Received: May 20, 2014
Accepted: June 20, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014050, DOI 10.4084/MJHID.2014.050
This article is available on PDF format at:

Antoine Thyss, Esma Saada, Lauris Gastaud, Frédéric Peyrade and Daniel Re
Haematology
Department; Centre Antoine-Lacassagne , 33 Avenue de Valombrose, 06189
Nice cedex 2, France.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Hodgkin
Lymphoma HL can be cured in the large majority of younger patients, but
prognosis for older patients, especially those with advanced-stage
disease, has not improved substantially. The percentage of HL patients
aged over 60 ranges between 15% and 35%.A minority of them is enrolled
into clinical trials. HL in the elderly have some specificities: more
frequent male sex, B-symptoms, advanced stage, sub diaphragmatic
presentation, higher percentage of mixed cellularity, up to 50% of
advanced cases associated to EBV. Very old age (>70) and
comorbidities are factor of further worsening prognosis. Like in
younger patients, ABVD is the most used protocol, but treatment outcome
remains much inferior with more frequent, severe and sometimes specific
toxicities. Few prospective studies with specific protocols are
available. The main data have been published by the Italian Lymphoma
Group with the VEPEMB schedule and the German Hodgkin Study Group with
the PVAG regimen. Recently, the Scotland and Newcastle Lymphoma Study
Group published the SHIELD program associating a prospective phase 2
trial with VEPEMB and a prospective registration of others patients.
Patients over 60y with early-stage disease received three cycles plus
radiotherapy and had 81% of 3-year overall survival (OS).Those with
advanced-stage disease received six cycles, with 3-year OS of 66%.The
role of geriatric and comorbidity assessment in the treatment’s choice
for HL in the elderly is a major challenge. The combination of loss of
activities of daily living combined with the age stratification more or
less 70y has been shown as a simple and effective survival model. Hopes
come from promising new agents like brentuximab-vedotin (BV) a novel
antibody-drug conjugate. The use of TEP to adapt the combination of
chemotherapy and radiotherapy according to the metabolic response could
also be way for prospective studies.
|
Introduction
Both
the clinical management of older patients with cancer and the design of
research studies pertinent to this population remain major challenges.
This statement is particularly true for Hodgkin’s Lymphoma (HL): in
younger patients, HL is cured in the vast majority, but results remain
disappointing for older patients, especially those with advanced-stage
disease.[1]
For the treatment of HL in elderly, there is a major contrast between
the relative disease frequency in older patients and the rarity of the
studies specially targeted at this population. HL in older patients is
not a very rare disease: in the epidemiological data from the US
government, the age adjusted incidence in 2006-2008 for HL ≥ 65
represents 17.7% of all HL.[2]
Population-based epidemiological studies typically show a bi-modal peak
of HL incidence:[1-11] the first
around 30-35 years old, the second beyond 55-60.[7]
The proportion of HL patients aged > 60 ranges in the different
reports between 15% and 35%;[6,9-12]
accordingly the 26.7% of biopsies of HL, collected between
2006-2011 in a database, grouping all pathologists of the department of
the Alpes-Maritimes in the south of France, and including also our
cancer centre, was from patients aged > 60
(unpublished data).
Several recent series have specified the relative incidence of HL in
the elderly and identified specific prognostic factors in this group of
patients;[1,3-7,10,11,13,14]
however, only a minority of them are enrolled in clinical trials.[10]
Specificity of HL in the Elderly
Age itself is one of the important prognostic factors in Hodgkin
Lymphoma (HL) and is included in the prognostic scoring system of some
of the main cooperative groups such as EORTC or Canadian-ECOG, as in
the international prognostic index for advanced HL[8]
but not for the German Hodgkin Study Group, GHSG. For example, age over
50 is an unfavourable prognosis factor for the EORTC group. Patients
over 60 are rarely eligible and/or included in the prospective trials
of these groups. Moreover, International Prognostic Index (IPS) is the
most used model for predicting prognosis in advanced-stage HL patients8
but it must be noted that no patient with an age > 65 was
enrolled
in the study. Patients aged > 60 years have a 5-year event-free
survival (EFS) and overall survival (OS) rate of 30-40% and 40%-50%,
respectively.[12,15,16]
These figures are significantly inferior to those reported in the adult
patient.[3]
However, HL survival for all age groups increased in the last two
decades, even in the age subset over 60, where no major advances in
treatment efficacy were recorded, indicating an overall improvement in
patient care.[1,3]
Several reports stressed the difference in pathogenetic mechanisms,
tumour pathobiology, host-related factors, clinical presentation,
symptoms and prognosis for HL affecting the elderly as compared to the
adult.[4,5,13]
There are many
differences between the presentation of HL in the elderly in comparison
to that of the younger patients: (a) mixed cellularity is more frequent
ranging from 31% to 50%;[1,13] (b) 34% of cases and up to 50% of
advanced cases show the presence of EBER or LMP-1 proteins in
Reed-Sternberg cells[6] (these two
points are not independent); (c) B-symptoms and advanced
stage are much more common than in adults.[7]
Male sex and sub-diaphragmatic presentation are also more frequent in
patients over 50. Age over 70 is a factor of further worsening
prognosis,[1,9]
as is co-morbidity.[1]
The latter seems to play an essential role in predicting treatment
outcome: in a retrospective analysis, stage, B symptoms and presence of
co-morbidity were independently associated with inferior survival.[12] Several recent retrospective
studies, mainly on population databases, have pointed out these
particularities (table 1).
