Received: July 31, 2014
Accepted: August 22, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014062, DOI 10.4084/MJHID.2014.062
This article is available on PDF format at:

Orietta Spinelli1, Manuela Tosi1, Barbara Peruta1, Marie Lorena Guinea Montalvo1, Elena Maino2, Anna Maria Scattolin2, Margherita Parolini1, Piera Viero2, Alessandro Rambaldi1 and Renato Bassan2
1 Hematology and Bone Marrow
Transplant Unit of Azienda Ospedaliera Papa Giovanni XXIII, Bergamo,
Italy.
2 Hematology and Bone Marrow Transplant Unit, Ospedale
dell’Angelo e SS. Giovanni e Paolo, Mestre-Venezia, Italy.

| This is an Open Access article distributed under the
terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Acute
lymphoblastic leukemia (ALL) is curable in about 40-50% of adult
patients, however this is subject to ample variations owing to several
host- and disease-related prognostic characteristics. Currently, the
study of minimal residual disease (MRD) following induction and early
consolidation therapy stands out as the most sensitive individual
prognostic marker to define the risk of relapse following the
achievement of remission, and ultimately that of treatment failure or
success. Because substantial therapeutic advancement is now being
achieved using intensified pediatric-type regimens, MRD analysis is
especially useful to orientate stem cell transplantation choices. These
strategic innovations are progressively leading to greater than 50%
cure rates.
|
Introduction
Philadelphia-negative
(Ph-) ALL in adults is a relatively rare neoplasm with an overall
survival rate of 40% or slightly higher in adult patients with an age
range between 15 to 60 years. Obtaining an early complete remission
(CR) and avoiding relapse are the two essential therapeutic steps to
achieve cure. Although the vast majority of patients will achieve CR,
nearly half of them are at risk of relapse in relation with the
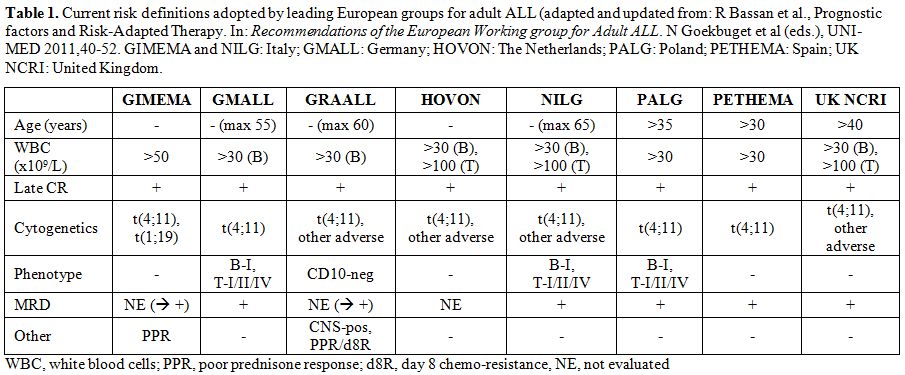
individual risk profile.[1] The several risk factors considered by leading European Groups are shown in Table 1.
Once CR is achieved, the risk-adapted approach is dedicated to identify
the patients who are (more) likely to benefit from conventional
chemotherapy, that carries the lowest risk of treatment-related
morbidity and mortality, and those for whom allogeneic stem cell
transplantation (allo-SCT) is indicated, despite the higher toxicity
associated with this procedure (at least 10-15% transplant-related
mortality at 1-3 years, and up to 30% in selected bad-risk subsets).[2]
The paradigm of therapy optimization is expected to be further improved
as much as our understanding of the mechanisms underlying resistance
and relapse is increased, and novel therapeutics for specific risk and
disease subsets are developed.
ALL Risk Subsets
Ph- ALL is a disease of B- or T-cell precursors and is mostly defined by a rapid immunophenotypic analysis of blast cell populations. B-lineage ALL (80%) usually expresses cytoplasmic CD22, CD19, CD10 in the “common” subset, and clonal surface immunoglobulin in Burkitt leukemia; T-lineage ALL (20%) does express cytoplasmic CD3, CD7, CD1a+ in cortical T-ALL, and surface CD3 in mature T-ALL. Cytogenetics and genetics are necessary to distinguish between Philadelphia chromosome/BCR-ABL1 positive (Ph+) and Ph- ALL, or identify other high-risk abnormalities such as t(4;11)/KMT2A rearrangements, monosomy 7, hypodiploidy and IKZF1 gene deletion. Recognizing Ph+ ALL and Burkitt leukemia is fundamental, because these formerly high-risk subsets require and can greatly benefit from different, highly specific treatments. ALL subsets are variously considered in the risk sub-classification of different study Groups (Table 1).
