AHSCT (entire cohort). |
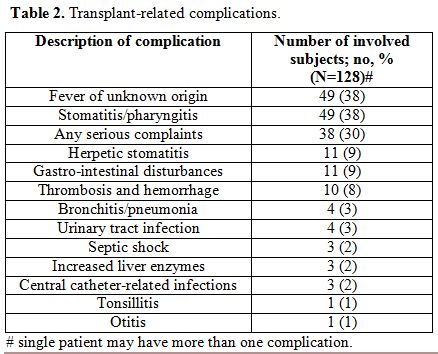 | Table 2. Transplant-related complications. |
No difference in the probabilities of LFS was found between patients transplanted before (n=71) and after 2000 (n=57). No statistical difference was found between patients with B-cell and T-cell ALL as well as between patients transplanted in CR1 vs CR2 in regards to demographic and laboratory data. There was no difference in estimated LFS between B-lineage and T-lineage ALL at 10 years; 24% vs 34%, respectively (p=0.48) as well as for patients transplanted in CR1 and CR2 (30% vs 9%; p=0.22). There was a strong trend towards better LFS at 10 years for patients with negative MRD at transplant when compared with subjects who had positive or unknown MRD (32% vs 23% vs 25%; p=0.06). The impact of MRD status on LFS has not been also observed when we restricted our study cohort to the patients with known MRD status and CR1 at transplant; the probabilities of LFS at 10 years were 35% for MRD-negative and 30% for MRD-positive subjects, p=0.5. Only 5 patients were found to be Ph+ at transplant, but they remained MRD negative by flow cytometry. LFS at 10 years for this small subgroup was 20% and this was comparable with that seen for subjects with undetectable and missing Ph status (26% and 28%; p=0.97). No factor was found to have a significant impact on LFS in multivariate Cox regression model.
Discussion
AHSCT for ALL has not shown a significant advantage over conventional
chemotherapy in a majority of randomized studies and that was proved
for any particular risk groups.[8,16,17] It may result from two main
aspects of AHSCT, namely 1) the contamination of graft by residual
leukemic cells and 2) the lack of graft-versus-leukemia effect. There
are only single reports on the use of different purging methods in
patients undergoing AHSCT, but none of these studies demonstrated
outcome benefits if compared with “no purging” procedure.[18-20]
Prior trials in high-risk ALL analyzed the efficacy of donor vs no
donor outcomes after consolidation treatment. Then, the subjects with
lacking donor were randomized between AHSCT and maintenance with
chemotherapy. Unexpectedly, two large studies of EORTC and PETHEMA
groups failed to prove that allogeneic sibling HSCT yielded a better
LFS than AHSCT or chemotherapy. There was also no difference if AHSCT
was compared with chemotherapy.[5,21] The LALA-94 trial was the first
that demonstrated the superiority of allogeneic HSCT in high risk ALL
patients, but the same study did not show any advantage of AHSCT over
chemotherapy.[2] Moreover, the
up-to-date largest international study
showed that patients randomized to AHSCT had a significantly lower
5-year overall survival than these treated with chemotherapy (37% vs
46%).[22] It should be emphasized
that no beneficial effect of AHSCT in
first complete remission was demonstrated if compared with chemotherapy
in meta-analysis study with 2962 Philadelphia-negative ALL patients.
The authors analyzed data from five randomized trials comparing AHSCT
and chemotherapy and they found no difference in relapse rate.
Moreover, there was a tendency toward higher TRM and inferior survival
in autografted subjects. The conclusion from this analysis is that
autograft has no advantage over chemotherapy in ALL transplanted in
CR1.[16] The results of our
analysis despite many drawbacks resulting
from a retrospective nature of the study, seem to be comparable with
data provided by others. The LALA-85, -87 and -94 trials reported on
10-year probability of LFS of 20% for AHSCT in CR1 of high-risk ALL.[9]
It should be mentioned that the comparison with other studies is
difficult, and it is probably due to the difference in conditioning
regimen (usually TBI and Cyclophosphamide) and purging methods.[23]
The advantages of our study were a quite large number of included
patients, a uniform conditioning regimen administered in most patients
as well as the homogeneous use of bone marrow as a source of stem
cells. However, the leukemic contamination was not examined in
transplanted marrow. There were single attempts to improve the results
of AHSCT in ALL setting by an intensification of conditioning regimen
and the preemptive results based on the small number of included
patients were found to be satisfactory. For transplanted subjects the
LFS at 5 year was 53% with TRM of 5%.[24]
One may consider to offer AHSCT or allogeneic unrelated donor (URD)
transplantation for high-risk ALL patients who lacking a family donor.
