Received: June 12, 2014
Accepted: June 30, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014052, DOI 10.4084/MJHID.2014.052
This article is available on PDF format at:

Josep-Maria Ribera1,2, Jordi Ribera2 and Eulàlia Genesca2
1
Clinical Hematology Department. Institut Catala' d’Oncologia-Hospital
Universitari Germans Trias i Pujol Universitat Autònoma de Barcelona.
PETHEMA Group, Spain.
2 Jose Carreras Leukemia Research Institute.
Badalona, Spain.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract The
primary objective of this review was to update and discuss the current
concepts and the results of the treatment of acute lymphoblastic
leukemia (ALL) in adolescents and young adults (AYA). After a brief
consideration of the epidemiologic and clinicobiologic characteristics
of ALL in the AYA population, the main retrospective comparative
studies stating the superiority of pediatric over adult-based protocols
were reviewed. The most important prospective studies in young adults
using pediatric inspired or pediatric unmodified protocols were also
reviewed emphasizing their feasibility at least up to the age of 40 yr
and their promising results, with event-free survival rates of 60-65%
or greater. Results of trials from pediatric groups have shown that the
unfavourable prognosis of adolescents is no more adequate. The majority
of the older adolescents with ALL can be cured with risk-adjusted and
minimal residual disease-guided intensive chemotherapy, without stem
cell transplantation. However, some specific subgroups, which are more
frequent in adolescents than in children (e.g., early pre-T, iAMP21,
and BCR-ABL-like), deserve particular attention. In summary, the
advances in treatment of ALL in adolescents have been translated to
young adults, and that explains the significant improvement in survival
of these patients in recent years.
|
Introduction
Acute
lymphoblastic leukemia (ALL) encompasses a heterogeneous group of
disorders. The results of clinical trials in adults have been
disappointing compared with those of the pediatric age group, with cure
rates of 90% in children compared with 30%–40% in adults.[1]
An analysis of the Surveillance, Epidemiology, and End Results (SEER)
database showed an improvement in survival in adults over the last two
decades, with the greatest significant improvement being in the
adolescent and young adult (AYA) group.[2,3]
ALL is relatively rare among the AYA populations, whereas it is the
most commonly diagnosed leukemia in childhood, accounting for 75% of
leukemias diagnosed in pediatric patients. Many retrospective analyses
of the adolescent age group with newly diagnosed ALL treated according
to either pediatric or adult protocols showed a statistically
significantly superior outcome for patients treated with pediatric
regimens. A lower relapse rate accounted for the superior results
observed in adolescents treated according to pediatric protocols. For
that reason, treatment of ALL in AYA has gained increasing interest in
recent years. This review will focus on the biology and treatment of
ALL in AYA.
The Concept of AYA
Although there is not a uniformly accepted definition of age subgroups
in ALL, it can be considered that classic pediatric ALL patients range
from 0 to 14 yr of age, adolescents from 15 to 19 years, young adults
from 20 to 39 years, adults from 40 to 60 years and older adults and
elderly patients include those beyond the age of 65 years. Thus, AYA
encompasses those patients from 15 to 39 years of age.
Biological Factors in ALL of AYA
Several clinical and biologic characteristics of ALL used for risk
stratification and prognostication (e.g., phenotype, cytogenetics and
molecular genetics) are age-dependent (Table 1).
Regarding the immunophenotype, T-cell ALL (T-ALL) is more frequent in
AYA than in children and is known to be associated with slightly poor
outcomes. In addition, the “early T-cell precursor ALL” (ETP-ALL)
subtype, which is associated with poor treatment response, is
frequently presented in AYA.[4]
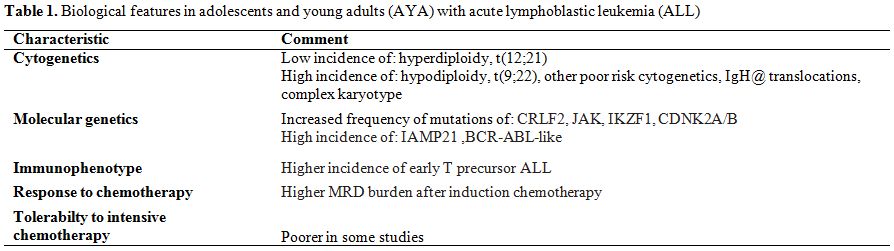 | Table 1. Biological features in adolescents and young adults (AYA) with acute lymphoblastic leukemia (ALL). |
One
of the substantial differences between children and AYA is the
difference in cytogenetics and molecular genetics. The genetic
abnormalities associated with good prognosis decrease with age.