Treatment Steps and the Role of MRD Analysis
The disease response to chemotherapy remains the primary
determinant of outcome. It would be possible as demonstrated by several
recent trials, to lower the rate of refractory/relapsed (R/R) ALL and
improve overall treatment results by moving from standard adult-type
programs to pediatric-derived therapy (PDT), as extensively reviewed by
J Ribera in this issue of the Journal. Apart from that, the response
kinetics to the early components of chemotherapy can be assessed
through MRD analysis at predefined treatment steps, using either flow
cytometry that identifies leukemia-associated immunophenotype or RQ-PCR
methodology that detects abnormal fusion genes or case-specific gene
rearrangements. Post-induction MRD is the most important prognostic
indicator, basically superseding any other pretreatment risk factor for
relapse.[3] For this reason, a prospective MRD
analysis can be used to enable recognition of “true” high-risk (HR)
patients with suboptimal MRD response, to whom offer allo-SCT.[4,5]
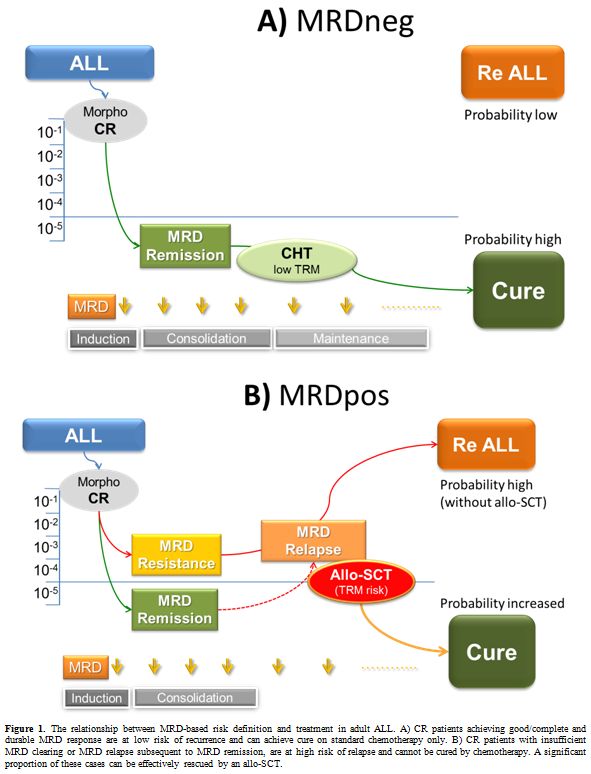
On the other hand patients showing complete MRD response following
induction/consolidation chemotherapy, at “true” standard-risk (SR), can
avoid allo-SCT (simultaneously lowering treatment mortality).[4] The general outline of this strategy is highlighted in Figure 1.
In this regard terms such as molecular complete remission (molCR) and
resistance (or relapse) indicate absent or low MRD signals (<10-4) versus persistence or rise of MRD above this critical threshold.[5]
The relationship between MRD positivity and relapse is a strict one, a
full blown relapse occurring within few weeks to months from MRD
reappearance despite intensive treatment.[6]
MRD Detection: Molecular Analysis of Gene Rearrangements
MRD Detection: Molecular Analysis of Gene Fusion Transcripts
Another method for molecular MRD detection and monitoring is based on
fusion transcript analysis. Forty percent of ALL samples bear
chromosomal translocations generating chimeric transcripts that can be
used to discriminate leukemic cells from normal cells. The most common
translocation product found in adult ALL is that of Ph+ ALL, i.e. the
BCR-ABL1 transcript (25-30%), whereas the most common chimeric
transcript in pediatric patients is represented by ETV6-RUNX1 that
accounts for 25-30% of translocated childhood ALL. Other fusion
transcripts are KMT2A-AFF1 and TCF3-PBX1 each accounting from 3-8% of
adult and pediatric ALL.[13] Due to the large DNA
portion in which translocation breakpoints occur, patient specific tool
for MRD evaluation cannot be easily obtained. Interestingly, the RNA
splicing process produces in all the patients the same fusion
transcript or few splicing variants. This offer the opportunity to
apply the same primer set to all the patients bearing the same
translocation leading to an easy and rapid fusion transcript evaluation
at diagnosis and during treatment.[14] Another
advantage of this approach is represented by the stable association
between the gene fusion and the leukemic clone because of its
involvement in neoplastic transformation.