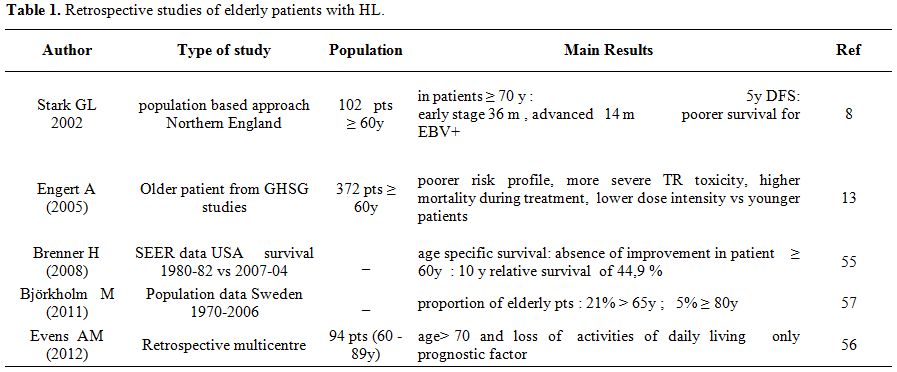 | Table 1. Retrospective studies of elderly patients with HL. |
Management of HL in the Elderly
There is no true consensus for treatment of HL in the elderly. After
full staging, including PET–scan, geriatric assessment and evaluation
of co-morbidities, the principles of treatment can be resumed as
follows: for stages 1A and 2A, a short chemotherapy program
of
two cycles, possibly prolonged to four courses in case of poor risk
features, followed by involved fields (IF) radiotherapy. For advanced
stage, it is often claimed that a prolonged use of ABVD (i.e. 6 cycles)
could be also given to patients in good general status, but elderly
patients are often incapable to tolerate the regimen of six cycles of
ABVD, particularly because of the lung toxicity induced by bleomycin.
There is no consensual approach for frail patients. The number of
chemotherapy cycles might be reduced depending on the result of interim
PET-scans, even if data for this approach in this elderly population
are lacking.
The ABVD regimen (doxorubicin, bleomycin, vinblastine, and dacarbazine)
has been considered the standard front-line treatment for HL patients[17]
and remains a gold standard, especially for limited-stage HL.
Obviously, treatment outcomes with ABVD in elderly patients remain
inferior to those obtained in younger patients.[18]
Moreover, toxicities are more frequent and severe in older patients,
and treatment dose intensity is often reduced because of toxicity
and/or co-morbidity and some specific toxicities are observed, as lung
toxicity of bleomycin reported as high as 46% in some series.[18,19]
A frequent approach in geriatric oncology or haematology to reduce
toxicity of chemotherapy is the development of specific protocols that
reduce dose intensity and/or omit or replace some drugs with
age-associated toxicity. This approach often permits a better tolerance
but is systematically associated with a reduction of response and
survival, as compared to younger adult patients. For example, the role
of relative dose intensity (RDI) has been questioned by the German
Hodgkin Study Group (GHSG) in the elderly HL patient: a significantly
lower RDI was observed in older HL patients[2]
and patients with a RDI < 65% had poorer outcomes than patients
receiving higher chemotherapy doses.[9]
Taken together these data stress the concept that in elderly patients,
non-HL events affect survival in a more significant way compared to
younger patients.