Hyperdiploidy and the t(12;21) [ETV6-RUNX1]
translocation decrease with older age,[5]
during poor-risk cytogenetics, such as t(9;22) [BCR-ABL1],
complex karyotype, and hypodiploidy increase in prevalence with age. In
addition, several reports have demonstrated that good risk cytogenetics
is associated with inferior survival in adults and in adolescents
compared with children.[6] A recent
study including a
large cohort of teenagers and young adults enrolled in the UKALL2003
and UKALLXII trials showed a higher frequency of IgH@ translocations in
AYA.[7] Although these
translocations are associated
with an adverse outcome in adults, they are not independent prognostic
factors in children and adolescents.
To date, there has been no specific study of genomic profiling of AYA
with ALL because these patients have been included in both pediatric
and adult ALL studies. However, JAK
mutations, CRLF2
alteration, iAMP21 and BCR-ABL-like
profile are among the frequent alterations reported in AYA, and all of
them are associated with a poor prognosis. In summary, the genetic
profile of AYA ALL patients seems to be similar to patients with
high-risk ALL, suggesting that distinct underlying genetic and biologic
characteristics account for part of the inferior results observed in
AYA.[8]
In addition to the difference in biological factors between ALL in
children and in AYA, there is evidence that adult ALL cells are less
susceptible to chemotherapy both in
vitro and in
vivo.
In some studies ALL patients between 15–30 years of age had a
significantly higher minimal residual disease (MRD) burden compared
with children.[9,10]
As far as host factors are concerned, several features are present in
less young patients, being responsible for increased treatment
toxicity. They include differences in the metabolism of
chemotherapeutic agents, depleted marrow reserve and increased
extramedullary toxicity. All these issues increase the frequency of
life-threatening infections, organ failure, treatment delays and dose
reductions in planned chemotherapy.
Pediatric-based vs. Adult-based Treatments. Retrospective Studies
Several retrospective reports have shown that adolescents (15-20 yr.)
and young adults treated by adult oncologists or hematologists with
adult ALL protocols have poorer outcomes than similarly aged patients
treated by pediatricians with pediatric protocols despite having the
same biologic disease.[11-20] The
cut-off point of
age for treatment of patients in pediatric or adult hemato-oncology
units varies among different countries but usually ranges between 15
and 18 years.
The first study, in which such different outcomes were reported, was
conducted in France.[12]
A comparison of AYA aged 15-20 yr, treated with the pediatric-based
protocol FRALLE-93 (n=77) with patients of the same age and comparable
clinical and biologic characteristics of ALL, who received the
adult-based protocol LALA-94 (n=100), showed a complete remission (CR)
rate of 94% vs. 83%, respectively. After a median follow-up of 3.5 yr,
the supposed event-free survival (EFS) was 67% vs. 41% at 5 years.
Multivariate analysis showed an independent influence of the protocol
on the outcome. The differences in the drugs employed and, especially
in the dose-intensity, might explain the better results of the
FRALLE-93 protocol. In this protocol the cumulated dose of prednisone
was five-fold higher, the vinca alkaloids three-fold and the
asparaginase 20 fold-higher than in the LALA-94 study. In addition, in
the FRALLE-93 study the dose of prednisone in induction was higher, and
asparaginase was also given in this period, in contrast with the
LALA-94 trial. Moreover, the time interval between CR and
post-remission therapy was 2 days in the FRALLE-93 vs. 7 days in the
LALA-94 study.
The North-American Cancer and Acute Leukemia Group B (CALGB) and the
Children’s Cancer Group (CCG) performed a retrospective comparison of
presenting features, planned treatment, CR rate, and outcome of 321
AYA, aged 16 to 20 years, which we treated in consecutive trials in
either the CCG or the CALGB from 1988 to 2001.[11]
CR
rates were identical, being 90% for both the CALGB and CCG AYA. The CCG
AYA had a 63% EFS and 67% overall survival (OS) at 7 years in contrast
to the CALGB AYA, in which the 7-year EFS was only 34%, and the OS was
46%. While the CALGB AYA aged 16 to 17 years achieved similar outcomes
to all the CCG AYA with a 7-year EFS of 55%, the EFS for 18- to
20-year-old CALGB patients was only 29%. Comparison of the regimens
showed that the CCG AYA received more intensive central nervous system
prophylaxis and higher cumulative doses of non myelosuppressive agents
earlier. There were no differences in outcomes in those who reached
maintenance therapy on time compared with those who were delayed.