Problems and Pitfalls of Molecular MRD Analysis
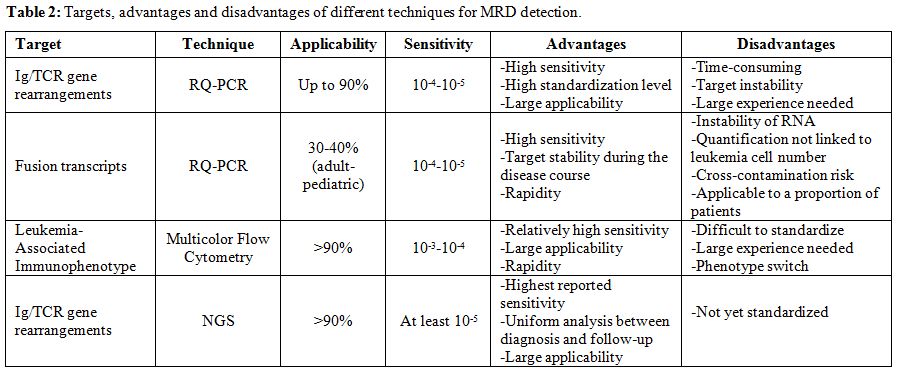
The generation of clone-specific Ig/TCR probes for MRD evaluation is quite expensive, time-consuming and requires experienced personnel. Therefore, this method can be successfully applied only by specialized laboratories processing several cases per year. Other pitfalls have to be considered: in lymphoid cells receptor-antigen rearrangements are not linked to the oncogenic process itself and can persist during the treatment of ALL, possibly leading to falsely positive MRD results.[15] The real occurrence of this phenomenon is unknown. Furthermore, leukemic sub-clones with a different rearrangement pattern already present at low, undetectable levels at diagnosis can emerge during treatment.[16,17] In addition, false positive results can be obtained when massive, post-chemotherapy lymphocyte regeneration is present.[18-20] Also the analysis of abnormal fusion gene transcripts presents some limitations. First, the utilization of RNA as RQ-PCR target that is more prone to degradation, impairing in some cases a correct MRD detection. Another risk is the cross-contamination during the RQ-PCR procedure possibly leading to false positive results. Furthermore, the amount of transcript is not directly related to leukemic cell number but rather depends on the transcription level that can differ among cases, rendering the MRD measurement less accurate and less comparable between patients.[14] Cooperative efforts are ongoing to optimize BCR-ABL1 detection and to harmonize MRD expression by the EWALL and ESG-MRD-ALL (now fused into the EuroMRD study group).[21]
MRD Detection: Flow Cytometry Analysis
A further MRD detection method is represented by multi-parametric flow
cytometry (MFC). This approach takes advantage from the presence of
proteic epitopes on the cell surface, that are differently expressed by
B- and T-lymphoblasts and are sequentially acquired during cell
development. The study of these molecules with specific diagnostic
antibodies can identify different stages of development of normal
lymphocytes as well as leukemic cells in which an aberrant or
asynchronous expression can be found. This leukemia-associated
immunophenotype (LAIP) has to be identified at diagnosis before any
therapy in each ALL case, by comparing the marker profile of leukemia
cells to reference bone marrow samples. This approach is successful in
a vast proportion of cases (>90%) and can reach a sensitivity of 10-3-10-4 (one leukemic cell out of 1000-10,000 normal cells).[22-24]
The MFC analysis is quick, can release MRD evaluations suitable for
clinical decisions in few hours, and is, therefore, particularly useful
to assess the therapeutic response following the first two induction
weeks.[25]
Problems and Pitfalls of MFC MRD Analysis
Despite its rapidity and high applicability rate, MFC also has some limitations. It requires fresh, viable cells, which could be a problem within multicenter studies in which samples are sent to a reference laboratory, as shipping can take more than 24 hours. Furthermore, the sensitivity is greatly dependent on the number of evaluated cells (i.e. number of acquired events), while specificity depends on the presence of true leukemic cells in the MRD samples. In post-induction, a regenerating bone marrow can contain a large number of immature normal lymphoid cells leading to false positive results.[18-20] Another phenomenon that can impair MRD detection by MFC is a phenotypic shift occurring with chemotherapy and modifying the leukemic antigen profile.[26] The latter problem can be partially overcome by using multiple sets of markers for each case. MFC-based MRD studies underwent a large effort to standardization of methodology during the last decade, again within the EuroFlow Consortium.[27,28] Nevertheless, a reliable MFC MRD evaluation can only be performed in a specialized laboratory run by highly experienced operators.