In the late 1990s, the GHSG carried out a prospectively randomized
study to compare the baseline BEACOPP regimen against COPP-ABVD, their
standard regimen at the time.[20]
Between February
1993 and 1998, 75 patients aged 66 – 75 years with newly diagnosed HL
in advanced stages were recruited into the HD9 trial as a separate
stratum (HD9 elderly). Patients were assigned to eight alternating
cycles of COPP and ABVD or eight cycles of BEACOPP in baseline doses.
Radiotherapy was given to initially bulky or residual disease. In
total, 68 of 75 registered patients were met assessment criteria: 26
with COPP-ABVD and 42 with BEACOPP baseline. There were no significant
differences in terms of complete remission (76%), overall survival
(50%), and freedom from treatment failure (FFTF) (46%) at 5 years. Two
patients (8%) treated with COPP-ABVD and nine patients (21%) treated
with BEACOPP died of acute toxicity. They concluded that both regimens
gave limited results in elderly patients, and that baseline BAECOPP was
too toxic. The use of the escalated BEACOPP, the standard regimen of
the GHSG for advanced stage HL in younger patients, is excluded for
patients over 60 years because of excessive toxicity.[21]
Prospective HL Studies Specifically Designed for Older Patients
Very few prospective HL studies designed specifically for older patients are available. All studies published since 2000 are listed in Table 2. Some non-ABVD chemotherapy regimens containing different alkylating agents have been published and are also listed in Table 2.
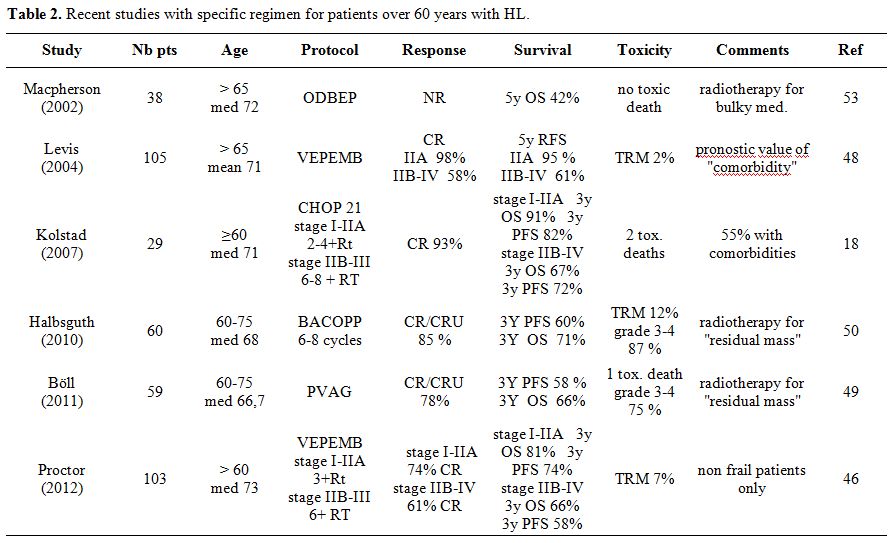 | Table 2. Recent studies with specific regimen for patients over 60 years with HL. |
Macpherson
et al. have proposed in 2002 a new protocol called ODBEP (vincristine,
doxorubicin, bleomycin, etoposide and prednisone).[22]
They compared 38 patients aged over 65, treated with this regimen, with
an historical control group of 17 patients treated with
MOPP/ABV-variant, a standard regimen in the 1980s. With a median
survival of 43 and 39 months, and five-year overall survival (OS) of
42% and 32%, respectively, there was no statistically significant
difference between the two treatments; however, ODBEP appeared to be
less toxic.
The Italian Lymphoma Group Results published the results with the
VEPEMB schedule (vinblastine, cyclophosphamide, prednisolone,
procarbazine, etoposide, mitoxantrone and bleomycin).[12]
This study included 105 evaluable patients over 65 (mean 71 years,
range 66–83), with 48 (46%) stages I-IIA, and 57 (54%) stages IIB-IV.