A similar Dutch study in patients aged 15-21 yr yielded similar
results,[13]
with a 5-yr EFS of 69% for comparable patients treated with the most
dose-intensive pediatric protocol DCOG vs. 34% for those treated with
adult protocols ALL-5 and ALL-18 from the HOVON Group. Likewise,
comparative retrospective studies from Italy also showed a poorer
prognosis for patients aged 14-18 yr treated with adult-type protocols.[14]
In turn, a Swedish study compared patients aged 10-40 yr treated with
the pediatric trial NOPHO-92 (n=144) vs. a similar group of patients
included in the Swedish Adult ALL Group (n=99).[15]
Significantly higher CR rate (99% vs. 90%) and EFS were observed in
patients treated with the pediatric protocol, with the type of
treatment being an independent prognostic variable on multivariate
analysis. However, it is of note that adults aged 26-40 yr had
significantly poorer prognosis than AYA (15-25 yr). Another study from
Denmark yielded similar results.[16]
In a
retrospective study from the British Medical Research Council (MRC),
performed only in adolescents (15-17 yr), who were enrolled in the
ALL97/revised99 (pediatric, n=61) or UKALLXII/E2993 (adult, n = 67)
trials between 1997 and 2002 (17), the EFS (65% vs. 49%) was higher,
and the rate of death in remission was lower in patients enrolled in
the pediatric trial.[17] In a
retrospective study
conducted in the Princess Margaret Hospital of Toronto restricted to
AYA with T-ALL 40 patients (median age 30 yr, range 17-69) were treated
with several adult type protocols and were compared with 32 patients
(median age 32 yr, range 17-64) treated with a DFCI protocol.[18]
Although there were no differences in CR attainment (93% vs. 84%), the
OS and relapse-free survival (RFS) probabilities were significantly
higher in patients treated with the DFCI trial (83% vs. 56% and 88% vs.
23%, respectively). On multivariate analysis the treatment group (DFCI
vs. non-DFCI) was the major prognostic factor influencing both RFS and
OS. Other studies from different countries[19]
have shown similar results (Table
2).
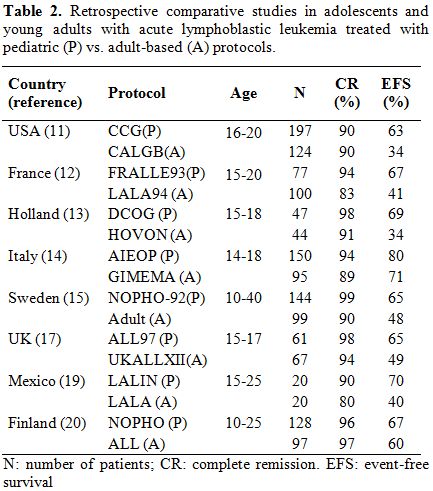 | Table 2. Retrospective comparative studies in adolescents and young adults with acute lymphoblastic leukemia treated with pediatric (P) vs. adult-based (A) protocols. |
Only
one population-based study from Finland showed that the outcomes of AYA
with ALL treated with pediatric or adult protocols were equal.[20]
One hundred and twenty-eight patients (10-16 yr.) were treated with the
pediatric Nordic (NOPHO) protocols and 97 patients (17-25 yr.) with
Finnish Leukemia Group National protocols. All patients were centrally
referred and treated in five academic centers. The 5-year EFS was 67%
for the pediatric treatment group and 60% for the adult treatment
group. There were no significant differences in the cumulative doses of
corticosteroids, vincristine and asparaginase between the pediatric and
adult protocols, whereas the pediatric protocols used a higher
cumulative dose of methotrexate and lower doses of anthracyclines than
the adult protocols. Epipodophyllotoxins and mitoxantrone were not
included in the pediatric protocols. The authors attributed the similar
results to the similarity of the pediatric and adult protocols and to
the centralized care of the patients in five academic centers, ensuring
good compliance and adherence to the protocols in both the two distinct
age groups.