MRD Detection: the Novelty of Next Generation Sequencing
The recent availability of high-throughput next generation sequencing (NGS) provides an opportunity to explore new methods for MRD detection and monitoring. Similar to the conventional molecular techniques, the first NGS studies were based on Ig/TCR gene rearrangements amplification by multiple sets of oligonucleotides. The difference is that amplicons are sequenced by NGS instead of Sanger sequencing, thus giving the opportunity to identify dominant clones, as well as minor clones. Follow up samples are then studied with the same amplification and sequencing approach without the need of performing a patient, clone–specific RQ-PCR assay. To calculate the absolute number of rearranged molecules, it is necessary to add specific reference sequences.[29-32] NGS based methods are potentially more accurate and sensitive than RQ-PCR based technology and can increase the accuracy of MRD analysis for clinical purposes, but some aspects remain to be specifically addressed. These refer to quantity and type of diagnostic material, internal controls, primer design and combination for multiplex reactions, background definition, maximal and reproducible sensitivity determination, sequence quality parameters, error correction and bioinformatics data analysis. A European Consortium, named EuroClonality-NGS Consortium, has been recently created to address all these aspects in a scientifically independent way. The consortium consists of laboratories already experienced in designing and testing assays for Ig/TCR rearrangements detection and their evaluation within clinical trials.[33]
Clinical and Therapeutic Implications of MRD Analysis
Since MRD analysis is adopted to refine the individual prognostic profile (Table 1), it is expected that a significant proportion of SR patients, without any known traditional risk factor, will be found MRD positive (MRDpos) at convenient, prefixed MRD study time-points. These patients, still harboring many ALL cells in remission marrows, usually more than 10-4 and up to 10-2, will experience disease relapse in an usually short lapse of time, despite continuation of intensive therapy (Figure 1). This is the case where MRD analysis can explain why up to 40% of clinically SR patients do eventually relapse. Because persistent MRD reflects chemo-resistance, there is little point in continuing the same therapy, given the increasing risk of recurrence over time. On the contrary there are patients with HR ALL, who exhibit prompt and complete MRD response, a finding that in all MRD-based trials is associated with a high probability of cure. Therefore, an allo-SCT may be therapeutically redundant in MRD negative (MRDneg) patients while it must be seriously considered in MRDpos ones, in order to overcome the poor outlook associated with the persistence and rise of residual disease. Terms such as molecular CR (molCR) and molecular failure (molFail) were coined to distinguish these opposite conditions.[5] In essence the MRD analysis refines our ability to recognize which patients can be rather safely excluded from allo-SCT, because obtaining a satisfactory cure rate with chemotherapy only and without the risk of SCT-related mortality. Instead, MRDpos patients would partially benefit from an allo-SCT. Against this background, we review the evidence gathered by prospective, MRD-based clinical trials in adult Ph- ALL.