Co-morbidity defined only as “the presence of a concomitant disease,
requiring specific treatment” was observed in 39 patients (37%). The CR
rate was 76%, respectively 98% for stage IIA and 58% for stage IIB-IV,
and the five-year RFS rate of patients entering CR was 82%,
(respectively 95% and 66%). In multivariate analysis, stage, systemic
symptoms, and co-morbidity had independent value in affecting OS, DSS,
and FFS. Surprisingly, despite the use of bleomycin, no pulmonary
toxicity is mentioned, raising the question of this systematic
evaluation.
The same regimen has been used by the Scotland and Newcastle Lymphoma
study group (SNLG) in the SHIELD program[23]
for 175 patients, associating a prospective phase 2 trial of VEPEMB
with 105 patients with a prospective registration study of patients not
treated as part of the VEPEMB study. This trial included patients too
frail to receive standard therapies and patients managed with curative
intent using alternative treatment regimens, including 35 patients
treated with ABVD. This somewhat complicated program was designed to
recruit all patients representative of the target population in
participating centers. Initial evaluation of the patients involved a
formalized assessment of co-morbidity using a modified ACE-27
co-morbidity scale. Patients designated as “non-frail,” were eligible
for the phase 2 VEPEMB protocol. Those designated as “frail” were
eligible for entry into the registration arm of the study and were
treated at their physician’s discretion. Of 103 VEPEMB patients over 60
(median age 73), 31 had early-stage disease (stage 1A/2A) and received
VEPEMB three times, along with radiotherapy. The median follow-up was
36 months. Complete remission (CR) rate (intention-to-treat) was 74%,
and three-year overall survival (OS) and progression free survival
(PFS) were 81% and 74%, respectively. A total of 72 patients had
advanced-stage disease (stage 1B/2B/3 or 4) and received VEPEMB six
times. CR rate was 61% with three-year OS and PFS of 66% and 58%,
respectively. Overall TRM was 7%.
Another new regimen has been developed by the GHSG in the same period
in early unfavorable and advanced stages, the PVAG regimen (prednisone,
vinblastine, doxorubicin, and gemcitabine).[24]
In a
phase 2 study, 59 patients with HL aged 60 to 75 (median, 68 years) and
with “normal organ function” and good general condition (WHO-Index ≤ 2)
received 6 to 8 cycles of PVAG and additional radiotherapy if they were
not in complete remission (CR) after chemotherapy. CR/CR uncertain was
obtained in 46 patients (78%). The three-year estimated for OS and PFS
were 58% (95% CI, 43%-71%) and 66% (95% CI, 50%-78%), respectively. WHO
grade 3/4 toxicities were documented in 43 patients (73%), with one
treatment-related death. These results are very close to those of their
previous BACOPP regimen for survival, with less toxicity.
A study of Kolstad et al.[25]
reported for the first
time the results of the CHOP-21 regimen, largely used in non-Hodgkin’s
lymphoma. In this single institution’s study with 29 patients aged 60
to 91, 11 stage-I/II patients received 2-4 cycle followed by
radiotherapy and 18 stage-IIB/IV patients received 6-8 cycles, without
radiotherapy for 13/18. Two treatment-related deaths occurred, but the
complete response rate of CHOP ± radiotherapy was 93% and OS and PFS at
three years were 79% and 76%, respectively. Unfortunately, these
impressive results of CHOP-21 in elderly HL as not been confirmed by
other groups.
Studies Designed for Younger Patients but Including Patients over 60 Years
Other studies designed for younger patients have included patients aged over 60. They are listed in Table 3.
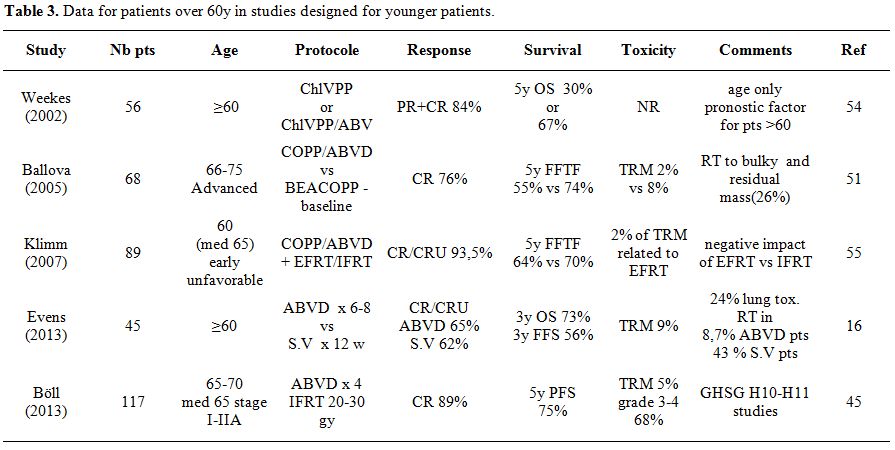 | Table 3. Data for patients over 60y in studies designed for younger patients. |
In
2002, the Nebraska Study Group in a retrospective comparison of ChlVPP
vs. hybrid ChlVPP/ABV in 56 patients over 60 years, showed a major
survival difference with a five-year OS of 30% vs. 67% respectively and
conclude that adriamycin should be considered as a major component of
the chemotherapy regimen in these patients.[15]
None of the usual clinical features were significant predictors of OS
in patients above 60.