Finally, the retrospective data from the MD Anderson Cancer Center
using the Hyper-CVAD regimen (not including asparaginase) have also
reported favorable results in 102 AYA (median age 19 yr), with CR of
97% and OS of 65%. Preliminary reports from 60 AYA patients aged 12-40
yr treated at the MD Anderson Cancer Center with modified augmented
Berlin-Frankfurt-Münster (BFM) therapy showed very promising results
(2-yr DFS and OS probabilities of 85% and 91%, respectively) in the
subset of patients younger than 25 yr,[21]
stressing the importance of treating these patients in large referral
centers.
In summary, the 5- to 6-yr EFS rate for AYA treated with pediatric
regimens ranges from 65% to 70% vs. 35% to 50% for adult regimens in
almost all retrospective comparative studies. However, it is of note
that the former included adolescents with a median age 16 or 17 yr. and
the latter were adolescents and young adults with a median age 19 yr.
or more. On the other hand, all the studies mentioned above have
focused on patients aged 15-21 years, but few have evaluated the
results in young adults up to 30 years or more, in which the frequency
of patients with adverse prognostic factors is progressively increasing.
A meta-analysis of trials was conducted comparing AYA patients treated
with pediatric versus adult regimens.[22]
This meta-analysis included a total of 11 such trials and 2489
patients. The AYA patients treated with pediatric regimens had
significantly lower all-cause mortality at three years (relative risk
[RR] 0.58, 0.51–0.67). The CR rate was significantly higher when AYA
patients received the pediatric regimen (RR 1.05, 1.01–1.1), and there
was a significant improvement in the 3-year EFS (RR 1.66, 1.39–1.99).
The relapse rate was also lower in patients receiving
pediatric-inspired regimens (RR 0.51, 0.39-0.66) with similar
non-relapse mortality (RR 0.53, 0.19-1.48).
Several factors may have contributed to explain this different outcome,
which cannot be exclusively interpreted in view of some existing
differences in the distribution of prognostic factors between the
populations enrolled in pediatric and adult trials. A major role is
certainly played by differences in protocol design and treatment
intensity, with pediatric protocols including more non myelosuppressive
drugs with demonstrated activity on ALL blasts, such as asparaginase,
glucocorticoids and vincristine. Moreover, central nervous system
prophylaxis was administered earlier, with greater frequency and for a
more prolonged period in pediatric trials and the duration of
maintenance therapy is shorter in some adult trials.[23-25]
A more accurate administration of therapy in pediatric Institutions may
also play a role, due to a peculiar attitude of pediatricians
concerning the need to maintain the doses and schedules prescribed, and
a possibly better compliance of adolescent patients treated in a
pediatric facility.
Results of Treatment of Adolescents with ALL by Pediatric Groups
The outcome of adolescents with ALL worldwide has significantly
improved over time. Barry et al. reported the outcome of adolescents
treated in the Dana-Farber Cancer Institute (DFCI) ALL Consortium
Protocols conducted from 1991 and 2000.[26]
A total
of 844 patients aged 1 to 18 years, with newly diagnosed ALL were
enrolled into two consecutive DFCI-ALL Consortium Protocols. Outcomes
were compared in three age groups: children aged 1 to 10 years (n =
685), young adolescents aged 10 to 15 years (n = 108), and older
adolescents aged 15 to 18 years (n = 51). With a median follow-up of
6.5 years, the 5-year EFS for those aged 1 to 10 years was 85%,
compared with 77% for those aged 10 to 15 years, and 78% for those aged
15 to 18 years. There was no difference in the rate of
treatment-related complications between the 10- to 15-year and 15- to
18-year age groups. The 5-year EFS of 78% is superior to published
outcomes for similarly aged patients treated with other pediatric and
adult ALL regimens.
Nachman et al. reported the results of the CCG1961 trial including AYA
up to 21 yr.[27]
The EFS and overall survival (OS) rates were 71.5% and 77.5%,
respectively. Rapid responder patients randomly assigned to augmented
therapy had a 5-year EFS of 81.8% vs 66.8% for patients receiving
standard therapy, but 1 versus 2 interim maintenance and delayed
intensification courses had no significant impact on EFS. A WBC count
over 50x109/L
was an adverse
prognostic factor. Given the excellent outcomes achieved with this
chemotherapy there seems to be no role for the routine use of stem cell
transplantation (SCT) in first remission.