A large study with 712 ALL patients compared the outcomes of autologous
and allogeneic URD HSCT performed in first or second CR. There was no
difference in engraftment rate, but TRM was significantly higher after
URD transplantation (42%) than after autologous transplant (20%). Of
note is that relapse rate was less frequently seen in the former group
(14% in CR1 and 25% in CR2) if compared with the latter one (49% in CR1
and 64% in CR2). Nevertheless, overall survival rates at 3 years were
comparable between URD HSCT and AHSCT for patients transplanted in CR1
(51% vs 44%) and CR2 (40% vs 32%).[25]
Some papers have shown that MRD status before transplant may have an
impact on LFS in high risk adult patients.[10,26] Patel et al. performed
a sub-analysis including 25 ALL patients with known MRD status before
AHSCT. It was demonstrated that the presence of MRD (≥10-4 ) was
associated with lower LFS at 5 years (25%) if compared with
MRD-negative (<10-4) subjects (77%).[10]
The other study included 123
high risk ALL patients autografted in CR1. The study patients were
collected from eight European centers and MRD assessment was based on
different methods (flow cytometry and polymerase chain reaction). The
estimated 5-year LFS was significantly higher for subjects with
negative MRD before transplant (57%) if compared with these
transplanted with positive MRD (17%). That was also true for T-lineage
ALL and a tendency was shown for B-origin ALL. In a multivariate
analysis, only negative MRD was associated with better post-transplant
outcome.[26] Our study did not
show a significant difference in LFS
between MRD-positive and MRD-negative ALL patients at transplant.
Moreover, the LFS was comparable between these two subgroups when the
analysis was restricted to the patients undergoing AHSCT in CR1.
However, our results should be interpreted with caution due to the
retrospective nature of the study and limited number of included
patients. The methodology of MRD assessment by flow cytometry has also
changed during the study period. It should be highlighted that the
prognostic value of MRD before transplant was statistically
demonstrated only for transplantations performed using peripheral blood
as a source of stem cells, but not for bone marrow.[26]
Another absorbing aspect of AHSCT relates to patients with
Philadelphia-positive ALL that has never been considered as an option
for this patient subgroup. One hundred and seventy seven
Philadelphia-positive subjects were autografted and the results have
recently been reported. It was demonstrated that year of
transplantation was the only factor influencing the risk of treatment
failure. The probability of LFS increased from 11% for transplants
performed between 1996-2001, to 39% in years 2002-2006 and finally it
reached 57% for years 2007-2010. The better results were shown for ALL
patients treated with tyrosine kinase inhibitors (TKI): LFS at 3 years
was 65%. The further studies are required to confirm these satisfactory
results.[27] The Ph status was
known for 53 patients from our study
cohort, and 9% of these subjects were Ph+ at transplant. None of these
Ph+ patients received TKI and all were transplanted before 2000. The
probability of 10-year LFS was 20% and this was comparable with
Ph-negative and unknown subgroup. The other pre-TKI studies did not
demonstrate any advantage of AHSCT over chemotherapy in this
population.[22,28]
Conversely, several recently published studies have
shown that AHSCT preceding by TKI and chemotherapy may result in
similar outcome to that achieved by allogeneic HSCT.[29,30]
Based on the
recent reports it seems reasonable to offer AHSCT for Ph+ ALL patients
without a family donor and no detectable BCR-ABL transcript at
transplant. However, there was lacking comparisons of matched URD HSCT
with AHSCT in this cohort. In conclusion, AHSCT for high-risk ALL may
constitute an alternative therapeutic option for patients who lacking a
family/alternative donor, however the long-term results remain
unsatisfactory. Prospective studies focusing on MRD status are required
to re-evaluate the role of AHSCT for high-risk ALL.
References





























[TOP]