In the multicenter HD8 study of the German Hodgkin’s Study Group, 1064
patients with early-stage unfavorable HL were randomized to
receive 4 cycles of chemotherapy (2 COPP alternated with 2 ABVD),
followed by either radiotherapy (RT) of 30 Gy extended field (EF) + 10
Gy to bulky disease or 30 Gy involved field (IF) + 10 Gy to bulky
disease. Of these, 89 patients (8.4%) were ≥60 years. Acute toxicity
from RT was more pronounced in elderly patients receiving EF-RT
compared with IF-RT. Freedom from treatment failure (FFTF) 64% vs. 87%,
and OS 70% vs. 94% after 5 years was lower in elderly patients compared
with younger patients. Elderly patients had poorer outcomes when
treated with EF-RT compared with IF-RT in terms of FFTF (58% versus
70%; P = 0.034) and OS (59% versus 81%; P = 0.008). The main conclusion
was that EF-RT after chemotherapy has a negative impact on elderly
patients with early-stage unfavorable HL and should be avoided.[26]
The German Hodgkin Study Group (GHSG) has tested the BACOPP regimen
that consists of bleomycin, doxorubicin, cyclophosphamide, vincristine,
procarbazine, and prednisone.[27]
Compared with the
BEACOPP baseline, a regiment largely studied by this group, etoposide
was omitted, and the anthracycline dose was increased as they
considered that anthracyclines might be one of the most relevant
components of chemotherapy for HL in older patients. Sixty five
patients with early unfavorable or advanced stage HL, aged between 60
and 75 years, were enrolled in this phase 2 trial. Treatment consisted
of 6 to eight cycles of BACOPP. Residual tumor masses were irradiated.
Of 60 eligible patients 51 (85%) achieved CR/CRu. With a median
observation time of 33 months, 18 patients died (30%), including 7
treatment-associated deaths. Grade 3/4 toxicities occurred in 52 (87%)
of patients. The PFS and OS rates for all patients at 3 years were 60%
(95% CI: 46%-73%), and 71% (95% CI: 59%-83%) respectively. Thus, they
considered this BACOPP regimen as active but compromised by a high
toxicity in older HL patients.
The U.S. intergroup trial E2496 comparing ABVD vs. Stanford V
(S.V) regimen has included 45 patients ≥ 60 years treated
with
ABVD (23 cases) or S.V (22 cases).[18]
Adjunctive
radiotherapy was delivered in 8.7% of ABVD patients and 43% S.V
patients with different criteria between these two groups. In these
patients, 5-year EFS and of 48% and 58%, respectively, were much
inferior to those obtained in younger patients (74% and 90%,
respectively). Moreover, in this trial, Bleomycin lung toxicity was
24%, mainly observed with ABVD (91%), and the death rate due to
Bleomycin toxicity was 18%. The overall treatment¬-related mortality
was 9% for older patients compared to 0.3% for patients aged <60
years. Overall, the death incidence without progression at two and five
years were respectively 13% and 22% for elderly patients, and
2%
and 9% for younger patients (p<.0001). These results stressed
that
both ABVD and S.V regimens are toxic and difficult to use for patients
aged above 60 and indicated the necessity of a precise evaluation and
selection of patients before the choice of treatment.