In turn, the results of the total therapy studies XIIIA, XIIIB, XIV 1
and XV from St Jude Children’s Research Hospital in the US including
963 pediatric patients, 89 of whom were older adolescents (aged 15 to
18 yr.), have been published.[28]
In the first three
studies the 44 older adolescents had a significantly poorer EFS and OS
than the 403 younger patients. On the contrary, in study XV (that
included the level of MRD to guide treatment, with featured intensive
methotrexate, vincristine, glucocorticoid and asparaginase and early
triple intrathecal chemotherapy for higher risk ALL) the EFS of 45
older adolescents was 86.4%, similar to 87.4% for the 453 younger
children. The OS was also comparable (87.9% vs. 94.1%, respectively).
The authors concluded that older adolescents with ALL can be cured with
risk-adjusted intensive chemotherapy without SCT.
Data from the Nordic NOPHO ALL92 protocol showed an EFS lower for
patients >10 yr with B-precursor ALL and WBC<50x109/L
(71% vs. 83%). Interestingly, for adolescents remaining in remission
the mean WBC count during maintenance therapy was correlated with the
risk of relapse, being this risk more pronounced in adolescents than in
non-adolescents.[29] Thus,
compliance to maintenance therapy may influence the risk of relapse in
adolescents with ALL.
In summary, with modern approaches of treatment of ALL with
pediatric-based protocols prognosis of adolescents cannot be considered
unfavorable since, hopefully, the improvement of pediatric patients can
be translated to young adults. This concept has led some groups[30]
to perform a treatment reduction, on the basis of rapid clearance of
MRD by the end of induction therapy, also in young adults (up to 25 yr)
with standard-risk ALL.
Pediatric-inspired and Unmodified Pediatric Treatments in Young Adults. Prospective Studies
Several prospective clinical trials using pediatric regimens for adults have been published (Table 3) or are currently ongoing. These studies are divided into two types according to their regimens and patients: a pediatric-inspired protocol planned by dose reduction of a pediatric protocol for adults up to 50 or 60 yr, and an unmodified pediatric protocol for AYA up to 30-40 years.
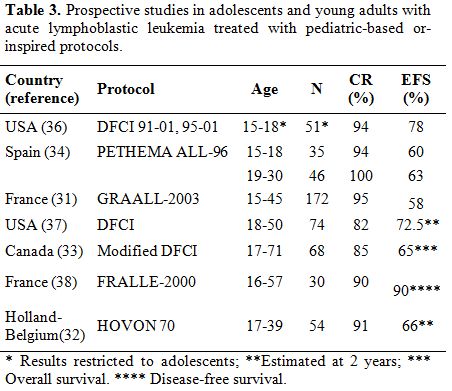 | Table 3. Prospective studies in adolescents and young adults with acute lymphoblastic leukemia treated with pediatric-based or-inspired protocols. |
Pediatric-inspired
protocols:
The French GRAALL group reported the results of the pediatric-inspired
GRAALL-2003 study including 215 patients aged 15-60 years.[31]
In this study there was an 8.6-fold, 3.7-fold and 16-fold increase in
cumulative doses of prednisone, vincristine and asparaginase,
respectively, compared with the previous adult-based LALA-94 protocol,
although the GRAALL-2003 trial retained some adult options, such as
allogeneic SCT for patients with high-risk ALL. The CR rate was 93.5%,
and at 42 months the EFS and OS rates were 55% and 60%, respectively.
The CR rate, EFS and OS compared favorably with the previous LALA-94
experience. It is of note, however, that in patients over 45 yr there
was a higher cumulative incidence of chemotherapy-related deaths (23%
vs. 5%) and deaths in first CR (22% vs. 5%), although the incidence of
relapse remained stable (30 vs. 32%). The results of this study suggest
that the pediatric-inspired therapy is feasible in young adults with
ALL in whom the outcome clearly improves at least until the age of 45
yr.