Clinical Application of MRD Study in Ph- Adult ALL: Prospective Clinical Trials
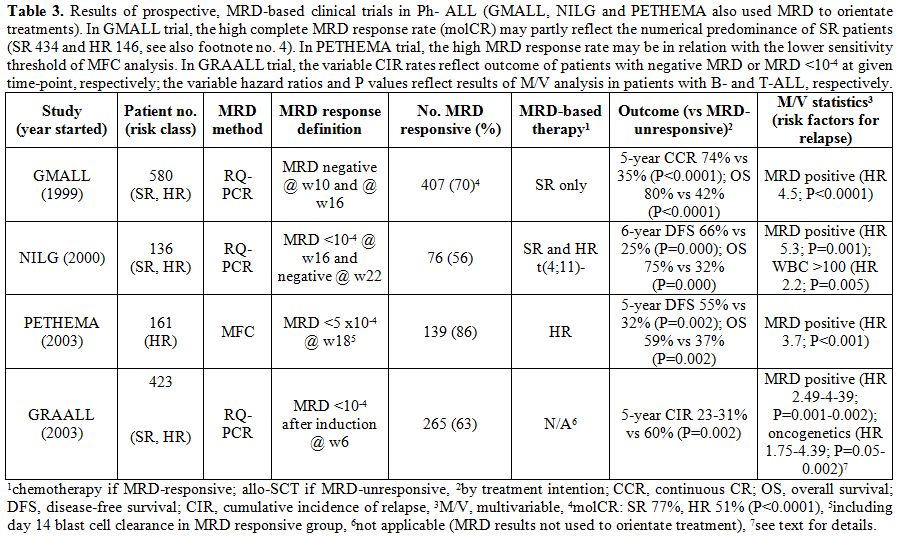
The published data concern 580 patients from GMALL trials,[5] 136 patients from a NILG trial,[4,34] and 161 patients from a PETHEMA trial,[35]
for a total of 877 adult patients with Ph- ALL valuable for MRD
response to induction/consolidation therapy and eligible to an
MRD-driven treatment strategy with regard to the final decision between
allo-SCT or standard chemotherapy in CR1. Notably, these patients are a
variable proportion of all patients in each study, the reasons for
exclusion from the MRD study being reported in the appropriate
reference and ranging from lack of suitable MRD markers to early
treatment failure, etc. While GMALL and NILG trials adopted the
molecular MRD analysis, the PETHEMA trial adopted the MFC analysis. In
addition, in a GRAALL trial the molecular MRD response was
prospectively assessed in a further 423 patients, although this
information was not used to allocate them to different treatments.[36]
Nevertheless selected results from this study will be reviewed because
highly relevant to this discussion. The main results from the trials
quoted are shown in Table 3.
As shown, the GMALL, NILG and PETHEMA studies had a different design
about patient selection, methods and timing of MRD analysis, and
therapeutic decisions based on MRD analysis results. In particular an
MRD- based treatment was planned for SR subsets only in the GMALL
trial, all risk subsets except t(4;11) positive ALL in the NILG trial,
and HR subsets only in the PETHEMA trial. Apart from that and the
different treatment protocols, MRD was confirmed in all studies as the
most significant risk factor for relapse, with a significantly better
outcome for MRDneg patients, over the MRDpos group, regardless of
allo-SCT being performed or not. Interestingly, the GRAALL study
confirmed an interaction between MRD and oncogene expression, the risk
of relapse being the highest for MRDpos patients with KMT2A positivity
or IKZF1-deleted B-ALL, or with NOTCH1/FBW7WT and/or N/K-RAS-mutated and/or PTEN-altered T-ALL.[36]
Again, a subset analysis of the NILG trial restricted to patients with
CD20+ B-ALL demonstrated that patients with this poor-risk ALL subset
have an excellent outcome even without allo-SCT when MRD negativity is
achieved.[37]
Effects of allo-SCT in MRDpos Subsets
As a rule MRDpos patients rely on allo-SCT for survival and will
relapse rapidly unless transplantation or other alternative treatments
are successfully applied. In order to do so, it is mandatory to search
for an HLA-compatible related or unrelated donor (or another source:
cord blood) as soon as possible, to have the shortest possible interval
between detection of MRD positivity and allotransplantation. To save
time, because the CR rate is about 90% in adult ALL, the donor search
should initiate at diagnosis in all patients, without waiting for CR.