Böll et al.[2] reported in 2013,
for the GHSG, 117
cases of patients aged from 60 to 75 years (median 65) in a
cohort of 1299 patients, included in the HD10 and HD11
trials,
and treated with 4 x ABVD for limited HL (stage I or II). Despite the
selection of a favourable subgroup, the dose intensity delivered was
reduced and only 59% of the patients achieved a relative dose intensity
>80% compared with 85% for younger patients. In 14%, major
protocol
violations occurred, mainly because of excessive toxicity. Toxicities
of WHO grade 3 or 4 occurred in 68%, with 5 % of treatment related
mortality. The author conclude: “in patients ≥ 60 years with HL, four
cycles of ABVD are associated with substantial dose reduction,
treatment delay, toxicity and treatment-related mortality."
The escalated BEACOPP, which is the standard protocol for
advanced-stage HL for the GHSG and is much more toxic that ABVD, could
not be proposed in patients aged over 60y: in their HD9 study
the
treatment related mortality (TRM) rate was high (14.3%) in patients
over 60 years and neutropenic infections were the main cause
of
TRM.[21]
Role of Geriatric and Comorbidity Assessment in the Treatment Choice for HL in the Elderly
While definitions of the elderly patient remain vague, one can describe
the ageing process as a gradual loss of adaptation to stress due to a
diminution of the functional reserves of different organs. The process
is variable from one individual to another and thus, the mean life
expectancy of a 70 year-old patient varies from 6.7 to 18 years, and
that of an 80-year old patient from 3.3 to 10.8 years. In addition to
chronic pathologies (co-morbidities), geriatric syndromes (dementia,
incontinence, under-nutrition, etc.) further complicate medical
management. Clinical studies regularly exclude patients who are very
old, frail or suffering from co-morbidities, which makes the results
difficult to apply to these patients.
The clinician thus confronted a dual dilemma: should he treat with a
proven but unpredictable risk of hyper toxicity, or undertreat a
potentially curable patient and waste an opportunity. A recent review
points out this dilemma in hematologic malignancies[28]
but data are very scarce for HL. A range of techniques have been
advanced for assessing geriatric patients with a view to singling out
those capable of receiving a potentially toxic dose of chemotherapy.
The first step is to identify those most likely to benefit from
comprehensive geriatric assessment (CGA). This method involves
evaluating comorbidities, physical and psychic autonomy, nutritional
and cognitive status, level of depression, mobility and balance.
Unfortunately, the process is long, costly, complex and difficult to
use in clinical units in the absence of a dedicated geriatrician.
Other, simpler methods have been suggested but remain open to criticism
and with contradictory results.
Recently, Soubeyran et al. reported interesting results of the Oncodage
study in 1688 patients with solid tumors. They validated the G8 score,
a relatively simple instrument to detect patients presenting at least
one abnormal geriatric test among the following: Activity of Daily
Living (ADL), Instrumental Activity of Daily Living (IADL), MMS,
Geriatric Depression Scale 15 (GDS-15questions), Mini Nutritional
Assessment (MNA), Timed Get up and Go, and evaluation of
co-morbidities.[29] Although
constructed from
patients with solid tumors, the G8 test appears to be useable in
malignant hematology. In non-Hodgkin’s lymphoma, the GELA group
reported a prospective series of 150 DLBCL patients over 80 years
treated with low doses of CHOP and full dose of rituximab. A good PS
non-bulky disease (<10 cm), albumin >35 g/l, extra-nodal
sites
<2, and high ADL level were associated with improved overall
survival (OS) but in multivariate analysis, only albumin levels
superior to 35g/l were associated with longer OS.[30]
In HL the only prospective study including a co-morbidity assessment in
a patient over 60 years seems to be the SHIELD study, previously
described:[23] this study seems to
be more applicable
to everyday life, and serves more as a reflection than a tool for the
choice of treatment for an individual patient. In their retrospective
study, Evens et al. showed that the combination of loss of activities
of daily living and the age stratification of about 70 years gives a
simple survival model in elderly HL.[1]
Several series have shown that the presence of co-morbidities is
independently prognostic.[12,31,32]
Further trials should be designed to permit some flexibility to modify
or adapt the drug dosing or the regimen, on the basis of firm objective
criteria to avoid prohibitive toxicities. These trials may be able to
optimize the duration of treatment to the response according to PET
response (cfr. infra).