The HOVON Group published the results of a study in adults with ALL up
to the age of 40 yr.,[32]
inspired by a pediatric regimen (FRALLE approach for high-risk ALL),
including intensified treatment with allogeneic SCT. Allogeneic SCT was
offered to the standard-risk patients with sibling donor and to all
high-risk patients. Fifty-four patients were included, with a median
age of 26 yr. Complete remission was achieved in 49 patients (91%), of
whom 33 (61%) completed treatment as scheduled. Side effects primarily
consisted of infections and occurred in 40% of patients. With a median
follow-up of 32 months, the estimated EFS was 66% and OS 72% at 24
months. In turn, the Princess Margaret Hospital used a modified
Dana-Farber Cancer Institute pediatric protocol in 68 adult patients
(17 to 71 years), with a CR rate of 85% and 3-year OS and DFS of 65%
and 77%, respectively.[33]
Unmodified
pediatric protocols:
The Spanish PETHEMA study was the first trial to compare the results of
the unmodified pediatric protocol ALL96 in adolescents (15-18 yr.,
n=35) and young adults (18-30 yr., n=46) with standard-risk (SR) ALL.[34]
Both groups were comparable for the main clinical and biologic
characteristics of ALL. The CR rate was 98% and after a median
follow-up of 4.2 yr., 6-year EFS and OS were 61% and 69%, with no
differences between adolescents and young adults. No significant
differences were observed in the timing of treatment delivery, although
the hematologic toxicity in consolidation and reinforcement cycles was
higher in young adults than in adolescents. These results suggest that
unmodified pediatric protocols can be efficiently and safely employed
in adult patients with SR ALL, at least up to the age of 30 yr.
Similarly, an Australian study has recently reported the results of the
FRALLE-93 pediatric protocol in 40 AYA up to 45 years, with no
treatment-related mortality and an OS probability of 75% in patients
with SR ALL, without the need for allogeneic SCT.[35]
However, until recently, the efficacy of a fully pediatric protocol for
AYA with high-risk ALL has not been analyzed. The Japan Adult Leukemia
Study Group (JALSG) conducted a phase 2 trial in which 129 patients
aged 15 to 24 with BCR-ABL-negative
ALL were treated with the same protocol developed for children with ALL
by the Japan Association of Childhood Leukemia Study (JACLS). The CR
rate was 94% and the 5-year DFS and OS rates were 67% (95% CI 58–75%)
and 73% (95% CI 64–80%), respectively.[36]
Severe
adverse events frequently occurred but the frequency was similar to
that observed in children treated with the same protocol. Only
insufficient maintenance therapy significantly worsened the DFS.
The DFCI Combined Adult/Pediatric ALL Consortium has applied a
pediatric protocol for adults aged 18-50 years.[37]
Specifically, the investigators used an extended course of asparaginase
for 30 weeks. The results in 74 patients have shown a CR rate of 82%
with promising 2-year EFS and OS probabilities of 72.5% and 73.2%,
respectively. This study proved that extended asparaginase treatment
was feasible in adults, and the drug-related toxicity was manageable,
although the incidence of pancreatitis (13%) and thrombosis/embolism
(19%) was a matter of concern. The University of South California group[38]
used an augmented BFM pediatric regimen with eight doses of pegylated
asparaginase to treat adults with ALL aged 19-57 years (median 33),
with a 3-year projected EFS of 65%. Toxicity attributable to
asparaginase was frequent but manageable. However, older patients
showed significantly less tolerance to asparaginase, vincristine and
steroids compared to children or adolescents. In the FRALLE group from
France 30 Philadelphia chromosome-negative adult ALL patients 16 to 57
years of age were treated in the FRALLE 2000 protocol consisting of a
prednisone pre-phase and a 4-drug induction including asparaginase,
consolidation, delayed intensification and maintenance chemotherapy.
The 4-yr DFS was 90% vs. 47% in matched historical controls.[39]
The undergoing U.S. intergroup trial C10403 (available at
www.clinicaltrials.gov. NCT00558519) has been developed to examine and
describe results among AYA with newly diagnosed ALL, treated using a
successful Children’s Oncology Group regimen (COG ALL0232), which give
rise to 78% survival rate for older adolescents aged 16–21 years.