Then, the conditioning regimen, whenever possible, should be
intensified using either total body irradiation (TBI) >13 Gy or
etoposide, which in a large retrospective analysis of
allotransplantation in advanced-stage ALL (of which MRDpos ALL may
represent a preclinical variant) were more effective than TBI <13 Gy
(with cyclophosphamide) or cyclophosphamide (with TBI >13 Gy),
respectively.[38] Subsequently, it is wise to
re-check MRD rapidly post-transplantation, to decide about tapering of
immune suppression and/or start of donor lymphocyte infusions. The
results of the three prospective European trials, albeit with some
differences, confirmed the partial success of allo-SCT in MRDpos
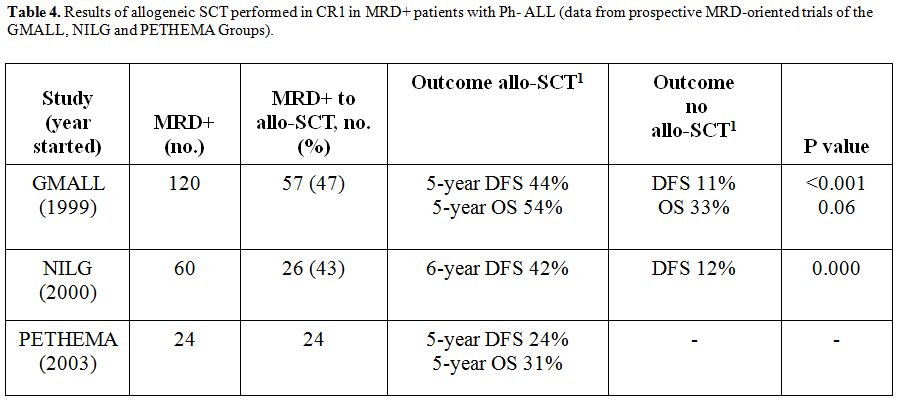
patients, with an average long-term survival rate around 50% (Table 4).
Notably, the GRAALL study contributed a significant information in
clinically HR patients aged 15-55 years (n=522), who were subject to
MRD analysis although an allo-SCT was prescribed by design.[39]
In this study, 238 of 522 total patients had a transplant (54%). When
outcome was considered by MRD status, available for 278 patients, it
was found that an allo-SCT did not benefit the MRD-responsive group
(molecular MRD <10-4 at week 6,
superimposable relapse-free survival rates close to 70% at 4 years),
whereas it improved outcome of MRD-pos group (P=0.04), the survival
rate increasing from about 30% without allo-SCT to about 50% at 4
years. The basic question is, therefore, how to predict cure by
allo-SCT in MRD-pos patients, in order to shift to new experimental
therapies prior to and/or instead of an allo-SCT in patients unlikely
to benefit from this procedure. The final long-term update of the NILG
trial[34] indicated how the risk of
post-transplantation relapse in MRDpos patients correlated with
post-induction quantitative MRD peaks at planned study time-points
(weeks 10, 16 and 22), regardless of the time elapsed from CR to MRD
analysis and/or subsequent SCT. Patients undergoing allo-SCT with one
or more post-induction MRD reads of 10-3 and greater had an inferior outcome (5-year survival 20% vs. 60% with all post-induction MRD reads <10-3). The 10-3 MRD level could be critical for the decision to transplant or not MRDpos patients.
The Future of MRD Analysis in Ph- ALL
Fifteen years of MRD-based clinical studies established the dominant
prognostic role of MRD, confirmed the value of MRD-based clinical
trials and allowed to identify the majority of the patients who are
likely to be cured by chemotherapy with no or little treatment-related
mortality. These latter become early and persistently MRDneg, with no
relationship to their clinical risk class. Instead, the patients
displaying either MRD persistence or relapse are not curable by
chemotherapy and may be cured by SCT (despite the higher death risk),
although in a lower proportion than unselected ALL patients, in
relation with quantitative MRD ranges and other undefined factors. The
predictive power of MRD analysis is not absolute because 20-30% of MRD
responsive patients will relapse, and certainly needs to be improved.
At the same time, the risk definition given by MRD is as yet
unrivalled, representing the disease itself, serially measured at the
submicroscopic level in response to the anti-leukemic therapy, which is
an extremely useful information. If well used, this instrument offers
the best chance to individualize and optimize treatments on a sound,
rational basis. In the end, coupling modern PDT concepts for
induction/consolidation therapy with an improved MRD-based and
risk-based transplantation strategy focusing on early, clinically
meaningful time-points, may open the way to real therapeutic progress.
As an example, in the recent PDT/MRD-based NILG study (n=140; age range
18-60 years), week 10 MRD response was increased to 72% and 4-year
survival rate was 64%.[40] For poor MRD responders
and all those unlikely to benefit from allo-SCT, cytotoxic monoclonal
antibodies like inotuzumab ozogamicin and blinatumomab as well as
chimeric antigen receptor-modified T cells represent new exciting
therapeutic possibilities.[41-43]
References






