New Drugs
Brentuximab
Vedotin
(BV) is a promising novel antibody-drug conjugate targeting CD30 linked
to a potent synthetic antitubulin chemotherapeutic agent, monomethyl
auristatin E (MME). BV acts as a cell cycle-specific agent, like Vinca
alkaloid drugs, and induces G2/M-phase growth arrest.[33]
A multicenter phase II trial of BV in HL patients, recurring after
ASCT, demonstrated an overall response rate (ORR) of 75% and a complete
response (CR) rate of 34% with a median duration of 20.5 months.[34]
The most common treatment-related adverse event was peripheral sensory
neuropathy, recorded in 42% of patients, but with complete resolution
after discontinuation of treatment in 50% of patients. A very similar
incidence of grade 2 or 3 peripheral polyneuropathy (52%) and symptom
resolution (54%) was reported by Gopal et al. in a small series (N=25)
of patients treated with BV for recurring HL after allogeneic bone
marrow transplantation.[35] A
higher rate of peripheral neuropathy was observed with weekly BV
treatment versus treatment delivered every 3 weeks.[36,37]
No association of more frequent or severe neuropathy with old age was
reported in any study, while a pre-existing neuropathy was found in
more than half of cases of neurotoxicity. The second most frequent
toxicity of grade 3 or more was haematological: anaemia (6-20%)
neutropenia (20% in both series), and thrombocytopenia (8%). A higher
rate of peripheral neuropathy was observed with weekly BV treatment
versus treatment delivered every 3 weeks.[36,37]
Bendamustine
hydrochloride
(Be) combines the antimetabolite activity of the purine-analogue
structure with the alkylating property of the nitrogen mustard group.
It is not a “new” drug, but its use in HL is recent. Because of its
distinctive activity profile, Be may show limited cross resistance with
agents usually employed for upfront and salvage treatments, and,
therefore, it has been proposed for the treatment of
relapsing/resistant HL. In two small phase II studies, enrolling nearly
70 patients aged 20-75, previously treated with 3-8 lines of
chemotherapy (including autologous or allogeneic stem cell transplant),
the ORR and CR rate, after a mean of 3.8 cycles of Bendamustine,
administered intravenously at the dose of 120 mg/m2 on day 1 and 2
every 28 days, were, respectively, 50-53% and 29-33%. The mean duration
of response was 5 and 5.7 months.[38,39]
Corazzelli
et al., in a third phase II study reported similar results with 6-8
courses of Be at 70-120mg/m² in 41 relapsed or refractory HL patients
after 1-8 lines of chemotherapy, aged 18-85 years. The ORR and CR rate
were 58% and 31%, respectively.[40]
Despite being relatively short PFS, these rates of response in such
heavily pre-treated patients are encouraging.
The feasibility of Be treatment in patients aged 70 or more has been
explored more frequently in Chronic Lymphocytic Leukemia (CLL). In a
large retrospective study of 465 CLL patients treated with Bendamustine
alone or in combination with rituximab, 91 were older than 70.[41] The most frequently administered
dose and schedule was 76-98 mg/m2,
twice per cycle every 21 days. Nearly 40% of patients experienced no
cycle delay, grade 3-4 blood/bone marrow AE occurred in 18.8% and
25.3%, respectively. In a multicenter phase II clinical trial from the
German CLL Study Group, among 117 previously untreated CLL patients
aged 34-78 years, 25.6% were aged > 70.[42]
Be schedule was 90 mg/m2
on days 1 and 2, every 28 days, associated to Rituximab. Grade 3 or 4
adverse events for neutropenia, thrombocytopenia, and anaemia were
documented in 19.7%, 22.2%, and 19.7% of patients, respectively.
However, in both reports, the myelosuppression, due to bone marrow
invasion by disease in all patients, and the association with Rituximab
could have negatively affected the haematological toxicity. Finally, in
a phase II trial in a small cohort of 20 elderly and frail patients
affected by Diffuse Large B-Cell Lymphoma (DLBCL), with a mean age of
72 (51-86), Be was administered at a dose of 90 mg/m2
on days 1 and 2 in association with Rituximab on day 0, every 28 days.[43]
The relative dose intensity (RDI) was 100%. Granulocyte colony
stimulating factor (GCS-F) was administered in only two patients and
only three grade 3 WHO (1 leukopenia and 2 anaemia) and one grade 4
(thrombocytopenia) toxicities occurred.
Taken together, all these data makes Be an interesting candidate for
use in combination for treatment of HL in elderly.