Individuals aged 16–39 with newly diagnosed ALL are eligible for
participation.[40] This trial will
test the
feasibility of treating young adults up to the age of 40 with a
pediatric regimen, assess adherence by patients and adult oncologists,
and describe the toxicities observed. A component of this trial will
analyze issues based on demographics, psychosocial characteristics, and
pretreatment features of the disease that are unique to the AYA
population. The results of this trial will be compared with patients up
to 29 years of age who were enrolled and treated by pediatric
oncologists on the COG AALL0232 study (now closed to accrual). The goal
of this trial is to demonstrate that the adult cancer cooperative
groups can deliver a “true pediatric’’ regimen to AYA patients and
achieve similar outcomes.
In recent years, the MRD research has been incorporated to the
therapeutic trials of adult AL, and it has been shown that a subgroup
of patients with good MRD clearance can be successfully treated without
SCT.[41-43] Although these trials
incorporate
elements of pediatric protocols, no specific data on the AYA
subpopulation were presented to date.
Allogeneic Stem Cell Transplantation (SCT) in AYA
The role of allogeneic SCT in AYA is not clearly defined, and there are
no prospective trials to assess the role of allogeneic SCT specifically
in this population. An older retrospective study, from 1995, included
patients between the ages of 15–45, from the International Bone Marrow
Transplant Registry, and showed no survival advantages compared with
chemotherapy alone. Transplanted patients had lower relapse rates, but
this was offset by the higher transplant-related mortality.[44]
The largest published study evaluating the role of allogeneic SCT in
ALL was a joint effort of Medical Research Council (MRC) in Great
Britain and Eastern Cooperative Oncology Group (ECOG).[45]
This trial enrolled nearly 2000 ALL patients and 234 patients younger
than 20 years of age. Based on this trial, there was an improvement in
the 5-year OS of all patients (53% vs. 45%) and standard-risk patients
with Philadelphia-negative ALL (62% vs. 52%). The 10-year cumulative
relapse rate was 24% when allogeneic SCT was utilized versus 49% when
patients were treated with chemotherapy alone or autologous SCT.
However, there was no significant survival advantage in the high-risk
group (41% vs. 35%) (P
= 0.2).
The high transplant-related mortality in this group (36%) offset the
lower relapse rate in the high-risk group. One of the major limitations
of this study was the use of adult regimens in treating AYA. A
meta-analysis from 13 studies including 2962 patients, excluding
Philadelphia chromosome-positive patients, showed a survival benefit
for having a matched sibling donor for patients < 35 years of
age
(OR = 0.79; 95% CI, 0.70-0.90, P = .0003) but not for those ≥ 35 years
of age (OR = 1.01; 95% CI, 0.85-1.19, P = .9) and concluded that
matched sibling donor myeloablative SCT improves survival only for
younger patients, with an absolute benefit of approximately 10% at 5
years.[46] Improved chemotherapy
outcomes and reduced
non-relapse mortality associated with allogeneic SCT may change the
relative effects of these treatments in the future. A recently
published retrospective study compared changes in survival after
myeloablative SCT for ALL among children (n = 981), AYA (n = 1218), and
older adults (n = 469) who underwent transplantation over 3 time
periods: 1990 to 1995, 1996 to 2001, and 2002 to 2007. Survival
improved over time in AYA and paralleled that seen in children and was
primarily related to lower rates of early treatment-related mortality
in the most recent era, whereas relapse rates did not change over time.[47]
A comparative study of outcomes of children and AYA undergoing allo-HCT
for B-ALL showed that AYA had a significantly inferior survival and a
greater transplantation-related mortality compared with children aged
<13 years, but with no differences in relapse, suggesting that
allo-SCT may overcome relapse in AYA.[48]
Therefore, the use of allogeneic SCT in standard-risk AYA patients
remains controversial and warrants further investigation.[49]
As MRD analysis plays a central role in defining the indications for
allo SCT using pediatric-type programs in adult patients,[50]
future studies should focus on more precisely identifying poor-risk
features, such as disease genomics and host pharmacogenomics, refining
MRD measurements, improving unrelated donor matching, reducing MRD
prior to alloHSCT, and developing post-alloHSCT humoral and cellular
therapy approaches.[51]
Acknowledgments
This work was supported in part by grants RD12/0036/0029 from RTICC, PI10/01417 from Fondo de Investigaciones Sanitarias and 2014 SGR225 (GRE), Generalitat de Catalunya.
References















































[TOP]