Other New Agents
Some other drugs are currently being studied and could potentially be used in the treatment of HL in the elderly because of a reduce toxicity compared to the usual chemotherapy regimen. Some epigenetic agents as HDACS inhibitors have shown encouraging results: for example, panobinostat, in a phase 2 trial, showed an ORR of 27%.[44] Lenalidomide an immune-modulatory agent with anti-angiogenic properties demonstrated a modest single agent activity, but may also be used in combination.[44,45]
Proposal for New Approaches
As the “gold standard” for younger patient, represented by ABVD, is more toxic and difficult to use with sufficient dose-intensity and efficacy in the elderly, there is room for new approaches in the treatment of HL in the elderly.
Treatment Adapted to the Positron Emission Tomography (PET)
Response.
The early complete metabolic response, evaluated by PET after two
cycles of ABVD, has been demonstrated to be the most important
prognostic factor in 260 patients with advanced stage HL.[46]
Many protocols are testing the possibility of shortening chemotherapy
and/or omitting radiotherapy in adult patients, especially when in
early favourable stage. In the elderly, this criterion could be used to
propose chemotherapy of shorter duration, to avoid unnecessary
toxicities. This strategy seems to be particularly attractive in the
limited stages (I or II) in which radiotherapy can be use thereafter
without excessive toxicity, even in the population of older patients.
The major limitation of this strategy is that most elderly patients
with HL have advanced stage,[7] reducing the indications of
radiotherapy.
Combination
of BV with other chemotherapy compounds.
As BV is the only truly new drug with a major activity in HL without
unexpected toxicities in the elderly, its use in a first line
combination should be tested on elderly HL patients. Several reasons
could account for the synergistic activity of BV with other
antineoplastic drugs. First, differences between the cell types
targeted by each class of compounds may account for improved activity.
BV action is targeted against cells expressing CD30. CD 30 is found in
Hodgkin’s and Reed Sternberg (HRS) cells, which typically comprise only
0.1–1% of the total cell population within HL lesions.[47]
Chemotherapeutic agents, in addition to tumour cells, also target
proliferating stromal cell infiltrates, which constitute the majority
of the cell populations within HL lesions. By contrast, the cytotoxic
effect of free MMAE diffused from CD30+ malignant cells on bystander
cells may in part account for the significant antitumor activity of BV.[48]
Secondly, differences in the mechanism of action between MME-based ADC
and chemotherapeutic compounds may help to explain the increase in
efficacy.
Frontline treatment of advanced-stage HL, with BV at the dose of 1.2
mg/Kg in association with ABVD (Doxorubicin, Vinblastine, Dacarbazine)
or AVD, proved very active in a phase I multicentre trial,
achieving respectively 95 and 96% of CR.[49]
The most
frequently reported (WHO grade ≥3) adverse effects were neutropenia and
peripheral sensory neuropathy present in 44% of the ABVD cohort, with 2
toxic deaths.[49] These results
with BV plus AVD are
promising, but it is possible that this combination will remain too
toxic for elderly patients.
Be seems to be a good candidate for such a combination as it have a
non-cross-resistant mechanism of action compared to BV combining the
antimetabolite activity of the purine-analogue structure with the
alkylating property of the nitrogen mustard group. In addition to
primary effects on tumour cells, Be has shown a peculiar activity in HL
by depleting the neoplastic microenvironment of tumour-supporting T-
and B-lymphocytes.[50,51] This
drug is already widely
used in patients over 70 with CL or low grade lymphoma, and toxicity is
easily manageable without dose reduction in case of renal
insufficiency.[52]
Conclusions
Existing data does not define an optimal first line treatment for
elderly HL patients. A comparison of existing trials is almost
impossible because of the small number of patients included in the few
published trials, lack of patient stratification (based on
co-morbidities or age cohorts, for example), heterogeneous
documentation of side effects such as lung toxicity, and varying
endpoints chosen to assess efficacy of treatment protocols.
Patient selection will be crucial for an effective treatment strategy
that could be modified based on interim PET imaging. In the absence of
clinical data, it is necessary to individualize treatment decisions
based on pre-treatment assessment scales and PET imaging. Based on our
clinical experience, it is of utmost importance to monitor patients
closely while on treatment in order to detect and control severe
treatment related complications.
Acknowledgments
Sandrine Pacquelet-Cheli provided editorial assistance in the preparation of the manuscript. Katelyn Gordon and Anastasia Glazunova provided assistance for the revision of the manuscript.
References


















































[TOP]